The Characterization of the Mitochondrial Genome of Fulgoraria rupestris and Phylogenetic Considerations within the Neogastropoda
Abstract
:1. Introduction
2. Materials and Method
2.1. Sample Preparation and DNA Extraction
2.2. Mitochondrial DNA Sequencing and Assembly
2.3. Genome Annotation and Bioinformatics Analysis
2.4. Phylogenetic Analysis
3. Results
3.1. Mitochondrial Genome Structural Features
3.2. Analysis of rRNA and tRNA in the F. rupestris Mitochondrial Genome
3.3. Nucleotide Composition and Base Skew Analysis
3.4. Amino Acid Composition and Codon Usage
3.5. Selection Pressure Analysis
3.6. Gene Order
3.7. Phylogenetic Relationships
4. Discussion
4.1. Basic Features of the Mitogenome of F. rupestris
4.2. Phylogenetic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponder, W.F.; Colgan, D.J.; Healy, J.; Nützel, A.; Simone, L.R.; Strong, E.E. Caenogastropod phylogeny. In Molluscan Phylogeny; University of California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Bernard, F. Recherches sur les Organes Palléaux des Gastéropodes Prosobranches; G. Masson: Paris, France, 1890. [Google Scholar]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Ponder, W.F. Brief introductions to higher groups of gastropods: Infraorder Neogastropoda. In Mollusca: The Southern Synthesis; CSIRO Publishing: Melbourne, Australia, 1998; pp. 808–854. [Google Scholar]
- Ponder, W.; Lindberg, D.R. Phylogeny and Evolution of the Mollusca; University of California Press: Berkeley, CA, USA, 2008; ISBN 0520250923. [Google Scholar]
- Kantor, Y.I.; Taylor, J.D. Phylogeny and Relationships of Neogastropoda; Taylor, J.D., Ed.; Oxford University Press: Oxford, NY, USA, 1996. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R. Towards a phylogeny of gastropod molluscs: An analysis using morphological characters. Zool. J. Linn. Soc. 1997, 119, 83–265. [Google Scholar] [CrossRef]
- Strong, E.E. Refining molluscan characters: Morphology, character coding and a phylogeny of the Caenogastropoda. Zool. J. Linn. Soc. 2003, 137, 447–554. [Google Scholar] [CrossRef]
- Bouvier, E. Système Nerveux, Morphologie Générale et Classification des Gastéropodes Prosobranches; G. Masson: Paris, France, 1887. [Google Scholar]
- Colgan, D.J.; Ponder, W.F.; Eggler, P.E. Gastropod evolutionary rates and phylogenetic relationships assessed using partial 28S rDNA and histone H3 sequences. Zool. Scr. 2000, 29, 29–63. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Beacham, E.; Macaranas, J.M. Gastropod phylogeny based on six segments from four genes representing coding or non-coding and mitochondrial or nuclear DNA. Molluscan Res. 2003, 23, 123–148. [Google Scholar] [CrossRef]
