Carboxypeptidase Inhibitor LXN Expression in Endometrial Tissue Is Menstrual Cycle Phase-Dependent and Is Upregulated in Endometriotic Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. mRNA Extraction and Gene Expression Analysis
2.3. Immunohistochemistry (IHC)
2.4. Cell Culture and siRNA Transfection
2.5. MTT (3-(4,5-Dimethyl-2-yl)-2,5-Diphenyltetrazolium Bromide) Cell Viability Assay
2.6. Migration Assay
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
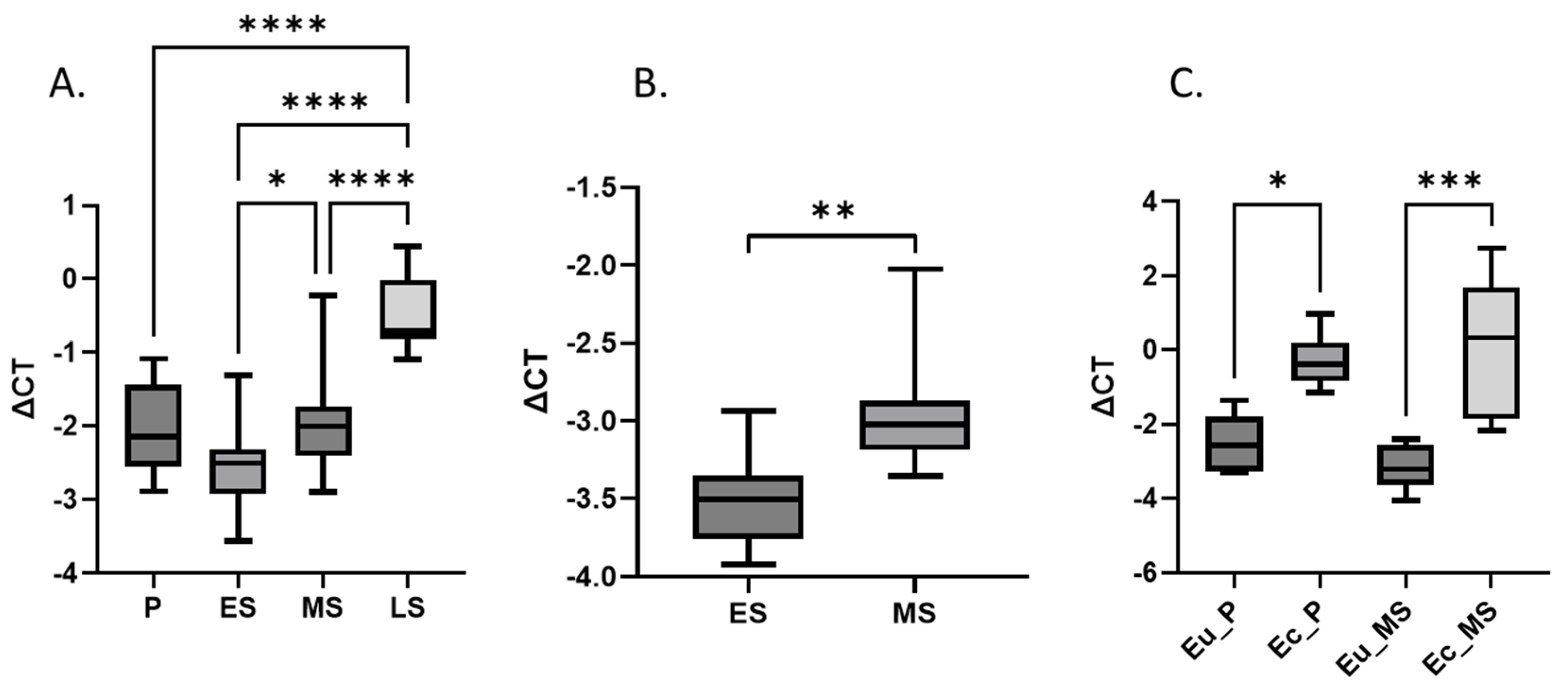
3.2. LXN Expression in Eutopic and Ectopic Endometrium
3.3. LXN Expression in Endometrial Tissue by IHC
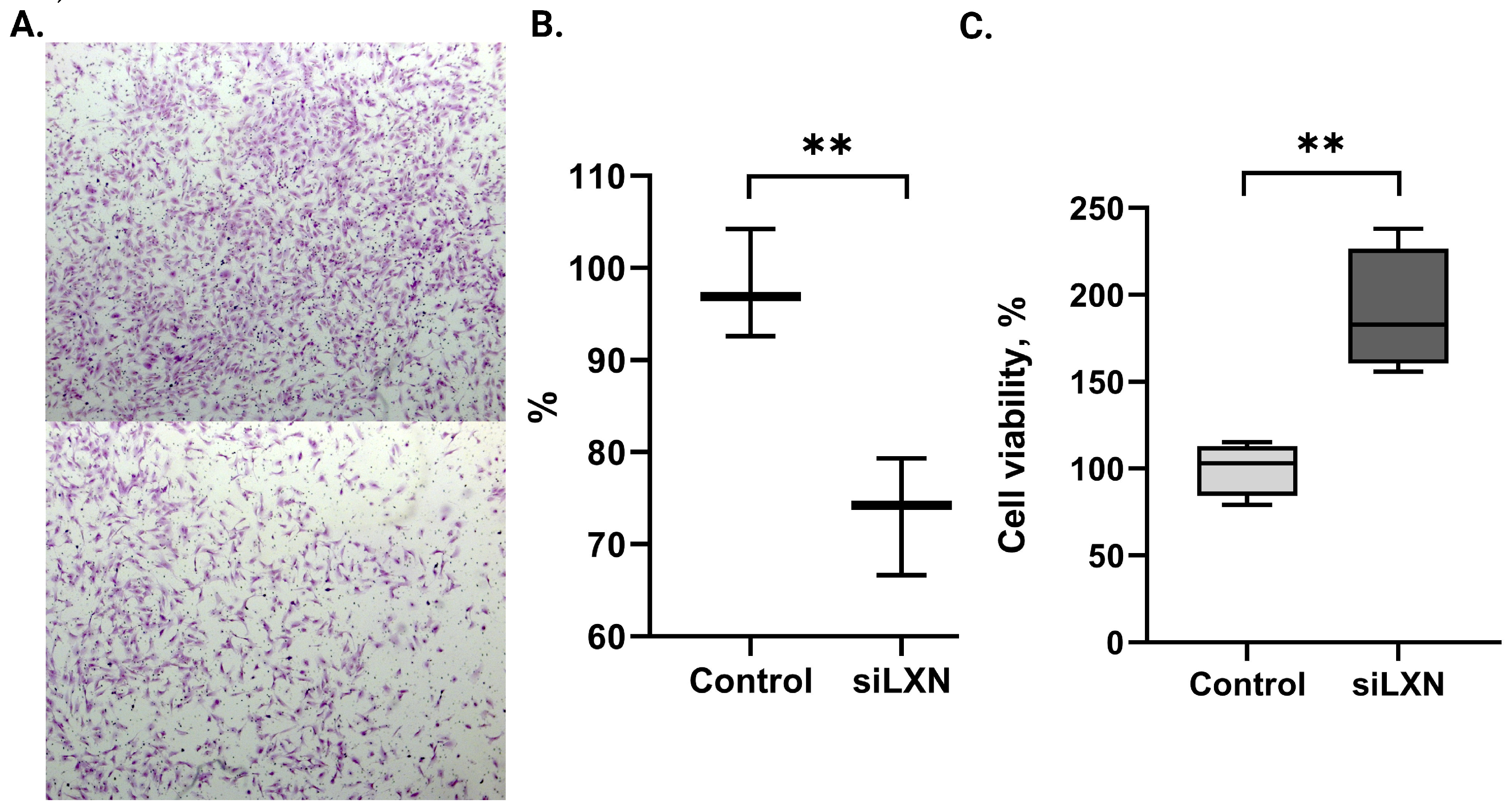
3.4. LXN Silencing Affects Endometrial Stromal Cell Migration and Viability
3.5. LXN Availability in Body Fluids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordts, S.; Koninckx, P.; Brosens, I. Pathogenesis of deep endometriosis. Fertil. Steril. 2017, 108, 872–885.e1. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.L.; Escareno, C.R.; Godsland, J.M.; Doig, J.R.; Johnson, C.M.; Phillips, S.C.; Smith, S.K.; Tavaré, S.; Print, C.G.; Charnock-Jones, D.S. Endometrial-Peritoneal Interactions during Endometriotic Lesion Establishment. Am. J. Pathol. 2008, 173, 700–715. [Google Scholar] [CrossRef]
- Rekker, K.; Saare, M.; Eriste, E.; Tasa, T.; Kukuškina, V.; Roost, A.M.; Anderson, K.; Samuel, K.; Karro, H.; Salumets, A.; et al. High-throughput mRNA sequencing of stromal cells from endometriomas and endometrium. Reproduction 2017, 154, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Normant, E.; Martres, M.-P.; Schwartz, J.-C.; Gros, C. Purification, cDNA cloning, functional expression, and characterization of a 26-kDa endogenous mammalian carboxypeptidase inhibitor. Proc. Natl. Acad. Sci. USA 1995, 92, 12225–12229. [Google Scholar] [CrossRef]
