Changes in the Transcriptome and Long Non-Coding RNAs but Not the Methylome Occur in Human Cells Exposed to Borrelia burgdorferi
Abstract
1. Introduction
2. Materials and Methods
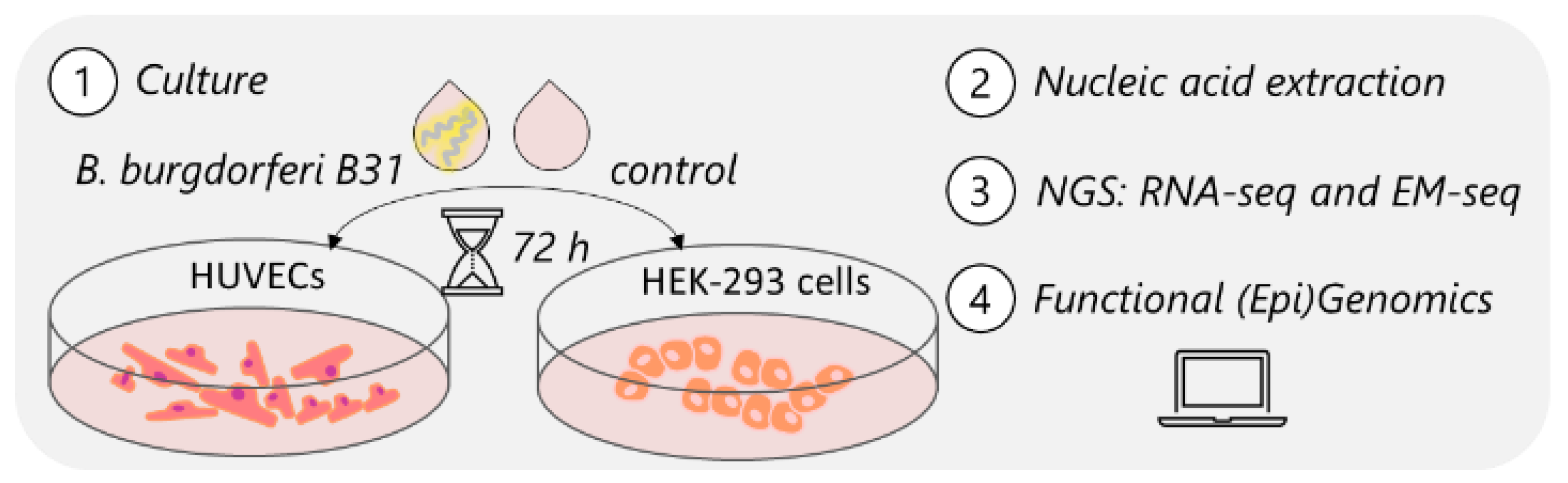
2.1. Human Cell Culture
2.2. Borrelia burgdorferi Culture
2.3. Human Cell Exposure Experiment
2.4. Nucleic Acid Extraction and Assessment
2.5. Library Preparation
2.5.1. RNA-Seq
2.5.2. Enzymatic Methyl-Seq (EM-Seq)
2.6. Sequencing
2.6.1. RNA-Seq
2.6.2. EM-Seq
2.7. Data Analysis
2.7.1. RNA-Seq
2.7.2. EM-Seq
3. Results
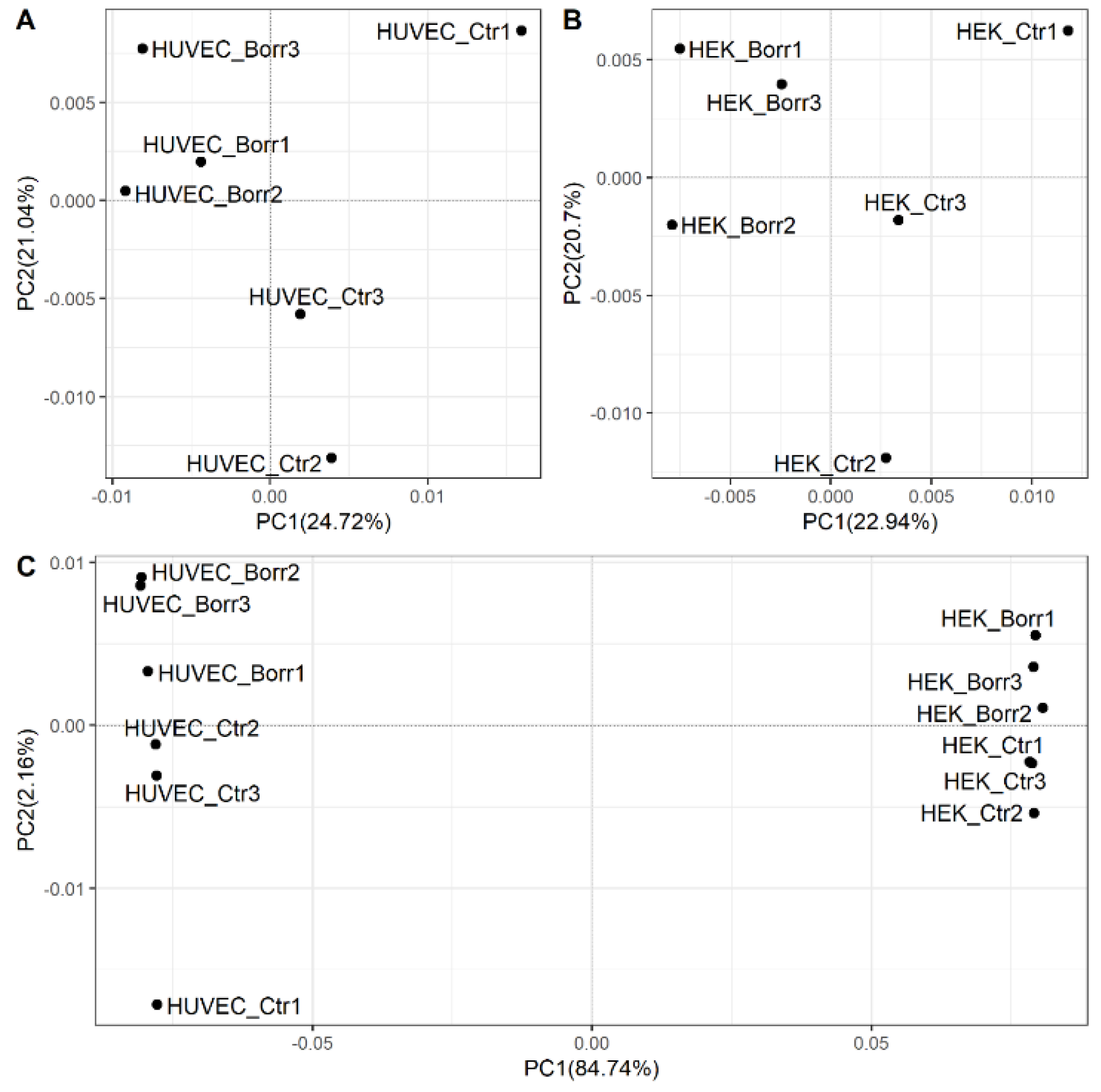
3.1. Human Cells Exposed to B. burgdorferi Strain B31 Show Altered Gene Expression
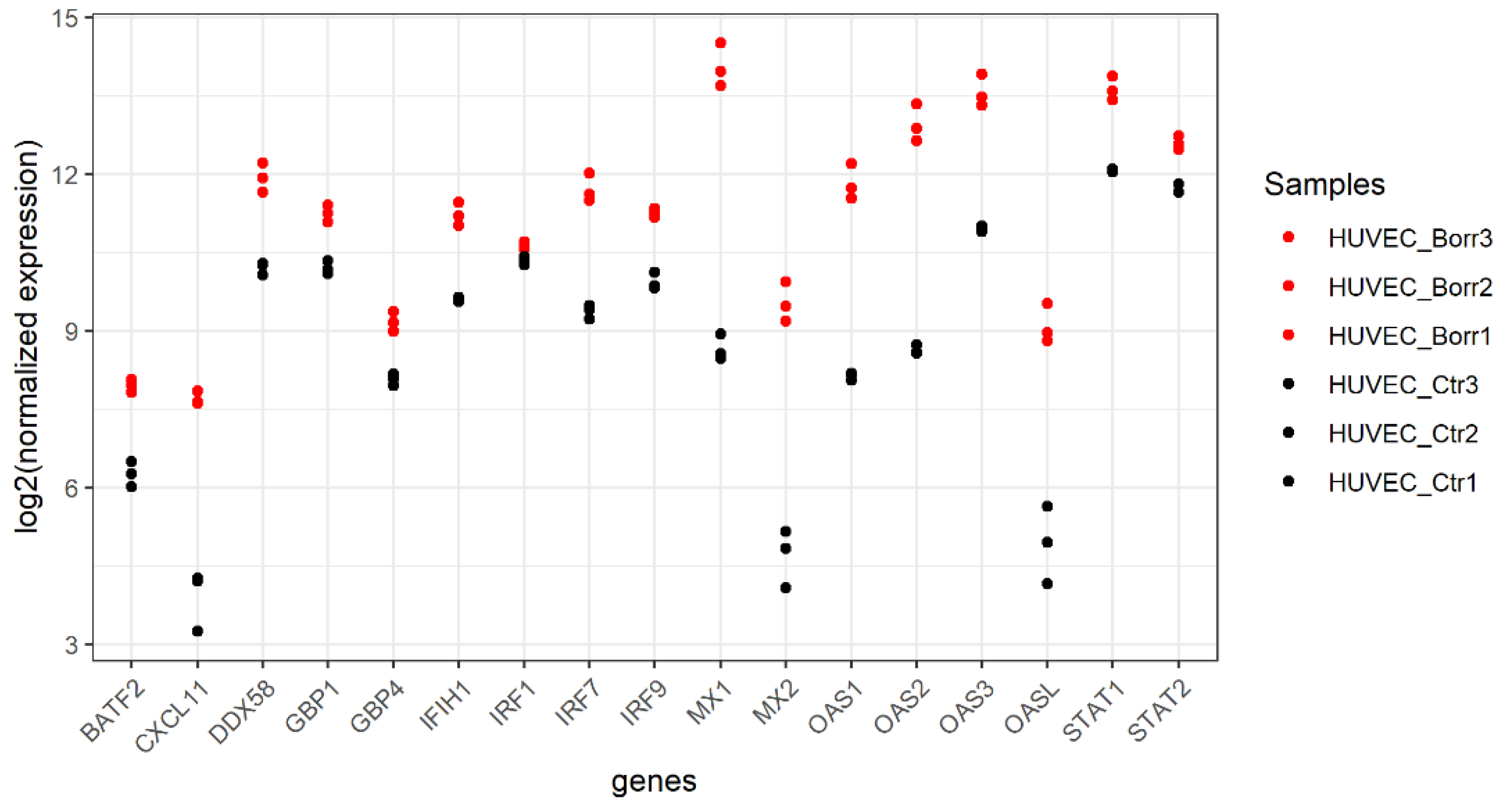
3.2. HUVECs Respond by Upregulating Host Defense Genes
3.3. Gene Expression Patterns in HEK-293 Cells Differ Distinctly from HUVECs
3.4. Transcriptional Changes Are Not Reflected by Methylome Changes
3.5. Changes in Non-Coding RNAs in Response to Borrelia-Exposed HUVECs
3.6. Overlap of Differentially Expressed Genes between This Study and Other Studies of Borrelia-Exposed Cells
4. Discussion
4.1. Immune Response of HUVECs
4.2. The Cellular Response of HEK-293 Cells Suggests ECM Remodelling That Could Promote Bacterial Survival
4.3. B. burgdorferi as an Instigator of Epigenetic Changes
4.4. Ticks as an Instigator of Epigenetic Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef]
- De la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Lyme Disease Charts and Figures: Historical Data. Available online: https://www.cdc.gov/lyme/stats/graphs.html (accessed on 18 May 2021).
- Public Health Agency of Canada Surveillance of Lyme Disease. Available online: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html#a3 (accessed on 18 May 2021).
- Smith, R.; Takkinen, J. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2006, 11, 2977. [Google Scholar] [CrossRef]
- Hinckley, A.F.; Connally, N.P.; Meek, J.I.; Johnson, B.J.; Kemperman, M.M.; Feldman, K.A.; White, J.L.; Mead, P.S. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 2014, 59, 676–681. [Google Scholar] [CrossRef]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of clinician-diagnosed lyme disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for lyme disease—United States, 2008–2015. MMWR Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef]
- Stone, B.L.; Tourand, Y.; Brissette, C.A. Brave New Worlds: The Expanding Universe of Lyme Disease. Vector-Borne Zoonotic Dis. 2017, 17, 619–629. [Google Scholar] [CrossRef]
- Bisanzio, D.; Fernández, M.P.; Martello, E.; Reithinger, R.; Diuk-Wasser, M.A. Current and Future Spatiotemporal Patterns of Lyme Disease Reporting in the Northeastern United States. JAMA Netw. Open 2020, 3, e200319. [Google Scholar] [CrossRef]
- Petrulionienė, A.; Radzišauskienė, D.; Ambrozaitis, A.; Čaplinskas, S.; Paulauskas, A.; Venalis, A. Epidemiology of lyme disease in a highly endemic European zone. Med. Lith. 2020, 56, 115. [Google Scholar] [CrossRef]