- Jiang, X.; Miao, J.; Li, J.; Ye, Y. Characterization of Lophiotoma leucotropis Mitochondrial Genome of Family Turridae and Phylogenetic Considerations within the Neogastropoda. Animals 2024, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.L.; Grande, C.; Zardoya, R. Neogastropod phylogenetic relationships based on entire mitochondrial genomes. BMC Evol. Biol. 2009, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, O.H. Handbuch der Paläozoologie. Nature 1938, 142, 1057. [Google Scholar]
- Thiele, J. Handbuch der Systematischen Weichtierkunde; A. Asher & Company: Berlin, Germany, 1963. [Google Scholar]
- Bouchet, P.; Rocroi, J.; Hausdorf, B.; Kaim, A.; Kano, Y.; Nützel, A.; Parkhaev, P.; Schrödl, M.; Strong, E.E. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 2017, 61, 1–526. [Google Scholar]
- Fedosov, A.; Puillandre, N.; Kantor, Y.; Bouchet, P. Phylogeny and systematics of Mitriform gastropods (Mollusca: Gastropoda: Neogastropoda). Zool. J. Linn. Soc. 2015, 175, 336–359. [Google Scholar] [CrossRef]
- Fedosov, A.E.; Caballer Gutierrez, M.; Buge, B.; Sorokin, P.V.; Puillandre, N.; Bouchet, P. Mapping the missing branch on the neogastropod tree of life: Molecular phylogeny of Marginelliform gastropods. J. Molluscan Stud. 2019, 85, 439–451. [Google Scholar] [CrossRef]
- Pilsbry, H.A.; Olsson, A.A.; Ithaca, N.P.R.I. Systems of the Volutidae; Paleontological Research Institution: New York, NY, USA, 1954. [Google Scholar]
- Harasewych, M.G.; Sei, M.; Wirshing, H.H.; González, V.L.; Uribe, J.E. The complete mitochondrial genome of Neptuneopsis gilchristi GB Sowerby III, 1898 (Neogastropoda: Volutidae: Calliotectinae). Nautilus 2019, 133, 67–73. [Google Scholar]
- Zou, S.; Li, Q.; Kong, L. Additional gene data and increased sampling give new insights into the phylogenetic relationships of Neogastropoda, within the caenogastropod phylogenetic framework. Mol. Phylogenet. Evol. 2011, 61, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bandel, K.; Dockery Iii, D.T. Protoconch characters of Late Cretaceous Latrogastropoda (Neogastropoda and Neomesogastropoda) as an aid in the reconstruction of the phylogeny of the Neogastropoda. Freib. Forschungshefte C 2012, 542, 93–128. [Google Scholar]
- Modica, M.V.; Holford, M. The Neogastropoda: Evolutionary innovations of predatory marine snails with remarkable pharmacological potential. In Evolutionary Biology–Concepts, Molecular and Morphological Evolution: 13th Meeting 2009; Springer: Berlin/Heidelberg, Germany, 2010; pp. 249–270. [Google Scholar]
- Uribe, J.E.; Fedosov, A.E.; Murphy, K.R.; Sei, M.; Harasewych, M.G. The complete mitochondrial genome of Costapex baldwinae (Gastropoda: Neogastropoda: Turbinelloidea: Costellariidae) from the Caribbean Deep-Sea. Mitochondrial DNA Part B 2021, 6, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Harasewych, M.G.; Sei, M.; Uribe, J.E. The complete mitochondrial genome of Harpovoluta charcoti (Gastropoda: Neogastropoda: Volutidae). Mitochondrial DNA Part B 2020, 5, 1986–1988. [Google Scholar] [CrossRef]
