No Evidence That Vitamin D Levels or Deficiency Are Associated with the Risk of Open-Angle Glaucoma in Individuals of European Ancestry: A Mendelian Randomisation Analysis
Abstract
:1. Introduction
2. Methods
2.1. Vitamin D Levels: The SUNLIGHT Consortium
2.2. Vitamin D Deficiency: The UK Biobank
2.3. Primary Open-Angle Glaucoma
2.4. Statistical Analysis
2.5. Vitamin D Metabolic Pathway Variants
2.6. Sensitivity Analysis
2.6.1. Replication Cohort: FinnGen R9
2.6.2. Vitamin D Levels p-Value Threshold
2.6.3. Vitamin D Deficiency Winter Samples
3. Results
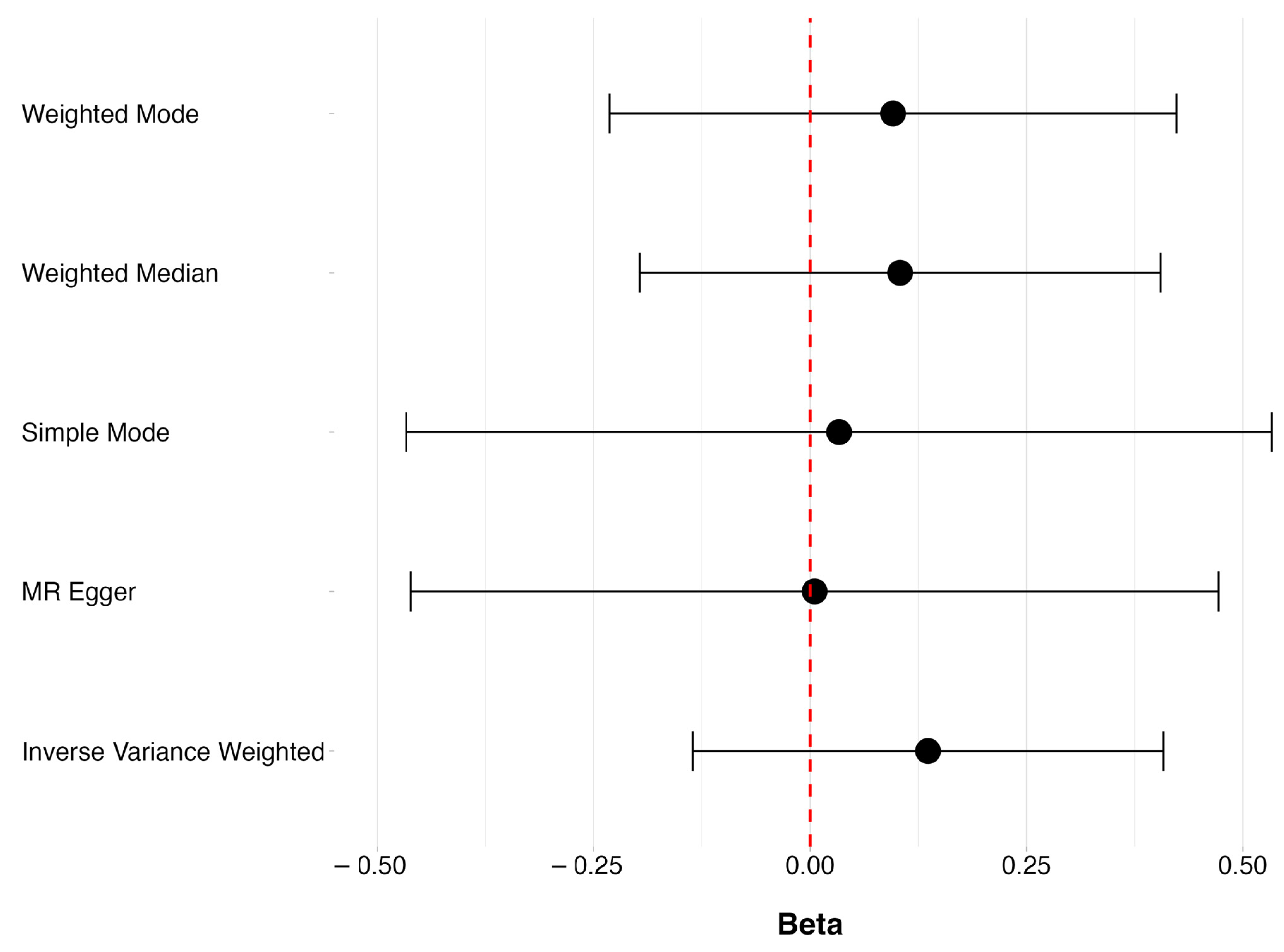
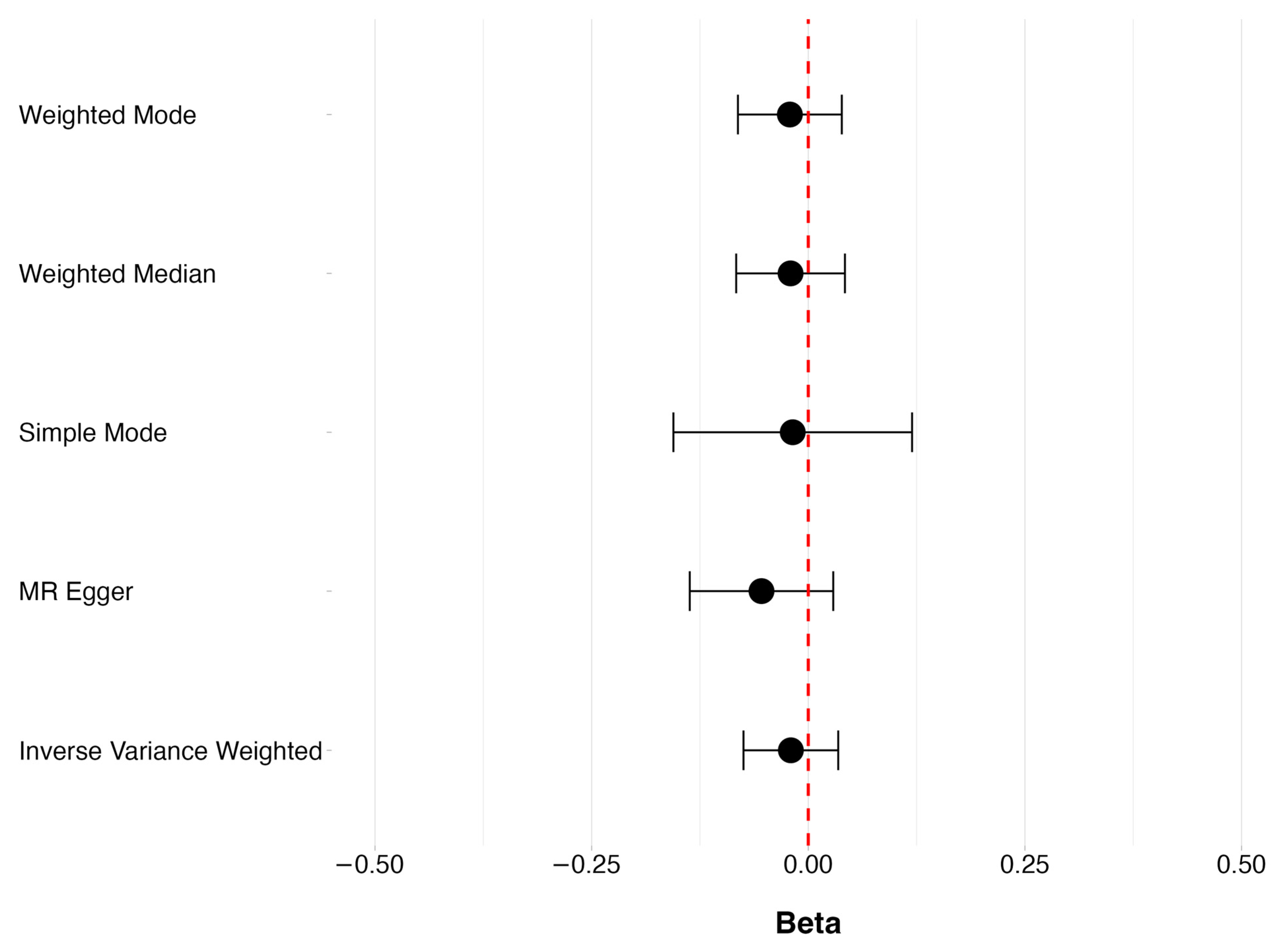
3.1. Levels of Vitamin D and POAG
3.2. Vitamin D Deficiency and POAG
3.3. Sensitivity Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Foster, P. Epidemiology of Glaucoma: What’s New? Can. J. Ophthalmol. 2012, 47, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Extrarenal Vitamin D Activation and Interactions Between Vitamin D 2, Vitamin D 3, and Vitamin D Analogs. Annu. Rev. Nutr. 2013, 33, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, C.; Tennakoon, S.; Kállay, E. Cytochrome P450 Vitamin D Hydroxylases in Inflammation and Cancer. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 74, pp. 413–458. ISBN 978-0-12-803119-3. [Google Scholar]
- Fraser, W.D.; Milan, A.M. Vitamin D Assays: Past and Present Debates, Difficulties, and Developments. Calcif. Tissue Int. 2013, 92, 118–127. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current Vitamin D Status in European and Middle East Countries and Strategies to Prevent Vitamin D Deficiency: A Position Statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- Samefors, M.; Östgren, C.J.; Mölstad, S.; Lannering, C.; Midlöv, P.; Tengblad, A. Vitamin D Deficiency in Elderly People in Swedish Nursing Homes Is Associated with Increased Mortality. Eur. J. Endocrinol. 2014, 170, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Snijder, M.B.; Van Dam, R.M.; Visser, M.; Deeg, D.J.H.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in Relation to Vitamin D Status and Parathyroid Hormone Levels: A Population-Based Study in Older Men and Women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef]