- Lindgren, E.; Jaenson, T.G.T. Lyme borreliosis in Europe: Influences of climate and climate change, epidemiology, ecology and adaptation measures. World Health Organ. 2006, 35, 157–188. [Google Scholar]
- Hubálek, Z. Epidemiology of Lyme Borreliosis. Curr. Probl. Dermatol. 2009, 37, 31–50. [Google Scholar] [CrossRef]
- Lloyd, V.; Hawkins, R. Under-Detection of Lyme Disease in Canada. Healthcare 2018, 6, 125. [Google Scholar] [CrossRef]
- Ogden, N.H.; Arsenault, J.; Hatchette, T.F.; Mechai, S.; Lindsay, L.R. Antibody responses to Borrelia burgdorferi detected by western blot vary geographically in Canada. PLoS ONE 2017, 12, e0171731. [Google Scholar] [CrossRef]
- Stanek, G.; Fingerle, V.; Hunfeld, K.P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef]
- Thompson, D.; Watt, J.A.; Brissette, C.A. Host transcriptome response to Borrelia burgdorferi sensu lato. Ticks Tick-Borne Dis. 2021, 12, 101638. [Google Scholar] [CrossRef]
- Kazimírová, M.; Štibrániová, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 2013, 4, 43. [Google Scholar] [CrossRef]
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]
- Borgermans, L.; Goderis, G.; Vandevoorde, J.; Devroey, D. Relevance of Chronic Lyme Disease to Family Medicine as a Complex Multidimensional Chronic Disease Construct: A Systematic Review. Int. J. Fam. Med. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Trevisan, G.; Bonin, S.; Ruscio, M. A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. 2020, 7, 265. [Google Scholar] [CrossRef]
- Rebman, A.W.; Aucott, J.N. Post-treatment Lyme Disease as a Model for Persistent Symptoms in Lyme Disease. Front. Med. 2020, 7, 57. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The role of epigenetics in autoimmune/inflammatory disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Matrone, C.; Singh, L.P. Epigenetic Modifications and Potential New Treatment Targets in Diabetic Retinopathy. J. Ophthalmol. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F.A. Impact of environmental signals on gene expression. Epigenetics 2012, 7, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Baedke, J. The epigenetic landscape in the course of time: Conrad Hal Waddington’s methodological impact on the life sciences. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2013, 44, 756–773. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Díaz, E.; Jordà, M.; Peinado, M.A.; Rivero, A. Epigenetics of Host-Pathogen Interactions: The Road Ahead and the Road Behind. PLoS Pathog. 2012, 8, e1003007. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Alberdi, P.; Ayllón, N.; Valdés, J.J.; Pierce, R.; Villar, M.; de la Fuente, J. Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 2016, 11, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D. Investigation of The Neurological Manifestations of Lyme Disease and The Impact of Borrelia Burgdorferi on The Epigenetic Landscape of Astrocytes. Ph.D. Thesis, University of North Dakota, Grand Forks, ND, USA, 2020. [Google Scholar]