- Zhong, S.; Huang, G.; Liu, Y.; Huang, L. The complete mitochondrial genome of marine gastropod Melo melo (neogastropoda: Volutoidea). Mitochondrial DNA Part B 2019, 4, 4161–4162. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.; Brey, T.; Mackensen, A.; Penchaszadeh, P.E. Age, growth, and mortality of the prosobranch Zidona dufresnei (Donovan, 1823) in the Mar del Plata area, south-western Atlantic Ocean. Mar. Biol. 2004, 145, 707–712. [Google Scholar]
- Bigatti, G.; Penchaszadeh, P.E. Imposex in Odontocymbiola magellanica (Caenogastropoda: Volutidae) in Patagonia. Comun. Soc. Malacol. Urug. 2005, 9, 371–375. [Google Scholar]
- Cledón, M.; Arntz, W.; Penchaszadeh, P.E. Gonadal cycle in an Adelomelon brasiliana (Neogastropoda: Volutidae) population of Buenos Aires province, Argentina. Mar. Biol. 2005, 147, 439–445. [Google Scholar] [CrossRef]
- Cledón, M.; Brey, T.; Penchaszadeh, P.E.; Arntz, W. Individual growth and somatic production in Adelomelon brasiliana (Gastropoda; Volutidae) off Argentina. Mar. Biol. 2005, 147, 447–452. [Google Scholar] [CrossRef]
- Gimenez, J.; Lasta, M.; Bigatti, G.; Penchaszadeh, P.E. Exploitation of the volute snail Zidona dufresnei in Argentine waters, southwestern Atlantic Ocean. J. Shellfish Res. 2005, 24, 1135–1140. [Google Scholar]
- Cledón, M.; Theobald, N.; Gerwinski, W.; Penchaszadeh, P. Imposex and organotin compounds in marine gastropods and sediments from the Mar del Plata coast, Argentina. J. Mar. Biol. Assoc. UK 2006, 86, 751–755. [Google Scholar] [CrossRef]
- Bigatti, G.; Carranza, A. Phenotypic variability associated with the occurrence of imposex in Odontocymbiola magellanica from Golfo Nuevo, Patagonia. J. Mar. Biol. Assoc. UK 2007, 87, 755–759. [Google Scholar] [CrossRef]
- Bigatti, G.; Ciocco, N.F. Volutid snails as an alternative resource for artisanal fisheries in northern patagonic gulfs: Availability and first suggestions for diving catches. J. Shellfish Res. 2008, 27, 417–421. [Google Scholar] [CrossRef]
- Penchaszadeh, P.E.; Antelo, C.S.; Zabala, S.; Bigatti, G. Reproduction and imposex in the edible snail Adelomelon ancilla from northern Patagonia, Argentina. Mar. Biol. 2009, 156, 1929–1939. [Google Scholar] [CrossRef]
- Márquez, F.; González-José, R.; Bigatti, G. Combined methods to detect pollution effects on shell shape and structure in Neogastropods. Ecol. Indic. 2011, 11, 248–254. [Google Scholar] [CrossRef]
- Roche, A.; Maggioni, M.; Narvarte, M. Predation on egg capsules of Zidona dufresnei (Volutidae): Ecological implications. Mar. Biol. 2011, 158, 2787–2793. [Google Scholar] [CrossRef]
