Notch-Dependent Expression of the Drosophila Hey Gene Is Supported by a Pair of Enhancers with Overlapping Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Strains and Genetics
2.2. Immunohistochemistry
2.3. DNA Constructs and Generation of Transgenic Lines
2.4. Generation of Df(2R)Hey7552/6656 Mutant
2.5. CRISPR-Cas9 Induced Deletions in the Hey Locus
- Adults surviving the injection were mated individually with yw67c23(wt) flies.
- 2.
- A total of 10-15 F1 progeny [yw;CR//+ or yw;+//+] from each of the positive P crosses were mated individually with Df(2R)Hey7552/6656[w+]//CyOwglacZ(w−) flies. DNA from each of the mated F1s was extracted after they had generated an adequate number of offspring (F2) and was used in the PCR screen for the CR event (positive F1).
- 3.
- Selection against the Df(2R)Hey7552/6656[w+] chromosome among the progeny of the positive F1s. Individual (w−) F2 progeny (yw; CR//CyOwglacZ or yw; +//CyOwglacZ) were mated with Df(2R)Hey7552/6656[w+]//CyOwglacZ(w−) flies. DNA from each of the mated F2s was extracted after they had generated an adequate number of offspring (F3) and was used in the PCR screen for the CR event (positive F2).
- 4.
- Selection against Df(2R)Hey7552/6656[w+] phenotype among the progeny of the positive F2s and inter-se cross of F3 yw; CR//CyOwglacZ virgin female and male siblings in order to establish the CRISPR deletion mutant line.
3. Results
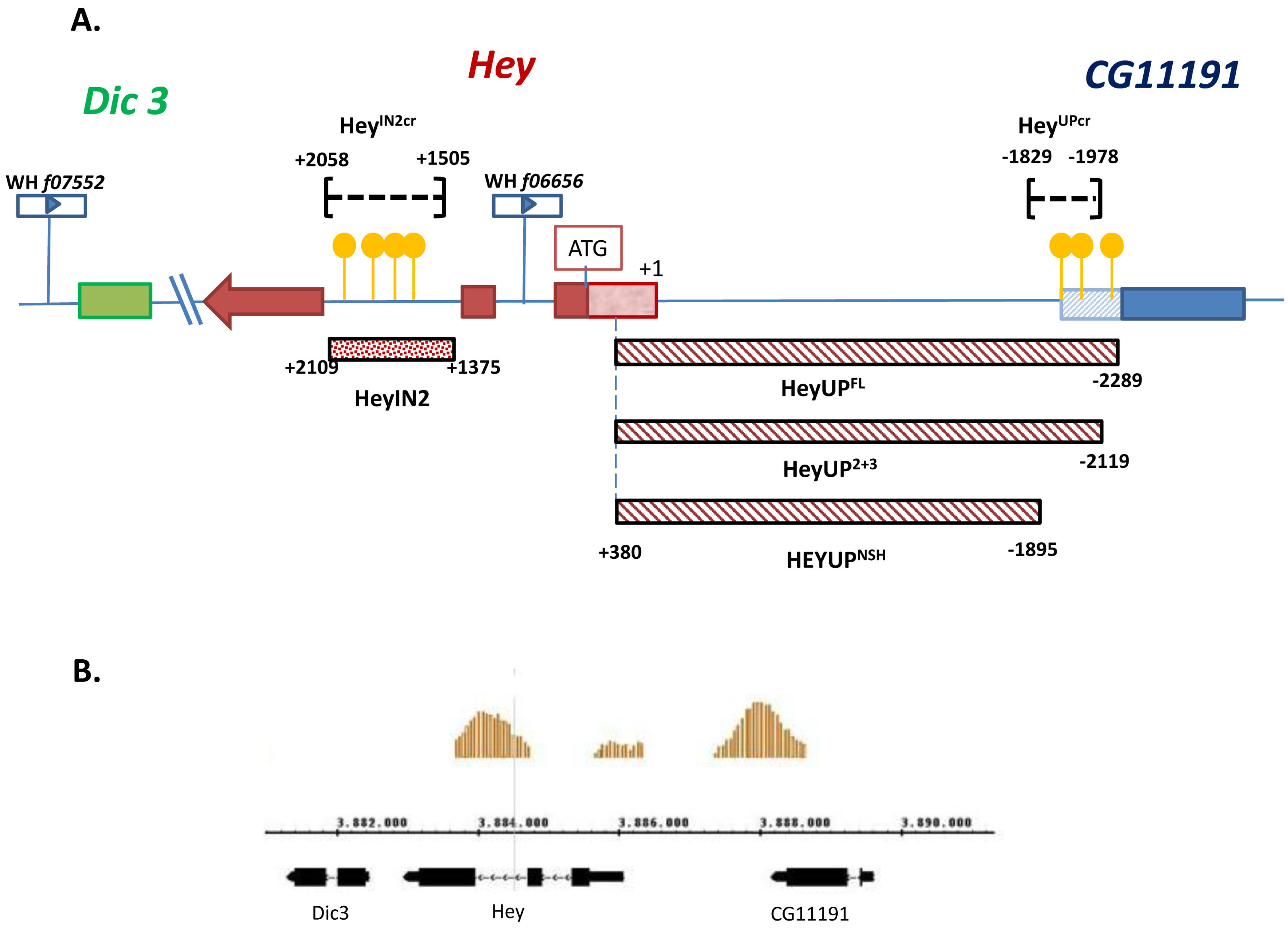
3.1. Identification of Two Enhancers from the Hey Locus
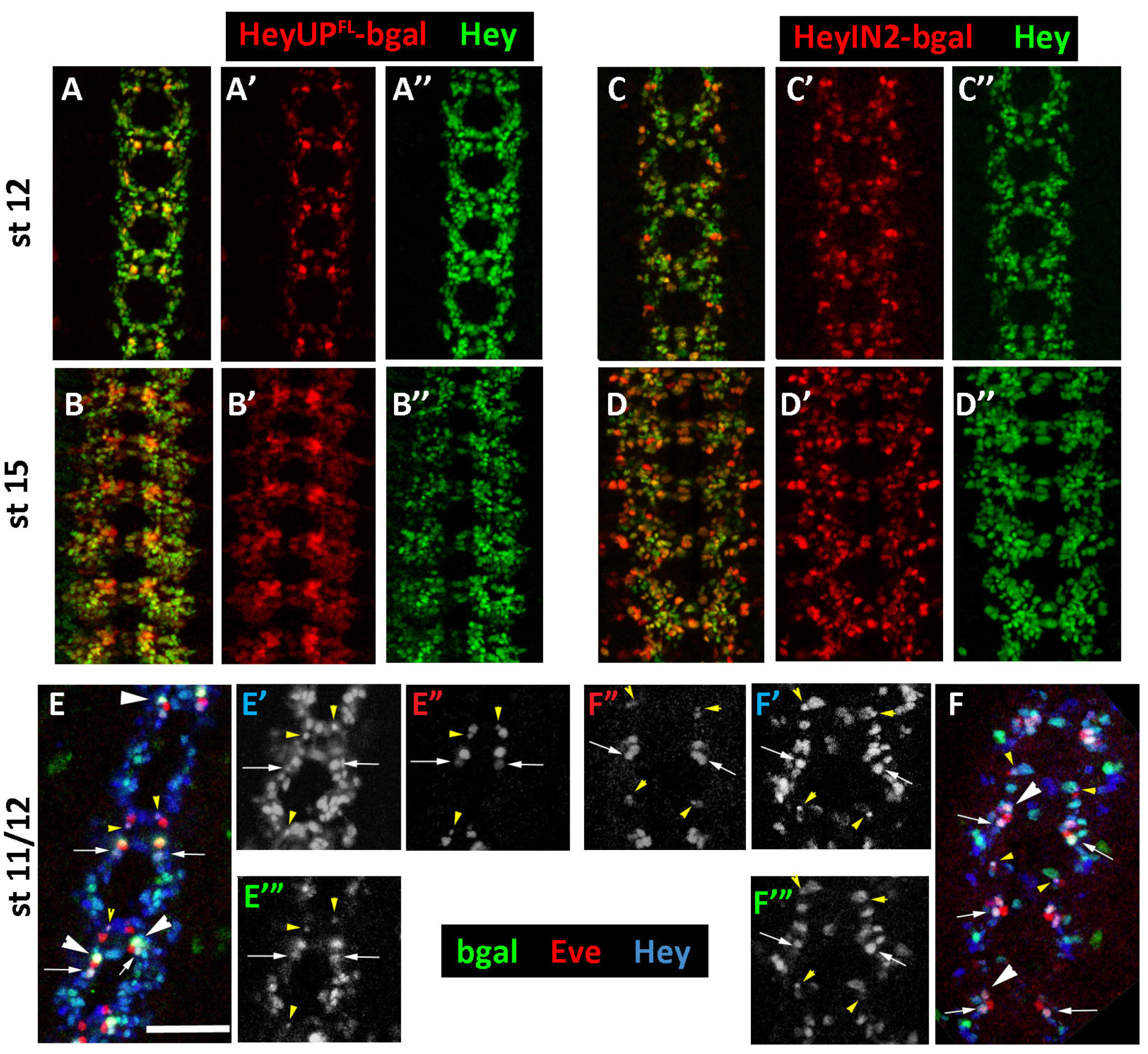
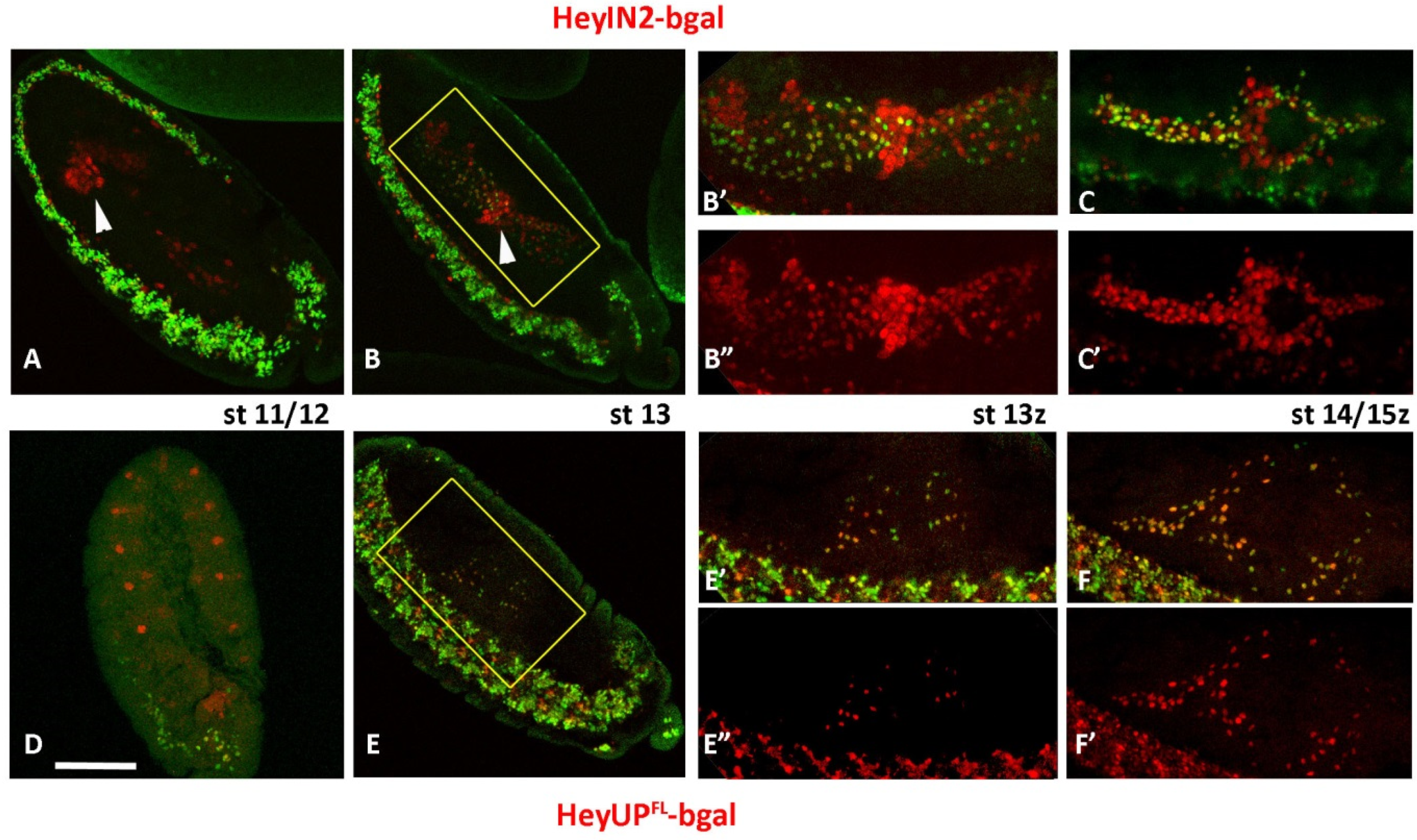
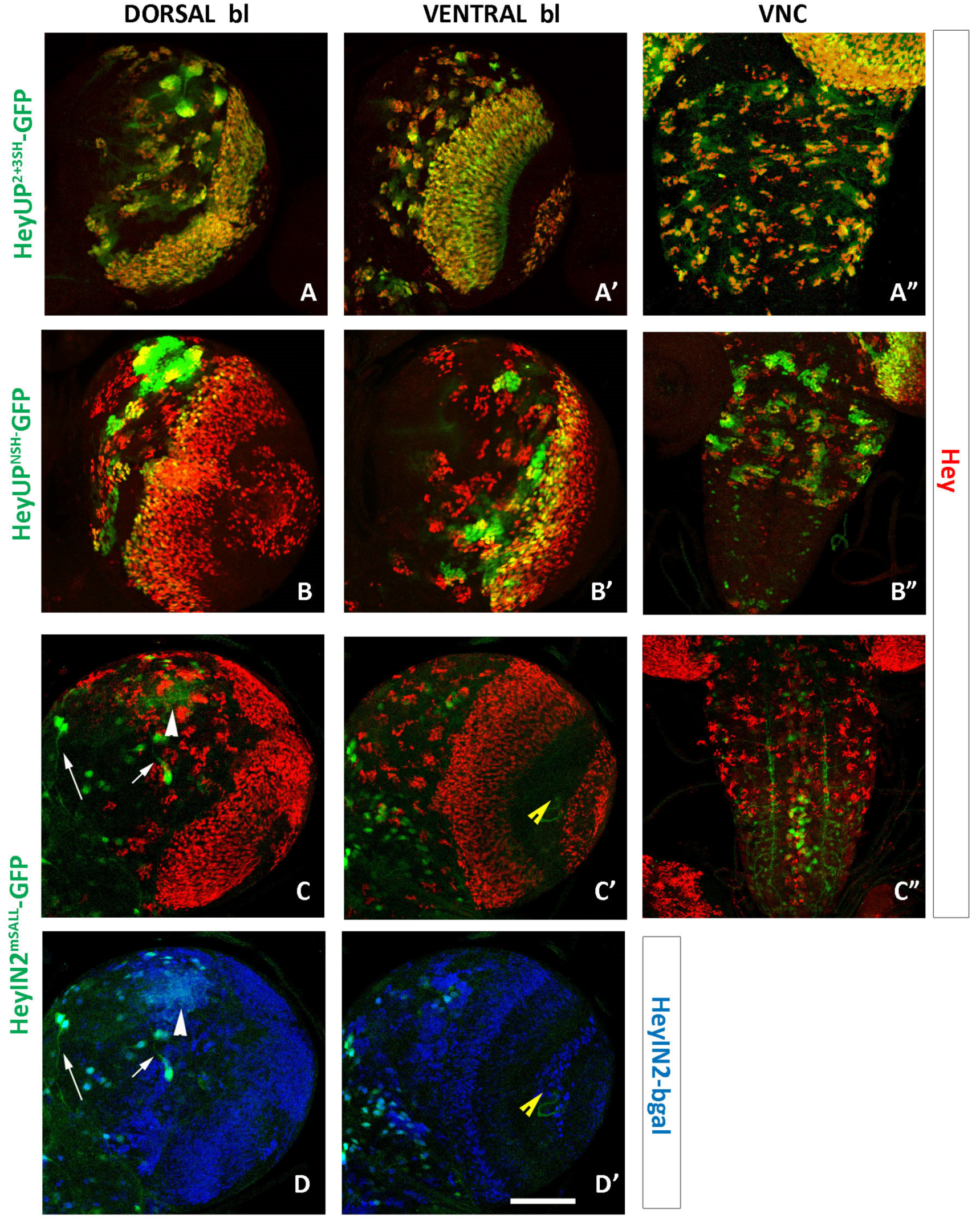
3.2. Both HeyUPFL and HeyIN2 Enhancers Are Active in the Embryonic CNS
3.3. HeyUPFL and HeyIN2 Enhancers Support Hey Expression in the Developing Midgut
3.4. HeyUPFL and HeyIN2 Enhancers Are Active in Larval CNS
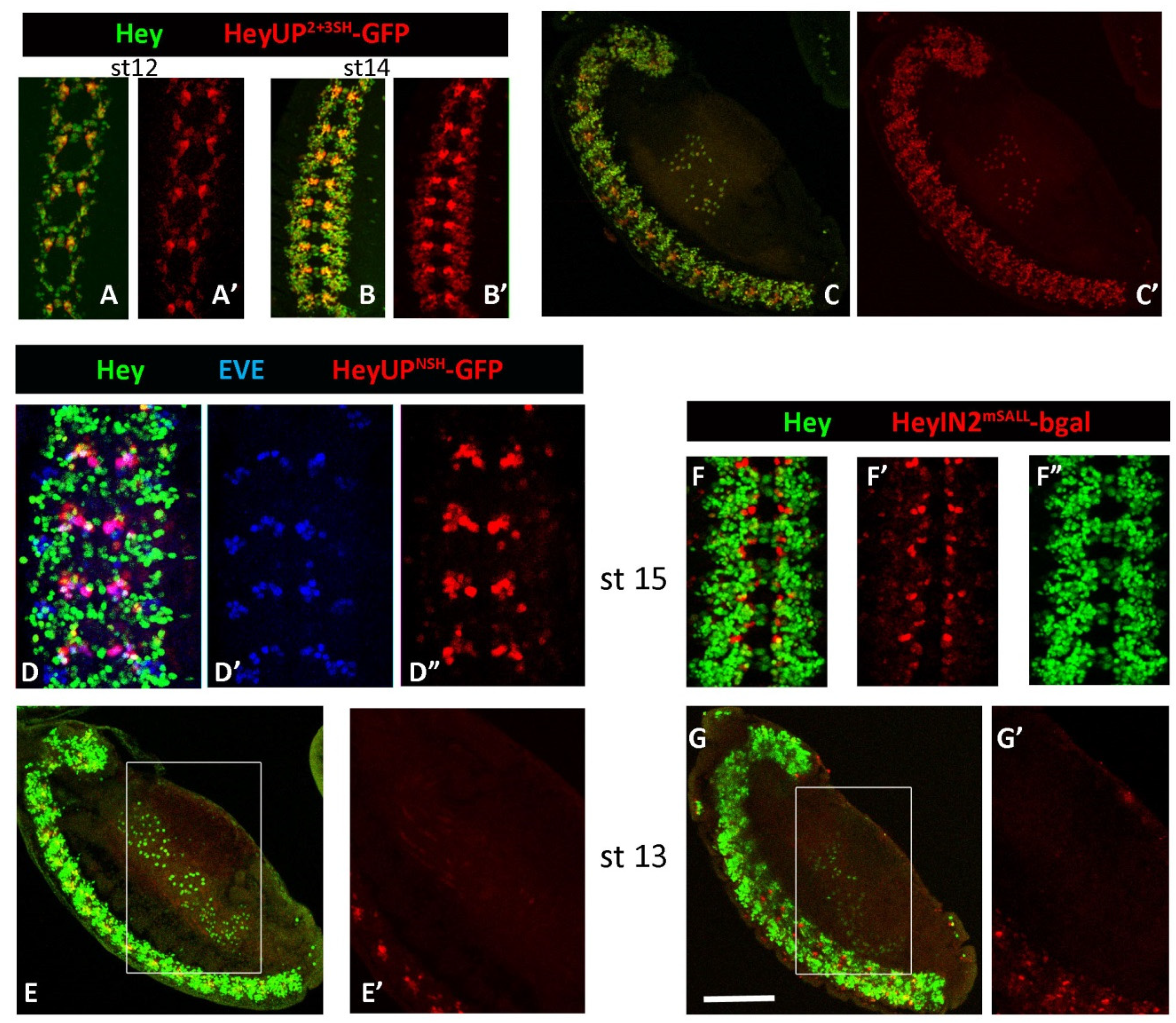
3.5. Notch-Dependence of HeyUPFL and HeyIN2 Regulatory Elements
3.5.1. Notch Deficient Background
3.5.2. Deletion/Mutational Analysis
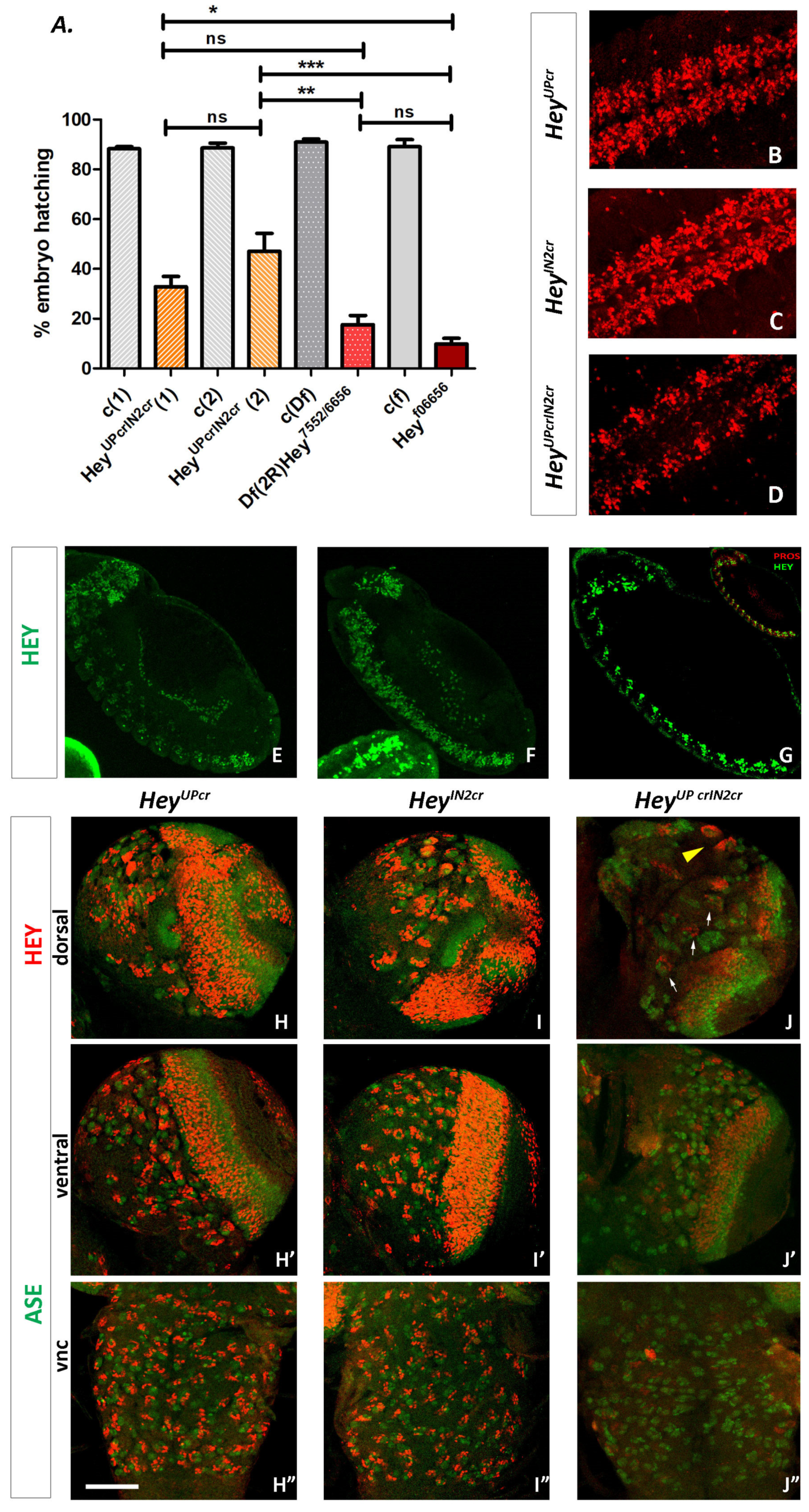
3.6. Functional Analysis of Hey Gene Using Genome Engineering
3.6.1. Generation of a Null Hey Allele