- De la Fuente, J.; Villar, M.; Cabezas-Cruz, A.; Estrada-Peña, A.; Ayllón, N.; Alberdi, P. Tick–Host–Pathogen Interactions: Conflict and Cooperation. PLoS Pathog. 2016, 12, e1005488. [Google Scholar] [CrossRef] [PubMed]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
- Gomez-Chamorro, A.; Hodžić, A.; King, K.C.; Cabezas-Cruz, A. Ecological and evolutionary perspectives on tick-borne pathogen co-infections. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100049. [Google Scholar] [CrossRef]
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. Tick-borne diseases and co-infection: Current considerations. Ticks Tick-Borne Dis. 2021, 12, 101607. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Vayssier-Taussat, M.; Greub, G. Tick-borne pathogen detection: What’s new? Microbes Infect. 2018, 20, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Bigelmayr, S.; Koenigs, A.; Kraiczy, P. Inter- and intraspecies-specific adhesion of Lyme borreliae to human keratinocytes. Ticks Tick-Borne Dis. 2019, 10, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Balderrama-Gutierrez, G.; Milovic, A.; Cook, V.J.; Islam, M.N.; Zhang, Y.; Kiaris, H.; Belisle, J.T.; Mortazavi, A.; Barbour, A.G. An Infection-Tolerant Mammalian Reservoir for Several Zoonotic Agents Broadly Counters the Inflammatory Effects of Endotoxin. mBio 2021, 12, e00588-21. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, A.; Schnell, G.; Bernard, Q.; Kern, A.; Westermann, B.; Ehret-Sabatier, L.; Grillon, A.; Schramm, F.; Jaulhac, B.; Boulanger, N. Dissociating effect of salivary gland extract from Ixodes ricinus on human fibroblasts: Potential impact on Borrelia transmission. Ticks Tick-Borne Dis. 2019, 10, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of Human Skin Fibroblasts by the Lyme Disease Spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, M.; Carpentier, W.; Cagnard, N.; Nadaud, S.; Grillon, A.; Barthel, C.; De Martino, S.J.; Jaulhac, B.; Boulanger, N.; Schramm, F. Homogeneous inflammatory gene profiles induced in human dermal fibroblasts in response to the three main species of Borrelia burgdorferi sensu lato. PLoS ONE 2016, 11, e0164117. [Google Scholar] [CrossRef]
- Wu, J.; Weening, E.H.; Faske, J.B.; Höök, M.; Skare, J.T. Invasion of eukaryotic cells by Borrelia burgdorferi requires β1 integrins and Src kinase activity. Infect. Immun. 2011, 79, 1338–1348. [Google Scholar] [CrossRef]
- Khatri, V.A.; Paul, S.; Patel, N.J.; Thippani, S.; Sawant, J.Y.; Durkee, K.L.; Murphy, C.L.; Aleman, G.O.; Valentino, J.A.; Jathan, J.; et al. Global transcriptomic analysis of breast cancer and normal mammary epithelial cells infected with Borrelia burgdorferi. Eur. J. Microbiol. Immunol. 2023, 13, 63–76. [Google Scholar] [CrossRef]