- Schumacher, C.F. Essai d’un Nouveau Système des Habitations des vers Testacés: Avec XXII Planches. Mr le directeur Schultz. 1817. [Google Scholar]
- Manoylov, K.M. Taxonomic identification of algae (morphological and molecular): Species concepts, methodologies, and their implications for ecological bioassessment. J. Phycol. 2014, 50, 409–424. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Singer, G.A.; Hebert, P.D.; Hickey, D.A. DNA barcoding: How it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 2007, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Xiao, L. Taxonomy and molecular taxonomy. In Cryptosporidium: Parasite and Disease; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–41. [Google Scholar]
- Fontanilla, I.K.; Naggs, F.; Wade, C.M. Molecular phylogeny of the achatinoidea (mollusca: Gastropoda). Mol. Phylogenet. Evol. 2017, 114, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Ingman, M.; Kaessmann, H.; Pääbo, S.; Gyllensten, U. Mitochondrial genome variation and the origin of modern humans. Nature 2000, 408, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.L. Evolutionary rates, divergence dates, and the performance of mitochondrial genes in Bayesian phylogenetic analysis. Syst. Biol. 2006, 55, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Feng, J.; Liu, X.; Yan, C.; Ye, Y.; Li, J.; Xu, K.; Guo, B.; Lü, Z. Sequence comparison of the mitochondrial genomes of five brackish water species of the family Neritidae: Phylogenetic implications and divergence time estimation. Ecol. Evol. 2022, 12, e8984. [Google Scholar] [CrossRef] [PubMed]
- Patrice, B. A new species of Fulgoraria Schumacher, 1817 (Gastropoda: Volutidae) from the bathyal Taiwanese water. Novapex 2008, 9, 161–163. [Google Scholar]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef]
- Hassanin, A.; Leger, N.; Deutsch, J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of Metazoa, and consequences for phylogenetic inferences. Syst. Biol. 2005, 54, 277–298. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-Delbarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP* Phylogenetic Analysis Using Parsimony (* and Other Methods). Version 4. 2003. Available online: http://paup.csit.fsu.edu/ (accessed on 10 November 2023).
- White, T.R.; Conrad, M.M.; Tseng, R.; Balayan, S.; Golding, R.; de Frias Martins, A.M.; Dayrat, B.A. Ten new complete mitochondrial genomes of pulmonates (Mollusca: Gastropoda) and their impact on phylogenetic relationships. BMC Evol. Biol. 2011, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Köhler, F.; Huang, X.; Wu, R.; Zhou, C.; Ouyang, S.; Wu, X. A novel gene arrangement among the Stylommatophora by the complete mitochondrial genome of the terrestrial slug Meghimatium bilineatum (Gastropoda, Arionoidea). Mol. Phylogenet. Evol. 2019, 135, 177–184. [Google Scholar] [CrossRef]