3.6.2. Generation of Lines with Deletions of the Su(H) Binding Sites from Hey Enhancers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spitz, F.; Furlong, E.E.M. Transcription Factors: From Enhancer Binding to Developmental Control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Hardison, R.C.; Taylor, J. Genomic Approaches towards Finding Cis-Regulatory Modules in Animals. Nat. Rev. Genet. 2012, 13, 469–483. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch Signaling: Cell Fate Control and Signal Integration in Development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Bray, S.J. Notch Signalling: A Simple Pathway Becomes Complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Gozlan, O.; Sprinzak, D. Notch Signaling in Development and Homeostasis. Development 2023, 150, dev201138. [Google Scholar] [CrossRef] [PubMed]
- Fryer, C.J.; White, J.B.; Jones, K.A. Mastermind Recruits CycC:CDK8 to Phosphorylate the Notch ICD and Coordinate Activation with Turnover. Mol. Cell 2004, 16, 509–520. [Google Scholar] [CrossRef]
- Wallberg, A.E.; Pedersen, K.; Lendahl, U.; Roeder, R.G. P300 and PCAF Act Cooperatively to Mediate Transcriptional Activation from Chromatin Templates by Notch Intracellular Domains In Vitro. Mol. Cell. Biol. 2002, 22, 7812–7819. [Google Scholar] [CrossRef]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch Signaling at a Glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cell 2017, 41, 228–241. [Google Scholar] [CrossRef]
- Zhou, S.; Fujimuro, M.; Hsieh, J.J.-D.; Chen, L.; Miyamoto, A.; Weinmaster, G.; Hayward, S.D. SKIP, a CBF1-Associated Protein, Interacts with the Ankyrin Repeat Domain of NotchIC To Facilitate NotchIC Function. Mol. Cell. Biol. 2000, 20, 2400–2410. [Google Scholar] [CrossRef]
- Barolo, S.; Stone, T.; Bang, A.G.; Posakony, J.W. Default Repression and Notch Signaling: Hairless Acts as an Adaptor to Recruit the Corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 2002, 16, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Nagel, A.C.; Krejci, A.; Tenin, G.; Bravo-Patiño, A.; Bray, S.; Maier, D.; Preiss, A. Hairless-Mediated Repression of Notch Target Genes Requires the Combined Activity of Groucho and CtBP Corepressors. Mol. Cell. Biol. 2005, 25, 10433–10441. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, H.; Krejčí, A.; Moshkin, Y.; Verrijzer, C.P.; Karch, F.; Bray, S.J. Gene-Specific Targeting of the Histone Chaperone Asf1 to Mediate Silencing. Dev. Cell 2007, 13, 593–600. [Google Scholar] [CrossRef]
- Moshkin, Y.M.; Kan, T.W.; Goodfellow, H.; Bezstarosti, K.; Maeda, R.K.; Pilyugin, M.; Karch, F.; Bray, S.J.; Demmers, J.A.A.; Verrijzer, C.P. Histone Chaperones ASF1 and NAP1 Differentially Modulate Removal of Active Histone Marks by LID-RPD3 Complexes during NOTCH Silencing. Mol. Cell 2009, 35, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Delidakis, C.; Monastirioti, M.; Magadi, S.S. E(Spl): Genetic, Developmental and Evolutionary Aspects of a Group of Invertebrate Hes Proteins with Close Ties to Notch Signaling In:Reshma Taneja, Editor. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 110, pp. 217–262. ISBN 978-0-12-405943-6. [Google Scholar]
- Housden, B.E.; Fu, A.Q.; Krejci, A.; Bernard, F.; Fischer, B.; Tavaré, S.; Russell, S.; Bray, S.J. Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by E(Spl)/Hes Genes. PLoS Genet. 2013, 9, e1003162. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.M.; Posakony, J.W. Suppressor of Hairless Directly Activates Transcription of Enhancer of Split Complex Genes in Response to Notch Receptor Activity. Genes Dev. 1995, 9, 2609–2622. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.; Furriols, M. Notch Pathway: Making Sense of Suppressor of Hairless. Curr. Biol. 2001, 11, R217–R221. [Google Scholar] [CrossRef] [PubMed]
- Lecourtois, M.; Schweisguth, F. The Neurogenic Suppressor of Hairless DNA-Binding Protein Mediates the Transcriptional Activation of the Enhancer of Split Complex Genes Triggered by Notch Signaling. Genes Dev. 1995, 9, 2598–2608. [Google Scholar] [CrossRef]
- Castro, B.; Barolo, S.; Bailey, A.M.; Posakony, J.W. Lateral Inhibition in Proneural Clusters: Cis-Regulatory Logic and Default Repression by Suppressor of Hairless. Development 2005, 132, 3333–3344. [Google Scholar] [CrossRef]