- Fischer, J.R.; Parveen, N.; Magoun, L.; Leong, J.M. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 2003, 100, 7307–7312. [Google Scholar] [CrossRef]
- Garcia-Monco, J.C.; Fernandez-Villar, B.; Benach, J.L. Adherence of the lyme disease spirochete to glial cells and cells of glial origin. J. Infect. Dis. 1989, 160, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Greenmyer, J.R.; Gaultney, R.A.; Brissette, C.A.; Watt, J.A. Primary human microglia are phagocytically active and respond to Borrelia burgdorferi with upregulation of chemokines and cytokines. Front. Microbiol. 2018, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.M.; Wang, H.; Magoun, L.; Field, J.A.; Morrissey, P.E.; Robbins, D.; Tatro, J.B.; Coburn, J.; Parveen, N. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect. Immun. 1998, 66, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Livengood, J.A.; Gilmore, R.D. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006, 8, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Tkáčová, Z.; Bhide, K.; Mochnáčová, E.; Petroušková, P.; Hruškovicová, J.; Kulkarni, A.; Bhide, M. Comprehensive Mapping of the Cell Response to Borrelia bavariensis in the Brain Microvascular Endothelial Cells in vitro Using RNA-Seq. Front. Microbiol. 2021, 12, 760627. [Google Scholar] [CrossRef] [PubMed]
- Galbe, J.L.; Guy, E.; Zapatero, J.M.; Peerschke, E.I.B.; Benach, J.L. Vascular clearance of borrelia burgdorferi in rats. Microb. Pathog. 1993, 14, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Georgilis, K.; Steere, A.C.; Klempner, M.S. Infectivity of Borrelia burgdorferi correlates with resistance to elimination by phagocytic cells. J. Infect. Dis. 1991, 163, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, K.A.; Brown, C.R. Treatment of Borrelia burgdorferi –Infected Mice with Apoptotic Cells Attenuates Lyme Arthritis via PPAR-γ. J. Immunol. 2019, 202, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, V.; Ingle, T.M.; Kilgore, N.; Zhang, G.; Hermann, B.P.; Seshu, J. Cellular and transcriptome signatures unveiled by single-cell RNA-Seq following ex vivo infection of murine splenocytes with Borrelia burgdorferi. Front. Immunol. 2023, 14, 1296580. [Google Scholar] [CrossRef]
- Salazar, J.C.; Duhnam-Ems, S.; La Vake, C.; Cruz, A.R.; Moore, M.W.; Caimano, M.J.; Velez-Climent, L.; Shupe, J.; Krueger, W.; Radolf, J.D. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-β. PLoS Pathog. 2009, 5, e1000444. [Google Scholar] [CrossRef]
- Servellita, V.; Bouquet, J.; Rebman, A.; Yang, T.; Samayoa, E.; Miller, S.; Stone, M.; Lanteri, M.; Busch, M.; Tang, P.; et al. A diagnostic classifier for gene expression-based identification of early Lyme disease. Commun. Med. 2022, 2, 92. [Google Scholar] [CrossRef] [PubMed]