- Feng, J.; Guo, Y.; Yan, C.; Ye, Y.; Li, J.; Guo, B.; Lü, Z. Comparative analysis of the complete mitochondrial genomes in two limpets from Lottiidae (Gastropoda: Patellogastropoda): Rare irregular gene rearrangement within Gastropoda. Sci. Rep. 2020, 10, 19277. [Google Scholar] [CrossRef]
- Lavrov, D.V.; Brown, W.M.; Boore, J.L. A novel type of RNA editing occurs in the mitochondrial tRNAs of the centipede Lithobius forficatus. Proc. Natl. Acad. Sci. USA 2000, 97, 13738–13742. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Qi, L.; Kong, L.; Li, Q. Complete mitochondrial genomes of two toxin-accumulated Nassariids (Neogastropoda: Nassariidae: Nassarius) and their implication for phylogeny. Int. J. Mol. Sci. 2020, 21, 3545. [Google Scholar] [CrossRef]
- Zhong, S.; Huang, L.; Huang, G.; Liu, Y.; Xu, W. The first complete mitochondrial genome of Melongenidae from Hemifusus tuba (Neogastropoda: Buccinoidea). Mitochondrial DNA Part B 2019, 4, 3400–3401. [Google Scholar] [CrossRef] [PubMed]
- Beagley, C.T.; Okimoto, R.; Wolstenholme, D.R. The mitochondrial genome of the sea anemone Metridium senile (Cnidaria): Introns, a paucity of tRNA genes, and a near-standard genetic code. Genetics 1998, 148, 1091–1108. [Google Scholar] [CrossRef]
- Searle, J.B. Phylogeography—The history and formation of species. Heredity 2000, 85, 201. [Google Scholar] [CrossRef]
- Shao, R.; Campbell, N.J.; Schmidt, E.R.; Barker, S.C. Increased rate of gene rearrangement in the mitochondrial genomes of three orders of hemipteroid insects. Mol. Biol. Evol. 2001, 18, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, P.; Rocroi, J.P.; Frýda, J.; Hausdorf, B.; Ponder, W.; Valdes, A.; Warén, A. A nomenclator and classification of gastropod family-group names. Malacologia 2005, 47, 1–368. [Google Scholar]
- Lemarcis, T.; Fedosov, A.E.; Kantor, Y.I.; Abdelkrim, J.; Zaharias, P.; Puillandre, N. Neogastropod (Mollusca, Gastropoda) phylogeny: A step forward with mitogenomes. Zool. Scr. 2022, 51, 550–561. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Beacham, E.; Macaranas, J. Molecular phylogenetics of Caenogastropoda (gastropoda: Mollusca). Mol. Phylogenet. Evol. 2007, 42, 717–737. [Google Scholar] [CrossRef]
- Oliverio, M.; Modica, M.V. Relationships of the haematophagous marine snail Colubraria (Rachiglossa: Colubrariidae), within the neogastropod phylogenetic framework. Zool. J. Linn. Soc. 2010, 158, 779–800. [Google Scholar] [CrossRef]