- Weber, D.; Wiese, C.; Gessler, M. Hey bHLH Transcription Factors. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 110, pp. 285–315. ISBN 978-0-12-405943-6. [Google Scholar]
- Hartenstein, V.; Wodarz, A. Initial Neurogenesis in Drosophila. WIREs Dev. Biol. 2013, 2, 823. [Google Scholar] [CrossRef]
- Skeath, J.B.; Doe, C.Q. Sanpodo and Notch Act in Opposition to Numb to Distinguish Sibling Neuron Fates in the Drosophila CNS. Development 1998, 125, 1857–1865. [Google Scholar] [CrossRef]
- Skeath, J.B.; Thor, S. Genetic Control of Drosophila Nerve Cord Development. Curr. Opin. Neurobiol. 2003, 13, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Monastirioti, M.; Giagtzoglou, N.; Koumbanakis, K.A.; Zacharioudaki, E.; Deligiannaki, M.; Wech, I.; Almeida, M.; Preiss, A.; Bray, S.; Delidakis, C. Drosophila Hey Is a Target of Notch in Asymmetric Divisions during Embryonic and Larval Neurogenesis. Development 2010, 137, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, T. Wiring the Drosophila Brain with Individually Tailored Neural Lineages. Curr. Biol. 2017, 27, R77–R82. [Google Scholar] [CrossRef] [PubMed]
- Skafida, E.; Delidakis, C.; Monastirioti, M. Expression of Hey Marks a Subset of Enteroendocrine Cells in the Drosophila Embryonic and Larval Midgut. Int. J. Dev. Biol. 2022, 66, 223–233. [Google Scholar] [CrossRef]
- Flint Brodsly, N.; Bitman-Lotan, E.; Boico, O.; Shafat, A.; Monastirioti, M.; Gessler, M.; Delidakis, C.; Rincon-Arano, H.; Orian, A. The Transcription Factor Hey and Nuclear Lamins Specify and Maintain Cell Identity. eLife 2019, 8, e44745. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.; Maeda, R.K.; Hediger, M.; Karch, F.; Basler, K. An Optimized Transgenesis System for Drosophila Using Germ-Line-Specific φC31 Integrases. Proc. Natl. Acad. Sci. USA 2007, 104, 3312–3317. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Luo, L. Mosaic Analysis with a Repressible Cell Marker (MARCM) for Drosophila Neural Development. Trends Neurosci. 2001, 24, 251–254. [Google Scholar] [CrossRef]