- Kasvandik, S.; Samuel, K.; Peters, M.; Eimre, M.; Peet, N.; Roost, A.M.; Padrik, L.; Paju, K.; Peil, L.; Salumets, A. Deep Quantitative Proteomics Reveals Extensive Metabolic Reprogramming and Cancer-Like Changes of Ectopic Endometriotic Stromal Cells. J. Proteome Res. 2016, 15, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Ni, Q.F.; Tian, Y.; Kong, L.L.; Lu, Y.T.; Ding, W.Z.; Kong, L.B. Latexin exhibits tumor suppressor potential in hepatocellular carcinoma. Oncol. Rep. 2014, 31, 1364–1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Basang, Z.; Ding, H.; Lu, Z.; Ning, T.; Wei, H.; Cai, H.; Ke, Y. Latexin expression is downregulated in human gastric carcinomas and exhibits tumor suppressor potential. BMC Cancer 2011, 11, 121. [Google Scholar] [CrossRef]
- He, G.; Kan, S.; Xu, S.; Sun, X.; Li, R.; Shu, W.; Chen, M. LXN deficiency regulates cytoskeleton remodelling by promoting proteolytic cleavage of Filamin A in vascular endothelial cells. J. Cell. Mol. Med. 2021, 25, 6815–6827. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Li, Z.; Wang, C.; Jia, J.; Gao, T.; Hildebrandt, G.; Zhou, D.; Bondada, S.; Ji, P.; et al. Latexin Inactivation Enhances Survival and Long-Term Engraftment of Hematopoietic Stem Cells and Expands the Entire Hematopoietic System in Mice. Stem Cell Rep. 2017, 8, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Kalkhof, S.; Büttner, P.; Krieg, L.; Wabitsch, M.; Küntzel, C.; Friebe, D.; Landgraf, K.; Hanschkow, M.; Schubert, K.; Kiess, W.; et al. In Depth Quantitative Proteomic and Transcriptomic Characterization of Human Adipocyte Differentiation Using the SGBS Cell Line. Proteomics 2020, 8, e1900405. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, A.; Listwan, P.; Cowieson, N.; Huber, T.; Ravasi, T.; Wells, C.A.; Flanagan, J.U.; Kellie, S.; Hume, D.A.; Kobe, B.; et al. An inflammatory role for the mammalian carboxypeptidase inhibitor latexin: Relationship to cystatins and the tumor suppressor TIG1. Structure Cell Press. 2005, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, H.; Tian, Y.; Wang, J.; Xu, S.; Huang, K.; Liang, H.; Chen, M. Covalent organic framework-based immunosensor to detect plasma Latexin reveals novel biomarker for coronary artery diseases. Anal. Chim. Acta 2023, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Saare, M.; Laisk, T.; Teder, H.; Paluoja, P.; Palta, P.; Koel, M.; Kirss, F.; Karro, H.; Sõritsa, D.; Salumets, A.; et al. A molecular tool for menstrual cycle phase dating of endometrial samples in endometriosis transcriptome studies. Biol. Reprod. 