- Barco, A.; Claremont, M.; Reid, D.G.; Houart, R.; Bouchet, P.; Williams, S.T.; Cruaud, C.; Couloux, A.; Oliverio, M. A molecular phylogenetic framework for the Muricidae, a diverse family of carnivorous gastropods. Mol. Phylogenet. Evol. 2010, 56, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Kantor, Y.I.; Fedosov, A.E.; Kosyan, A.R.; Puillandre, N.; Sorokin, P.A.; Kano, Y.; Clark, R.; Bouchet, P. Molecular phylogeny and revised classification of the Buccinoidea (Neogastropoda). Zool. J. Linn. Soc. 2022, 194, 789–857. [Google Scholar] [CrossRef]
- Galindo, L.A.; Puillandre, N.; Utge, J.; Lozouet, P.; Bouchet, P. The phylogeny and systematics of the Nassariidae revisited (Gastropoda, Buccinoidea). Mol. Phylogenet. Evol. 2016, 99, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Samadi, S.; Boisselier, M.; Sysoev, A.V.; Kantor, Y.I.; Cruaud, C.; Couloux, A.; Bouchet, P. Starting to unravel the toxoglossan knot: Molecular phylogeny of the “turrids”(Neogastropoda: Conoidea). Mol. Phylogenet. Evol. 2008, 47, 1122–1134. [Google Scholar] [CrossRef]
- Yang, M.; Dong, D.; Li, X. The complete mitogenome of Phymorhynchus sp. (Neogastropoda, Conoidea, Raphitomidae) provides insights into the deep-sea adaptive evolution of Conoidea. Ecol. Evol. 2021, 11, 7518–7531. [Google Scholar] [CrossRef]
- Parins-Fukuchi, C.; Stull, G.W.; Smith, S.A. Phylogenomic conflict coincides with rapid morphological innovation. Proc. Natl. Acad. Sci. USA 2021, 118, e2023058118. [Google Scholar] [CrossRef]
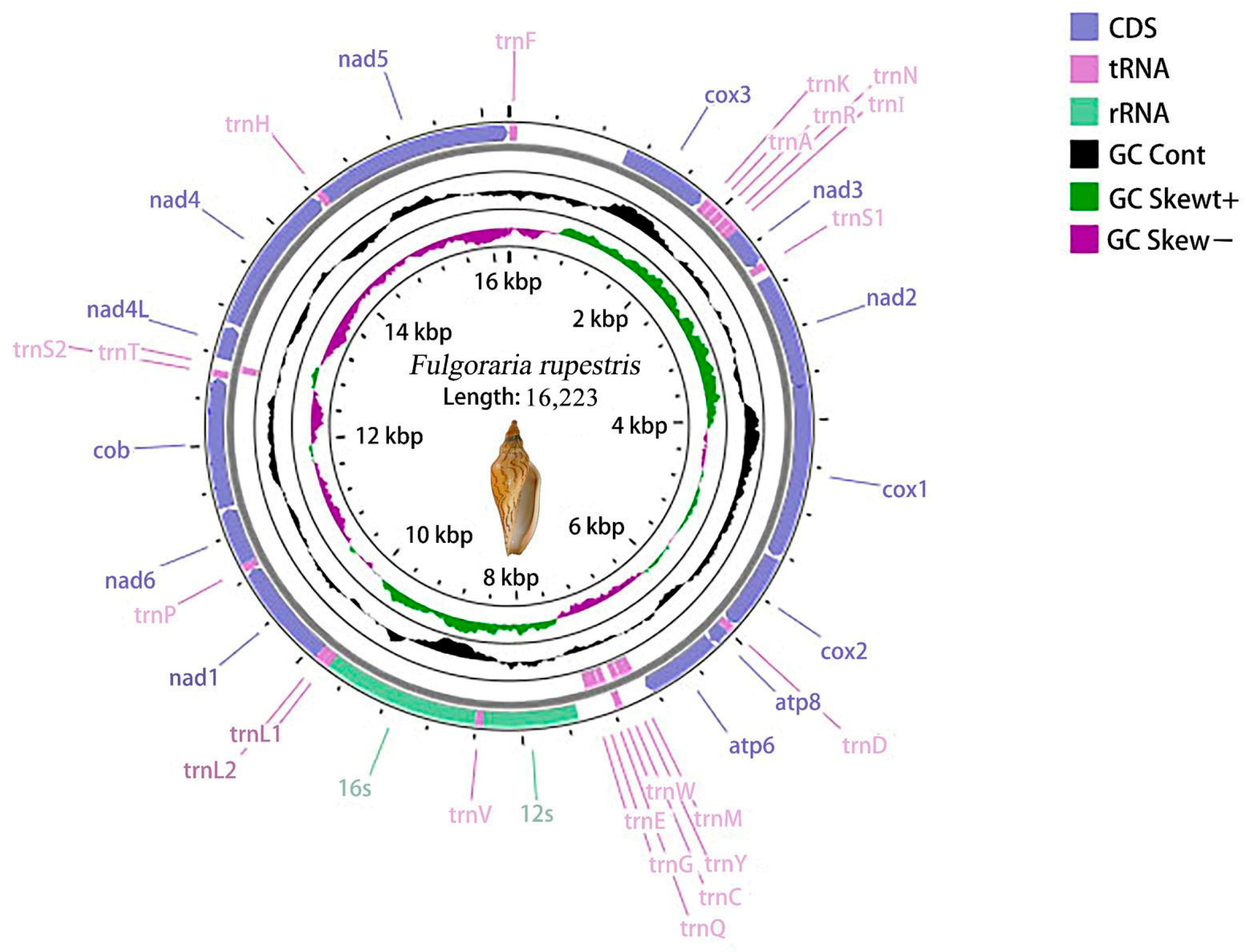
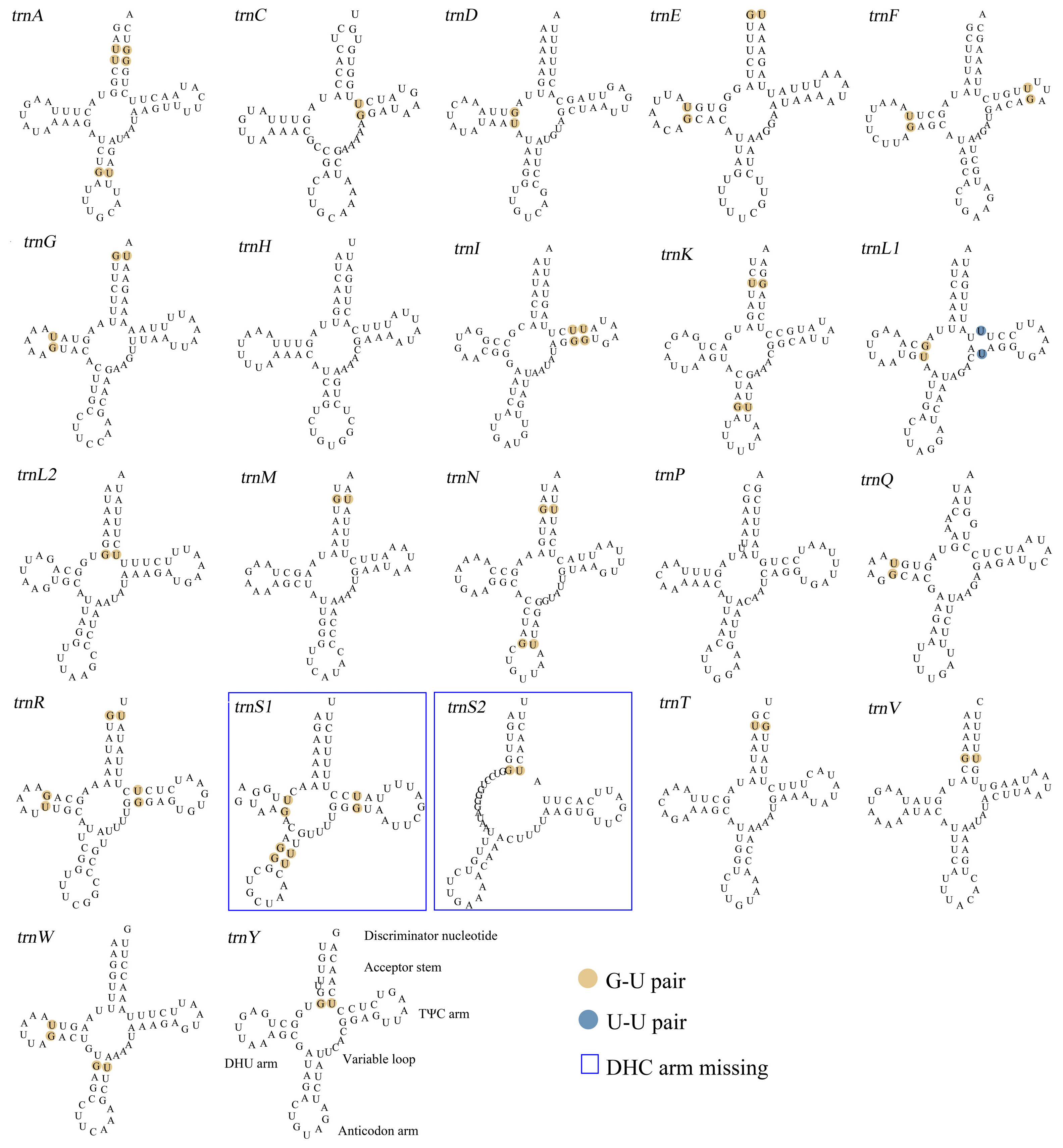

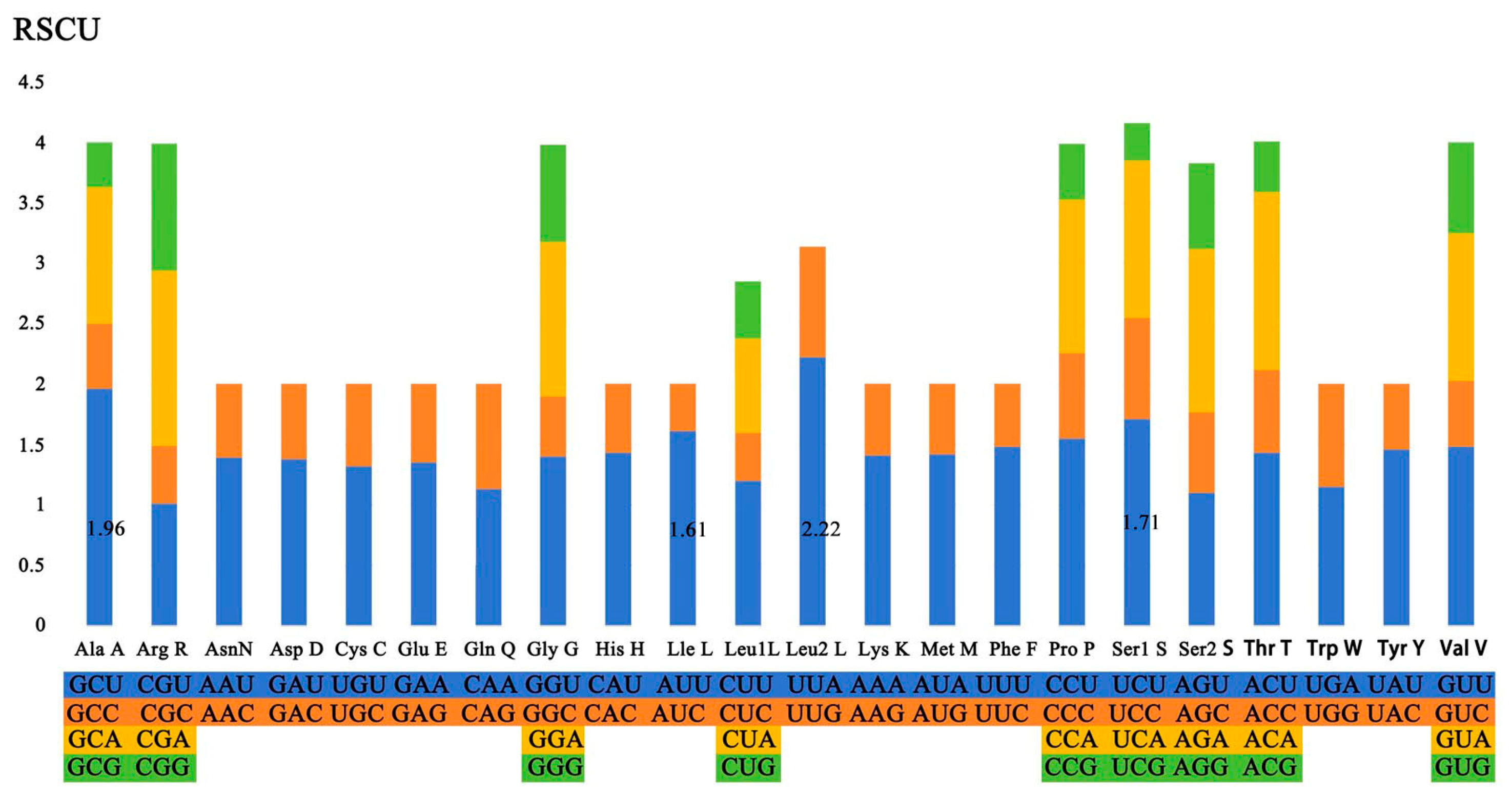



| Superfamily | Family | Species | Size (bp) | Accession No. |
|---|---|---|---|---|
| Olivoidea | Ancillariidae | Amalda mucronata | 15,353 | MN385249 |
| Amalda northlandica | 15,354 | NC014403 | ||
| Volutoidea | Volutidae | Fulgoraria rupestris * | 16,223 | OR588873 |
| Cymbium olla | 15,375 | NC013245 | ||
| Neptuneopsis gilchristi | 15,312 | MN125492 | ||
| Melo melo | 15,721 | MN462590 | ||
| Buccinoidea | Melongenidae | Brunneifusus ternatanus | 16,254 | MW548267 |
| Hemifusus tuba | 15,483 | MN462591 | ||
| Buccinidae | Volutharpa ampullacea | 16,177 | NC067974 | |
| Buccinum undatum | 15,265 | NC040940 | ||
| Buccinulum pertinax | 15,247 | NC039124 | ||
| Penion ormesi | 15,234 | NC039126 | ||
| Aeneator elegans | 15,254 | NC039120 | ||
| Buccinum tsubai | 15,262 | MW664905 | ||
| Penion chathamensis | 15,227 | MH140428 | ||
| Neptunea subdilatata | 15,393 | MG827217 | ||
| Penion maximus | 15,249 | MG211144 | ||
| Neptunea cumingii | 15,254 | NC062790 | ||
| Nassariidae | Nassarius foveolatus | 15,343 | MH346209 | |
| Nassarius javanus | 15,325 | NC041547 | ||
| Nassarius pullus | 15,278 | NC041311 | ||
| Tritia reticulata | 15,337 | NC038169 | ||
| Nassarius siquijorensis | 15,337 | NC048962 | ||
| Nassarius glans | 15,296 | NC049091 | ||
| Nassarius gregarius | 15,171 | NC062791 | ||