- Parks, A.L.; Cook, K.R.; Belvin, M.; Dompe, N.A.; Fawcett, R.; Huppert, K.; Tan, L.R.; Winter, C.G.; Bogart, K.P.; Deal, J.E.; et al. Systematic Generation of High-Resolution Deletion Coverage of the Drosophila Melanogaster Genome. Nat. Genet. 2004, 36, 288–292. [Google Scholar] [CrossRef]
- Thibault, S.T.; Singer, M.A.; Miyazaki, W.Y.; Milash, B.; Dompe, N.A.; Singh, C.M.; Buchholz, R.; Demsky, M.; Fawcett, R.; Francis-Lang, H.L.; et al. A Complementary Transposon Tool Kit for Drosophila Melanogaster Using P and piggyBac. Nat. Genet. 2004, 36, 283–287. [Google Scholar] [CrossRef]
- Port, F.; Chen, H.-M.; Lee, T.; Bullock, S.L. Optimized CRISPR/Cas Tools for Efficient Germline and Somatic Genome Engineering in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, E2967–E2976. [Google Scholar] [CrossRef] [PubMed]
- Menne, T.V.; Klämbt, C. The Formation of Commissures in the Drosophila CNS Depends on the Midline Cells and on the Notch Gene. Development 1994, 120, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.; Björklund, M.; Furger, E.; Schertel, C.; Taipale, J.; Basler, K. A Versatile Platform for Creating a Comprehensive UAS-ORFeome Library in Drosophila. Development 2013, 140, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Piwko, P.; Vitsaki, I.; Livadaras, I.; Delidakis, C. The Role of Insulators in Transgene Transvection in Drosophila. Genetics 2019, 212, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudaki, E.; Housden, B.E.; Garinis, G.; Stojnic, R.; Delidakis, C.; Bray, S. Genes Implicated in Stem-Cell Identity and Temporal-Program Are Directly Targeted by Notch in Neuroblast Tumours. Development 2016, 143, 219–231. [Google Scholar] [CrossRef]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome Engineering of Drosophila with the CRISPR RNA-Guided Cas9 Nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef]
- Odenwald, W.F.; Rasband, W.; Kuzin, A.; Brody, T. EVOPRINTER, a Multigenomic Comparative Tool for Rapid Identification of Functionally Important DNA. Proc. Natl. Acad. Sci. USA 2005, 102, 14700–14705. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R.M.; Rickert, C.; Altenhein, B.; Technau, G.M. Subtypes of Glial Cells in the Drosophila Embryonic Ventral Nerve Cord as Related to Lineage and Gene Expression. Mech. Dev. 2008, 125, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Adams, K.L.; Ortiz, P.A.; Ying, C.T.; Moridzadeh, R.; Younossi-Hartenstein, A.; Hartenstein, V. Development of the Drosophila Entero-Endocrine Lineage and Its Specification by the Notch Signaling Pathway. Dev. Biol. 2011, 353, 161–172. [Google Scholar] [CrossRef]
- Takashima, S.; Hartenstein, V. Genetic Control of Intestinal Stem Cell Specification and Development: A Comparative View. Stem Cell Rev. Rep. 2012, 8, 597–608. [Google Scholar] [CrossRef]
- Dubreuil, R.R.; Frankel, J.; Wang, P.; Howrylak, J.; Kappil, M.; Grushko, T.A. Mutations of α Spectrin andlabialBlock Cuprophilic Cell Differentiation and Acid Secretion in the Middle Midgut ofDrosophilaLarvae. Dev. Biol. 1998, 194, 1–11. [Google Scholar] [CrossRef]
- Overend, G.; Luo, Y.; Henderson, L.; Douglas, A.E.; Davies, S.A.; Dow, J.A.T. Molecular Mechanism and Functional Significance of Acid Generation in the Drosophila Midgut. Sci. Rep. 2016, 6, 27242. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-T.; Zirin, J.; Kanca, O.; Lin, W.-W.; Schulze, K.L.; Li-Kroeger, D.; Tao, R.; Devereaux, C.; Hu, Y.; Chung, V.; et al. A Gene-Specific T2A-GAL4 Library for Drosophila. eLife 2018, 7, e35574. [Google Scholar] [CrossRef] [PubMed]
- Hartenstein, V.; Takashima, S.; Hartenstein, P.; Asanad, S.; Asanad, K. bHLH proneural genes as cell fate determinants of entero-endocrine cells, an evolutionarily conserved lineage sharing a common root with sensory neurons. Dev. Biol. 2017, 431, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.W.; Rebeiz, M.; Atanasov, J.E.; Posakony, J.W. Neural Precursor-Specific Expression of Multiple Drosophila Genes Is Driven by Dual Enhancer Modules with Overlapping Function. Proc. Natl. Acad. Sci. USA 2014, 111, 17194–17199. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Hanson, K.A.; Kim, S.H.; Tare, A.; Tibbetts, R.S. Identification of Genetic Modifiers of TDP-43 Neurotoxicity in Drosophila. PLoS ONE 2013, 8, e57214. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Abed, M.; Kenyagin, D.; Werwie, T.R.; Boico, O.; Orian, A.; Parkhurst, S.M. The Drosophila STUbL Protein Degringolade Limits HES Functions during Embryogenesis. Development 2011, 138, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, S.; Klein, T. Regulation of Expression of Vg and Establishment of the Dorsoventral Compartment Boundary in the Wing Imaginal Disc by Suppressor of Hairless. Dev. Biol. 2006, 289, 77–90. [Google Scholar] [CrossRef]
- Barolo, S. Shadow Enhancers: Frequently Asked Questions about Distributed Cis-regulatory Information and Enhancer Redundancy. BioEssays 2012, 34, 135–141. [Google Scholar] [CrossRef]
- Waymack, R.; Fletcher, A.; Enciso, G.; Wunderlich, Z. Shadow Enhancers Can Suppress Input Transcription Factor Noise through Distinct Regulatory Logic. eLife 2020, 9, e59351. [Google Scholar] [CrossRef]
- Kvon, E.Z.; Waymack, R.; Gad, M.; Wunderlich, Z. Enhancer Redundancy in Development and Disease. Nat. Rev. Genet. 2021, 22, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Preger-Ben Noon, E.; Sabarís, G.; Ortiz, D.M.; Sager, J.; Liebowitz, A.; Stern, D.L.; Frankel, N. Comprehensive Analysis of a Cis-Regulatory Region Reveals Pleiotropy in Enhancer Function. Cell Rep. 2018, 22, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Dunipace, L.; Ákos, Z.; Stathopoulos, A. Coacting Enhancers Can Have Complementary Functions within Gene Regulatory Networks and Promote Canalization. PLoS Genet. 2019, 15, e1008525. [Google Scholar] [CrossRef] [PubMed]


| Study of the Hey Upstream Region |
|---|
| attp40: HeyUPFL-nbgal |
| attp40: HeyUPFL-n GFP |
| attp40: HeyUP2+3SH-nGFP |
| attp40: HeyUPNSH-nGFP |
| Study of the Hey intron 2 |
| attp40: HeyIN2-nGFP |
| attp2: HeyIN2-n bgal |
| attp40: HeyIN2-n bgal |
| attp40: Heyin2mSALL-nGFP |
| attp40: Heyin2mSALL-nlacZ |
| Hey mutants |
| Df(2R)Hey7552/6656/CyO twistG > UASGFP |
| HeyUPcr |
| HeyIN2cr |
| HeyUPcrIN2cr/CyO,wglacZ |
| HeyUPcrIN2cr/CyO,twistG > UASGFP |
| Name | Sequence (5′ to 3′) | |
|---|---|---|
| ||
| FOR Heyup | CAGAATTCCGGCGGCTTTGTAACCTCCACTTTGCG | [−2289 to −2264] |
| REV Heyup | CTGGTACCGAGGCAGATGCAGTTGGCGGCTAG | [+356 to +380] |
| heyPf-E1 | GGAATTCGTGTGTATGTGTGTGCGGC’ | [−2119 to −2101] |