- Wikel, S. Ticks and tick-borne pathogens at the cutaneous interface: Host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front. Microbiol. 2013, 4, 337. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Comstock, L.E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect. Immun. 1989, 57, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, A.; Furie, M.B.; Benach, J.L.; Lane, B.P.; Fleit, H.B. Interaction between Borrelia burgdorferi and endothelium in vitro. J. Clin. Investig. 1990, 85, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sturrock, A.; Weis, J.J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 1991, 59, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Dame, T.M.; Orenzoff, B.L.; Palmer, L.E.; Furie, M.B. IFN-g Alters response of Bb activated endotheliom to Favor Chronic Inflammation. J. Immunol. 2007, 178, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Robbins, D.; Leong, J.M. Strain variation in glycosaminoglycan recognition influences cell-type- specific binding by lyme disease spirochetes. Infect. Immun. 1999, 67, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Baranton, G.; Postic, D.; Saint Girons, I.; Boerlin, P.; Piffaretti, J.C.; Assous, M.; Grimont, P.A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 1992, 42, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Schmid, G.P.; Hyde, F.W.; Steigerwalt, A.G.; Brenner, D.J. Borrelia burgdorferi sp. nov.: Etiologic Agent of Lyme Disease. Int. J. Syst. Bacteriol. 1984, 34, 496–497. [Google Scholar] [CrossRef]
- Berthold, A.; Faucillion, M.-L.; Nilsson, I.; Golovchenko, M.; Lloyd, V.; Bergström, S.; Rudenko, N. Cultivation Methods of Spirochetes from Borrelia burgdorferi Sensu Lato Complex and Relapsing Fever Borrelia. J. Vis. Exp. 2022, 189, 64431. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasit. Vectors 2020, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aberer, E.; Surtov-Pudar, M.; Wilfinger, D.; Deutsch, A.; Leitinger, G.; Schaider, H. Co-culture of human fibroblasts and Borrelia burgdorferi enhances collagen and growth factor mRNA. Arch. Dermatol. Res. 2018, 310, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, K.; Nykky, J.; Marjomäki, V.; Gilbert, L. Distinctive Evasion Mechanisms to Allow Persistence of Borrelia burgdorferi in Different Human Cell Lines. Front. Microbiol. 2021, 12, 711291. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.M.K.; Koetsveld, J.; Trentelman, J.J.A.; Kaptein, T.M.; Hoornstra, D.; Wagemakers, A.; Fikrig, M.M.; Ersoz, J.I.; Oei, A.; Geijtenbeek, T.B.H.; et al. Borrelia miyamotoi Activates Human Dendritic Cells and Elicits T Cell Responses. J. Immunol. 2020, 204, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Krueger, F. Trim Galore. Babraham Bioinforma. 2016. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 20 May 2021).