| Fasciolariidae | Fusinus longicaudus | 16,319 | NC045906 | |
| Columbellidae | Columbella adansoni | 16,272 | KP716637 | |
| Mitrella albuginosa | 16,244 | MZ618619 | ||
| Conoidea | Conidae | Conus striatus | 15,738 | KX156937 |
| Conus borgesi | 15,536 | EU827198 | ||
| Conus imperialis | 15,505 | NC080963 | ||
| Conus litteratus | 15,569 | NC080962 | ||
| Conus marmoreus | 15,579 | OR033162 | ||
| Conus virgo | 15,594 | OR033159 | ||
| Conus ventricosus | 16,307 | ON968979 | ||
| Fusiturridae | Fusiturris similis | 15,595 | EU827197 | |
| Turridae | Gemmuloborsonia moosai | 15,541 | NC038183 | |
| Lophiotoma cerithiformis | 15,380 | NC008098 | ||
| Pseudomelatomidae | Leucosyrinx sp. | 15,358 | NC038185 | |
| Terebridae | Oxymeris dimidiata | 16,513 | EU827196 | |
| Raphitomidae | Phymorhynchus buccinoides | 15,764 | MN583349 | |
| Typhlosyrinx sp. | 15,804 | NC038186 | ||
| Clavatulidae | Turricula nelliae | 16,453 | MK251986 | |
| Muricoidea | Muricidae | Bolinus brandaris | 15,380 | EU827194 |
| Boreotrophon candelabrum | 15,265 | MK361104 | ||
| Ceratostoma burnetti | 15,334 | MK411749 | ||
| Chicoreus torrefactus | 15,359 | MG786489 | ||
| Purpura bufo | 15,239 | MW550291 | ||
| Tylothais aculeata | 17,024 | ON018806 | ||
| Unionoidea | Unionidae | Anodonta euscaphys | 15,741 | KP187851 |
| Anodonta arcaeformis | 15,672 | KF667530 |
| Gene | Position(bp) | Direction | Length (bp) | Intergenic Nucleotides (bp) | Start/Stop Codons | Anticodon | |
|---|---|---|---|---|---|---|---|
| From | To | ||||||
| trnF | 1 | 70 | + | 70 | 0 | GAA | |
| D−loop | 71 | 1,046 | + | 976 | 0 | ||
| cox3 | 1,047 | 1,826 | + | 780 | 18 | ATG/TAG | |
| trnK | 1,845 | 1,910 | + | 66 | 5 | TTT | |
| trnA | 1,916 | 1,984 | + | 69 | 7 | TGC | |
| trnR | 1,992 | 2,060 | + | 69 | 14 | TCG | |
| trnN | 2,075 | 2,142 | + | 68 | 6 | GTT | |
| trnI | 2,149 | 2,214 | + | 66 | 3 | GAT | |
| nad3 | 2,218 | 2,571 | + | 354 | 0 | ATG/TAA | |
| trnS1 | 2,572 | 2,639 | + | 68 | 0 | GCT | |
| nad2 | 2,640 | 3,719 | + | 1,080 | −23 | ATT/TAA | |
| cox1 | 3,697 | 5,232 | + | 1,536 | 16 | ATG/TAA | |
| cox2 | 5,249 | 5,935 | + | 687 | −2 | ATG/TAA | |
| trnD | 5,934 | 6,002 | + | 69 | 1 | GTC | |
| atp8 | 6,004 | 6,162 | + | 159 | 6 | ATG/TAA | |
| atp6 | 6,169 | 6,864 | + | 696 | 37 | ATG/TAG | |
| trnM | 6,902 | 6,967 | − | 66 | 2 | CAT | |
| trnY | 6,970 | 7,032 | − | 65 | 13 | GTA | |
| trnC | 7,046 | 7,110 | − | 65 | 0 | GCA | |
| trnW | 7,111 | 7,178 | − | 68 | −2 | TCA | |
| trnQ | 7,177 | 7,241 | − | 65 | 10 | TTG | |
| trnG | 7,252 | 7,318 | − | 67 | 0 | TCC | |
| trnE | 7,319 | 7,386 | − | 68 | 122 | TTC | |
| 12SrnA | 7,509 | 8,341 | + | 833 | −3 | ||
| trnV | 8,339 | 8,407 | + | 69 | 4 | TAC | |
| 16SrnA | 8,412 | 9,777 | + | 1,366 | −3 | ||
| trnL1 | 9,775 | 9,843 | + | 69 | 2 | TAG | |