- Krieg, M.-A.; Cornuz, J.; Jacquet, A.F.; Thiébaud, D.; Burckhardt, P. Influence of Anthropometric Parameters and Biochemical Markers of Bone Metabolism on Quantitative Ultrasound of Bone in the Institutionalized Elderly. Osteoporos. Int. 1998, 8, 115–120. [Google Scholar] [CrossRef]
- Izzotti, A.; Bagnis, A.; Sacca, S. The Role of Oxidative Stress in Glaucoma. Mutat. Res. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Tezel, G. The Role of Glia, Mitochondria, and the Immune System in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative Stress and Reactive Oxygen Species: A Review of Their Role in Ocular Disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The Controversial Role of Vitamin D as an Antioxidant: Results from Randomised Controlled Trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Tohari, A.M.; Alhasani, R.H.; Biswas, L.; Patnaik, S.R.; Reilly, J.; Zeng, Z.; Shu, X. Vitamin D Attenuates Oxidative Damage and Inflammation in Retinal Pigment Epithelial Cells. Antioxidants 2019, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, R.; Chen, Y.I.; Zangwill, L.M.; Holman, M.; Dirkes, K.; Hai, Y.; Arzumanyan, Z.; Slight, R.; Hammel, N.; Girkin, C.A.; et al. Association of Severity of Primary Open-Angle Glaucoma with Serum Vitamin D Levels in Patients of African Descent. Mol. Vis. 2019, 25, 438–445. [Google Scholar] [PubMed]
- Yoo, T.K.; Oh, E.; Hong, S. Is Vitamin D Status Associated with Open-Angle Glaucoma? A Cross-Sectional Study from South Korea. Public Health Nutr. 2014, 17, 833–843. [Google Scholar] [CrossRef]
- Goncalves, A.; Milea, D.; Gohier, P.; Jallet, G.; Leruez, S.; Baskaran, M.; Aung, T.; Annweiler, C. Serum Vitamin D Status Is Associated with the Presence but Not the Severity of Primary Open Angle Glaucoma. Maturitas 2015, 81, 470–474. [Google Scholar] [CrossRef]
- Vuković Arar, Ž. ASSOCIATION BETWEEN SERUM VITAMIN D LEVEL AND GLAUCOMA IN WOMEN. Acta Clin. Croat. 2016, 55, 203–208. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, J.M.; Kim, J.H.; Lee, M.Y.; Won, Y.S.; Lee, J.Y.; Park, K.H. The Relationship between Vitamin D and Glaucoma: A Kangbuk Samsung Health Study. Korean J. Ophthalmol. 2016, 30, 426. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Shao, M.; Cao, W.; Sun, X. Lack of Association between Serum Vitamin B6, Vitamin B12, and Vitamin D Levels with Different Types of Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 636. [Google Scholar] [CrossRef]