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna Austria, 2020. [Google Scholar]
- Love, M.I.; Soneson, C.; Hickey, P.F.; Johnson, L.K.; Tessa Pierce, N.; Shepherd, L.; Morgan, M.; Patro, R. Tximeta: Reference sequence checksums for provenance identification in RNA-seq. PLoS Comput. Biol. 2020, 16. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Various R programming tools for plotting data. Compr. R Arch. Netw. CRAN 2015, 1–66. [Google Scholar]
- Wickham, H. ggplot2; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Akalin, A.; Kormaksson, M.; Li, S.; Garrett-Bakelman, F.E.; Figueroa, M.E.; Melnick, A.; Mason, C.E. MethylKit: A comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Carreras-González, A.; Navasa, N.; Martín-Ruiz, I.; Lavín, J.L.; Azkargorta, M.; Atondo, E.; Barriales, D.; Macías-Cámara, N.; Pascual-Itoiz, M.A.; Sampedro, L.; et al. A multi-omic analysis reveals the regulatory role of CD180 during the response of macrophages to Borrelia burgdorferi article. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schramm, F.; Kern, A.; Barthel, C.; Nadaud, S.; Meyer, N.; Jaulhac, B.; Boulanger, N. Microarray analyses of inflammation response of human dermal fibroblasts to different strains of Borrelia burgdorferi sensu stricto. PLoS ONE 2012, 7, e40046. [Google Scholar] [CrossRef]
- Thompson, D.; Sorenson, J.; Greenmyer, J.; Brissette, C.A.; Watt, J.A. The Lyme disease bacterium, Borrelia burgdorferi, stimulates an inflammatory response in human choroid plexus epithelial cells. PLoS ONE 2020, 15, e0234993. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Konradt, C.; Hunter, C.A. Pathogen interactions with endothelial cells and the induction of innate and adaptive immunity. Eur. J. Immunol. 2018, 48, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Duray, P.H. The surgical pathology of human Lyme disease. An enlarging picture. Am. J. Surg. Pathol. 1987, 11 (Suppl. S1), 47–60. [Google Scholar] [CrossRef]
- Müllegger, R.R.; Means, T.K.; Shin, J.J.; Lee, M.; Jones, K.L.; Glickstein, L.J.; Luster, A.D.; Steere, A.C. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: Predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect. Immun. 2007, 75, 4621–4628. [Google Scholar] [CrossRef]
- Salazar, J.C.; Pope, C.D.; Sellati, T.J.; Feder, H.M.; Kiely, T.G.; Dardick, K.R.; Buckman, R.L.; Moore, M.W.; Caimano, M.J.; Pope, J.G.; et al. Coevolution of Markers of Innate and Adaptive Immunity in Skin and Peripheral Blood of Patients with Erythema Migrans. J. Immunol. 2003, 171, 2660–2670. [Google Scholar] [CrossRef]
- Müllegger, R.R.; McHugh, G.; Ruthazer, R.; Binder, B.; Kerl, H.; Steere, A.C. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Investig. Dermatol. 2000, 115, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Dixit, E.; Kagan, J.C. Intracellular Pathogen Detection by RIG-I-Like Receptors. Adv. Immunol. 2013, 117, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Guler, R.; Roy, S.; Suzuki, H.; Brombacher, F. Targeting Batf2 for infectious diseases and cancer. Oncotarget 2015, 6, 26575–26582. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Lasagni, L.; Annunziato, F.; Serio, M.; Romagnani, S. CXC chemokines: The regulatory link between inflammation and angiogenesis. Trends Immunol. 2004, 25, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, N.; Mandhana, R.; Horvath, C.M. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. Jak-Stat 2013, 2, e23931. [Google Scholar] [CrossRef] [PubMed]
- Leisching, G.; Wiid, I.; Baker, B. The Association of OASL and Type I Interferons in the Pathogenesis and Survival of Intracellular Replicating Bacterial Species. Front. Cell. Infect. Microbiol. 2017, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The oligoadenylate synthetase family: An ancient protein family with multiple antiviral activities. J. Interferon Cytokine Res. 2011, 31, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Leisching, G.; Cole, V.; Ali, A.T.; Baker, B. OAS1, OAS2 and OAS3 restrict intracellular M. tb replication and enhance cytokine secretion. Int. J. Infect. Dis. 2019, 80, S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Woitzik, P.; Linder, S. Molecular mechanisms of borrelia burgdorferi phagocytosis and intracellular processing by human macrophages. Biology 2021, 10, 567. [Google Scholar] [CrossRef]
- Decker, T.; Müller, M.; Stockinger, S. The Yin and Yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 2005, 5, 675–687. [Google Scholar] [CrossRef]