| trnL2 | 9,846 | 9,914 | + | 69 | 1 | TAA | |
| nad1 | 9,916 | 10,857 | + | 942 | 4 | ATG/TAG | |
| trnP | 10,862 | 10,931 | + | 70 | 1 | TGG | |
| nad6 | 10,933 | 11,433 | + | 501 | 13 | ATG/TAA | |
| cob | 11,447 | 12,586 | + | 1,140 | 9 | ATG/TAG | |
| trnS2 | 12,596 | 12,661 | + | 66 | 8 | TGA | |
| trnT | 12,670 | 12,738 | − | 69 | 21 | TGT | |
| nad4L | 12,760 | 13,056 | + | 297 | −7 | ATG/TAG | |
| nad4 | 13,050 | 14,423 | + | 1,338 | 3 | ATG/TAA | |
| trnH | 14,427 | 14,495 | + | 69 | 0 | GTG | |
| nad5 | 14,496 | 16,214 | + | 1,722 | 8 | ATT/TAA | |
| F. rupestris | A (%) | T (%) | G (%) | C (%) | A + T (%) | G + C (%) | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|---|---|
| Mitogenome | 30.55 | 38.12 | 16.01 | 15.32 | 68.67 | 31.33 | −0.11 | 0.02 |
| cox1 | 26.82 | 37.70 | 18.62 | 16.86 | 64.52 | 35.48 | −0.17 | 0.05 |
| cox2 | 30.71 | 35.37 | 17.76 | 16.16 | 66.08 | 33.92 | −0.07 | 0.05 |
| atp8 | 34.59 | 38.99 | 13.84 | 12.58 | 73.58 | 26.42 | −0.06 | 0.05 |
| atp6 | 26.72 | 42.39 | 14.08 | 16.81 | 69.11 | 30.89 | −0.23 | −0.09 |
| cox3 | 23.08 | 38.97 | 21.79 | 16.15 | 62.05 | 37.95 | −0.26 | 0.15 |
| nad3 | 25.71 | 39.55 | 20.06 | 14.69 | 65.25 | 34.75 | −0.21 | 0.15 |
| nad1 | 28.24 | 39.49 | 15.61 | 16.67 | 67.73 | 32.27 | −0.17 | −0.03 |
| nad5 | 29.55 | 39.44 | 13.44 | 17.57 | 68.99 | 31.01 | −0.14 | −0.13 |
| nad4 | 29.62 | 40.61 | 13.03 | 16.74 | 70.23 | 29.77 | −0.16 | −0.12 |
| nad4l | 28.96 | 38.72 | 18.18 | 14.14 | 67.68 | 32.32 | −0.14 | 0.13 |
| nad6 | 28.74 | 43.31 | 12.38 | 15.57 | 72.06 | 27.94 | −0.20 | −0.11 |
| cob | 26.93 | 39.04 | 18.62 | 16.86 | 65.96 | 35.48 | −0.18 | 0.05 |
| nad2 | 28.24 | 41.76 | 18.06 | 11.94 | 70.00 | 30.00 | −0.19 | 0.20 |
| tRNAs | 35.69 | 34.68 | 17.14 | 12.50 | 70.36 | 29.64 | 0.01 | 0.16 |
| rRNAs | 37.02 | 33.20 | 17.19 | 12.60 | 70.21 | 29.79 | 0.05 | 0.15 |
| PCGs | 27.84 | 39.37 | 16.59 | 16.21 | 67.21 | 32.80 | −0.17 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Dong, X.; Xu, K.; Zeng, J.; Wang, Z.; Li, J. The Characterization of the Mitochondrial Genome of Fulgoraria rupestris and Phylogenetic Considerations within the Neogastropoda. Genes 2024, 15, 1076. https://doi.org/10.3390/genes15081076
Ma J, Dong X, Xu K, Zeng J, Wang Z, Li J. The Characterization of the Mitochondrial Genome of Fulgoraria rupestris and Phylogenetic Considerations within the Neogastropoda. Genes. 2024; 15(8):1076. https://doi.org/10.3390/genes15081076
Chicago/Turabian StyleMa, Jiale, Xiangli Dong, Kaida Xu, Jiaying Zeng, Zhongming Wang, and Jiji Li. 2024. "The Characterization of the Mitochondrial Genome of Fulgoraria rupestris and Phylogenetic Considerations within the Neogastropoda" Genes 15, no. 8: 1076. https://doi.org/10.3390/genes15081076