- Lee, K.; Lim, C.-Y. Mendelian Randomization Analysis in Observational Epidemiology. J. Lipid Atheroscler. 2019, 8, 67. [Google Scholar] [CrossRef]
- Thanassoulis, G.; O’Donnell, C.J. Mendelian Randomization: Nature’s Randomized Trial in the Post–Genome Era. JAMA 2009, 301, 2386. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian Randomization: Genetic Anchors for Causal Inference in Epidemiological Studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.-H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-Wide Association Study in 79,366 European-Ancestry Individuals Informs the Genetic Architecture of 25-Hydroxyvitamin D Levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; Van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Amin, H.A.; Drenos, F. No Evidence That Vitamin D Is Able to Prevent or Affect the Severity of COVID-19 in Individuals with European Ancestry: A Mendelian Randomisation Study of Open Data. BMJ Nutr. Prev. Health 2021, 4, 42–48. [Google Scholar] [CrossRef]
- Allen, N.; Sudlow, C.; Downey, P.; Peakman, T.; Danesh, J.; Elliott, P.; Gallacher, J.; Green, J.; Matthews, P.; Pell, J.; et al. UK Biobank: Current Status and What It Means for Epidemiology. Health Policy Technol. 2012, 1, 123–126. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-Wide Meta-Analysis Identifies 127 Open-Angle Glaucoma Loci with Consistent Effect across Ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. 2023. Available online: https://www.r-project.org/ (accessed on 27 June 2024).
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for Performing Mendelian Randomization Investigations: Update for Summer 2023. Wellcome Open Res. 2023, 4, 186. [Google Scholar] [CrossRef]