- Ning, S.; Pagano, J.S.; Barber, G.N. IRF7: Activation, regulation, modification and function. Genes Immun. 2011, 12, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Tretina, K.; Park, E.S.; Maminska, A.; MacMicking, J.D. Interferon-induced guanylate-binding proteins: Guardians of host defense in health and disease. J. Exp. Med. 2019, 216, 482–500. [Google Scholar] [CrossRef]
- Santos, J.C.; Boucher, D.; Schneider, L.K.; Demarco, B.; Dilucca, M.; Shkarina, K.; Heilig, R.; Chen, K.W.; Lim, R.Y.H.; Broz, P. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kutsch, M.; Sistemich, L.; Lesser, C.F.; Goldberg, M.B.; Herrmann, C.; Coers, J. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 2020, 39, 1–22. [Google Scholar] [CrossRef]
- Zav’Yalov, V.P.; Hämäläinen-Laanaya, H.; Korpela, T.K.; Wahlroos, T. Interferon-inducible myxovirus resistance proteins: Potential biomarkers for differentiating viral from bacterial infections. Clin. Chem. 2019, 65, 739–750. [Google Scholar] [CrossRef]
- Meriläinen, L.; Brander, H.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Pleomorphic forms of Borrelia burgdorferi induce distinct immune responses. Microbes Infect. 2016, 18, 484–495. [Google Scholar] [CrossRef]
- Klose, M.; Scheungrab, M.; Luckner, M.; Wanner, G.; Linder, S. FIB-SEM-based analysis of Borrelia intracellular processing by human macrophages. J. Cell Sci. 2021, 134, jcs252320. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef]
- Inada, M.; Izawa, G.; Kobayashi, W.; Ozawa, M. 293 Cells Express Both Epithelial As Well As Mesenchymal Cell Adhesion Molecules. Int. J. Mol. Med. 2016, 37, 1521–1527. [Google Scholar] [CrossRef]
- Kirschning, C.J.; Wesche, H.; Merrill Ayres, T.; Rothe, M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998, 188, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; DuMontelle, J.L.; Zolodz, M.; Deora, A.; Mozier, N.M.; Golding, B. Use of toll-like receptor assays to detect and identify microbial contaminants in biological products. J. Clin. Microbiol. 2009, 47, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Najmeh, S.; Martel, G.; MacFadden-Murphy, E.; Farias, R.; Savage, P.; Leone, A.; Roussel, L.; Cools-Lartigue, J.; Gowing, S.; et al. Activation of the pattern recognition receptor NOD1 augments colon cancer metastasis. Protein Cell 2020, 11, 187–201. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Dunham-Ems, S.M.; La Vake, C.J.; Petzke, M.M.; Sahay, B.; Sellati, T.J.; Radolf, J.D.; Salazar, J.C. Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-β. Proc. Natl. Acad. Sci. USA 2011, 108, 3683–3688. [Google Scholar] [CrossRef]
- Wooten, R.M.; Ma, Y.; Yoder, R.A.; Brown, J.P.; Weis, J.H.; Zachary, J.F.; Kirschning, C.J.; Weis, J.J. Toll-Like Receptor 2 Is Required for Innate, But Not Acquired, Host Defense to Borrelia burgdorferi. J. Immunol. 2002, 168, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Wijmenga, C.; O’Neill, L.A.J. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 2012, 13, 535–542. [Google Scholar] [CrossRef]
- Cinco, M.; Murgia, R.; Presani, G.; Perticarari, S. Integrin CR3 mediates the binding of nonspecifically opsonized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect. Immun. 1997, 65, 4784–4789. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Cugini, C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin αvβ3. Proc. Natl. Acad. Sci. USA 2003, 100, 7301–7306. [Google Scholar] [CrossRef]
- Behera, A.K.; Hildebrand, E.; Uematsu, S.; Akira, S.; Coburn, J.; Hu, L.T. Identification of a TLR-Independent Pathway for Borrelia burgdorferi -Induced Expression of Matrix Metalloproteinases and Inflammatory Mediators through Binding to Integrin α 3 β 1. J. Immunol. 2006, 177, 657–664. [Google Scholar] [CrossRef]
- Gebbia, J.A.; Coleman, J.L.; Benach, J.L. Selective Induction of Matrix Metalloproteinases by Borrelia burgdorferi via Toll-Like Receptor 2 in Monocytes. J. Infect. Dis. 2004, 189, 113–119. [Google Scholar] [CrossRef][Green Version]
- Isogai, Z.; Ono, R.N.; Ushiro, S.; Keene, D.R.; Chen, Y.; Mazzieri, R.; Charbonneau, N.L.; Reinhardt, D.P.; Rifkin, D.B.; Sakai, L.Y. Latent transforming growth factor β-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003, 278, 2750–2757. [Google Scholar] [CrossRef]