| UPFip | GCGGAATTCCCTCCACTTTGCGTCCAGCGATA | [−2275 to −2252] |
| UPRip | GCGGGTACCTTGACTATAATGGAGCCCCGTATCAA | [−1656 to −1631] |
| UPMB-F | GCGGAATTCCCTCCACTTTGCGTCCAGCGATA | [−1895 to −1871] |
| CDS-F | CTGAGCTCATGGATCACAACATGCACGTCAATG | [+481 to +496] |
| heyIr-K1 | GCGGTACCCTGCAACAAGATACGAGGAGG | [+2089 to +2109] |
| heyIf-X1 | CGCTCTAGAATTACCAAGCCCACTTGAGC | [+1376 to +1395] |
| ||
| 1st Su(H)-NruI | CTAAACGCAAGCCGCTGTCGCGAACAATTGAGTAACG | |
| 2nd Su(H)-StuI | GTTGTCCCGAACAGGGTCGAATTGAGGCCTAATTATGATTGC | |
| 3rd Su(H)-SmaI | CTCGAATCTCGGGGCTCCCGGGGCTCAGTAGC | |
| 4th Su(H)-ApaLI | GGGTTGTTCTTTATGCTCGTCGCGTGCACCCAGCTATTTTAATTG | |
| ||
| f07552GP fwd | GGTGCCACCATGAACCAAACT | upstream of WH f07552 (+) |
| f06656GP REV | TCGCGCACACGATAAGTCAC | Hey exon1(−) [+308 to +328] |
| Tn1 REV | TCCAAGCGGCGACTGAGATG | WH5′ plus-left primer [31] |
| Tn2 FWD | CCTCGATATACAGACCGATAAAAC | WH3′ plus-right primer [31] |
| ||
| INT2 5′gRNA | CTTC GAGCAAATAGAGGGTTAACT AAAC AGTTAACCCTCTATTTGCTC | |
| INT2 3′gRNA | AAAC GAACTGGGATTAGACAGCTC CTTC GAGCTGTCTAATCCCAGTTC | |
| UP 5′gRNA | CTTC GTGCGGGTCGTGCGCCGGAA AAAC TTCCGGCGCACGACCCGCAC | |
| UP 3′gRNA | CTTC GCTTGATTTGGGGTGGGTTC AAAC GAACCCACCCCAAATCAAGC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monastirioti, M.; Koltsaki, I.; Pitsidianaki, I.; Skafida, E.; Batsiotos, N.; Delidakis, C. Notch-Dependent Expression of the Drosophila Hey Gene Is Supported by a Pair of Enhancers with Overlapping Activities. Genes 2024, 15, 1071. https://doi.org/10.3390/genes15081071
Monastirioti M, Koltsaki I, Pitsidianaki I, Skafida E, Batsiotos N, Delidakis C. Notch-Dependent Expression of the Drosophila Hey Gene Is Supported by a Pair of Enhancers with Overlapping Activities. Genes. 2024; 15(8):1071. https://doi.org/10.3390/genes15081071
Chicago/Turabian StyleMonastirioti, Maria, Ioanna Koltsaki, Ioanna Pitsidianaki, Emilia Skafida, Nikolaos Batsiotos, and Christos Delidakis. 2024. "Notch-Dependent Expression of the Drosophila Hey Gene Is Supported by a Pair of Enhancers with Overlapping Activities" Genes 15, no. 8: 1071. https://doi.org/10.3390/genes15081071