- Slob, E.A.W.; Burgess, S. A Comparison of Robust Mendelian Randomization Methods Using Summary Data. Genet. Epidemiol. 2020, 44, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davies, N.M.; Hemani, G.; Davey Smith, G. Two-Sample Mendelian Randomization: Avoiding the Downsides of a Powerful, Widely Applicable but Potentially Fallible Technique. Int. J. Epidemiol. 2016, 45, 1717–1726. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- FinnGen Project FinnGen: Documentation of R9 Release 2023. Available online: https://r9.finngen.fi/ (accessed on 27 June 2024).
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen Provides Genetic Insights from a Well-Phenotyped Isolated Population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Tomei, S.; Singh, P.; Mathew, R.; Mattei, V.; Garand, M.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Role of Polymorphisms in Vitamin D-Related Genes in Response to Vitamin D Supplementation. Nutrients 2020, 12, 2608. [Google Scholar] [CrossRef] [PubMed]
- Krefting, E.A.; Jorde, R.; Christoffersen, T.; Grimnes, G. Vitamin D and Intraocular Pressure—Results from a Case -Control and an Intervention Study. Acta Ophthalmol. 2014, 92, 345–349. [Google Scholar] [CrossRef]
- Hennis, A.; Wu, S.-Y.; Nemesure, B.; Honkanen, R.; Leske, M.C. Awareness of Incident Open-Angle Glaucoma in a Population Study. Ophthalmology 2007, 114, 1816–1821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanso, N.; Hashimi, M.; Amin, H.A.; Day, A.C.; Drenos, F. No Evidence That Vitamin D Levels or Deficiency Are Associated with the Risk of Open-Angle Glaucoma in Individuals of European Ancestry: A Mendelian Randomisation Analysis. Genes 2024, 15, 1084. https://doi.org/10.3390/genes15081084
Kanso N, Hashimi M, Amin HA, Day AC, Drenos F. No Evidence That Vitamin D Levels or Deficiency Are Associated with the Risk of Open-Angle Glaucoma in Individuals of European Ancestry: A Mendelian Randomisation Analysis. Genes. 2024; 15(8):1084. https://doi.org/10.3390/genes15081084
Chicago/Turabian StyleKanso, Nour, Munisa Hashimi, Hasnat A. Amin, Alexander C. Day, and Fotios Drenos. 2024. "No Evidence That Vitamin D Levels or Deficiency Are Associated with the Risk of Open-Angle Glaucoma in Individuals of European Ancestry: A Mendelian Randomisation Analysis" Genes 15, no. 8: 1084. https://doi.org/10.3390/genes15081084