- Schiller, M.; Javelaud, D.; Mauviel, A. TGF-β-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004, 35, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Takemura, T.; Hino, S.; Yoshioka, K. Cloning, expression, and chromosomal localization of a human tubulointerstitial nephritis antigen. Biochem. Biophys. Res. Commun. 2000, 268, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, Y.Z.; Wei, Y.; Ell, B.; Sheng, X.; Esposito, M.; Kang, J.; Hang, X.; Zheng, H.; Rowicki, M.; et al. Tinagl1 Suppresses Triple-Negative Breast Cancer Progression and Metastasis by Simultaneously Inhibiting Integrin/FAK and EGFR Signaling. Cancer Cell 2019, 35, 64–80.e7. [Google Scholar] [CrossRef]
- Khodosevich, K.; Lazarini, F.; vonEngelhardt, J.; Kaneko, H.; Lledo, P.M.; Monyer, H. Connective Tissue Growth Factor Regulates Interneuron Survival and Information Processing in the Olfactory Bulb. Neuron 2013, 79, 1136–1151. [Google Scholar] [CrossRef]
- Hall-Glenn, F.; de Young, R.A.; Huang, B.L.; van Handel, B.; Hofmann, J.J.; Chen, T.T.; Choi, A.; Ong, J.R.; Benya, P.D.; Mikkola, H.; et al. CCN2/Connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS ONE 2012, 7, e30562. [Google Scholar] [CrossRef] [PubMed]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Seeger, T.S.; Frank, D.; Rohr, C.; Will, R.; Just, S.; Grund, C.; Lyon, R.; Luedde, M.; Koegl, M.; Sheikh, F.; et al. Myozap, a novel intercalated disc protein, activates serum response factor-dependent signaling and is required to maintain cardiac function in vivo. Circ. Res. 2010, 106, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Pieperhoff, S.; Rickelt, S.; Heid, H.; Claycomb, W.C.; Zimbelmann, R.; Kuhn, C.; Winter-Simanowski, S.; Kuhn, C.; Frey, N.; Franke, W.W. The plaque protein myozap identified as a novel major component of adhering junctions in endothelia of the blood and the lymph vascular systems. J. Cell. Mol. Med. 2012, 16, 1709–1719. [Google Scholar] [CrossRef]
- Rickelt, S.; Kuhn, C.; Winter-Simanowski, S.; Zimbelmann, R.; Frey, N.; Franke, W.W. Protein myozap—A late addition to the molecular ensembles of various kinds of adherens junctions. Cell Tissue Res. 2011, 346, 347–359. [Google Scholar] [CrossRef]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.A.; Haltiwanger, R.S. Fringe benefits: Functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr. Opin. Struct. Biol. 2011, 21, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, S.W.; Faulkner, G.J.; Dinger, M.E. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat. Rev. Genet. 2020, 21, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Aznaourova, M.; Schulte, L.N. Non-coding RNA Networks in Infection. Syst. Med. 2021, 2, 565–572. [Google Scholar] [CrossRef]
- Arnold, W.K.; Savage, C.R.; Brissette, C.A.; Seshu, J.; Livny, J.; Stevenson, B. RNA-Seq of Borrelia burgdorferi in multiple phases of growth reveals insights into the dynamics of gene expression, transcriptome architecture, and noncoding RNAs. PLoS ONE 2016, 11, e0164165. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.A. Wonders of tick saliva. Ticks Tick-Borne Dis. 2019, 10, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, M.; Langenberger, D.; Schwarz, A.; Erhart, J.; Kotsyfakis, M. In silico target network analysis of de novo-discovered, tick saliva-specific microRNAs reveals important combinatorial effects in their interference with vertebrate host physiology. RNA 2017, 23, 1259–1269. [Google Scholar] [CrossRef]
- Lewis, L.A.; Radulović, Ž.M.; Kim, T.K.; Porter, L.M.; Mulenga, A. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks Tick-Borne Dis. 2015, 6, 424–434. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthold, A.; Lloyd, V.K. Changes in the Transcriptome and Long Non-Coding RNAs but Not the Methylome Occur in Human Cells Exposed to Borrelia burgdorferi. Genes 2024, 15, 1010. https://doi.org/10.3390/genes15081010
Berthold A, Lloyd VK. Changes in the Transcriptome and Long Non-Coding RNAs but Not the Methylome Occur in Human Cells Exposed to Borrelia burgdorferi. Genes. 2024; 15(8):1010. https://doi.org/10.3390/genes15081010
Chicago/Turabian StyleBerthold, Anne, and Vett K. Lloyd. 2024. "Changes in the Transcriptome and Long Non-Coding RNAs but Not the Methylome Occur in Human Cells Exposed to Borrelia burgdorferi" Genes 15, no. 8: 1010. https://doi.org/10.3390/genes15081010
APA StyleBerthold, A., & Lloyd, V. K. (2024). Changes in the Transcriptome and Long Non-Coding RNAs but Not the Methylome Occur in Human Cells Exposed to Borrelia burgdorferi. Genes, 15(8), 1010. https://doi.org/10.3390/genes15081010