2019, 101, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rekker, K.; Altmäe, S.; Suhorutshenko, M.; Peters, M.; Martinez-Blanch, J.F.; Codoñer, F.M.; Vilella, F.; Simón, C.; Salumets, A.; Velthut-Meikas, A. A two-cohort RNA-seq study reveals changes in endometrial and blood mirnome in fertile and infertile women. Genes 2018, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Samalecos, A.; Reimann, K.; Wittmann, S.; Schulte, H.M.; Brosens, J.J.; Bamberger, A.M.; Gellersen, B. Characterization of a novel telomerase-immortalized human endometrial stromal cell line, St-T1b. Reprod. Biol. Endocrinol. 2009, 7, 76. [Google Scholar] [CrossRef]
- Kasvandik, S.; Saarma, M.; Kaart, T.; Rooda, I.; Velthut-Meikas, A.; Ehrenberg, A.; Gemzell, K.; Lalitkumar, P.G.; Salumets, A.; Peters, M. Uterine Fluid Proteins for Minimally Invasive Assessment of Endometrial Receptivity. J. Clin. Endocrinol. Metab. 2020, 105, dgz019. [Google Scholar] [CrossRef] [PubMed]
- Seed, R.I.; Taurozzi, A.J.; Wilcock, D.J.; Nappo, G.; Erb, H.H.H.; Read, M.L.; Gurney, M.; Archer, L.K.; Ito, S.; Rumsby, M.G.; et al. The putative tumour suppressor protein Latexin is secreted by prostate luminal cells and is downregulated in malignancy. Sci. Rep. 2019, 9, 5120. [Google Scholar] [CrossRef]
- Kasvandik, S. The Role of Proteomic Changes in Endometrial Cells—From the Perspective of Fertility and Endometriosis. Ph.D. Thesis, University of Tartu, Tartu, Estonia, 2020. Available online: http://hdl.handle.net/10062/67345 (accessed on 5 March 2024).
- Eyster, K.M.; Hansen, K.A.; Winterton, E.; Klinkova, O.; Drappeau, D.; Mark-Kappeler, C.J. Reciprocal Communication Between Endometrial Stromal Cells and Macrophages. Reprod. Sci. 2010, 17, 809–822. [Google Scholar] [CrossRef][Green Version]
- Oldridge, E.E.; Walker, H.F.; Stower, M.J.; Simms, M.S.; Mann, V.M.; Collins, A.T.; Pellacani, D.; Maitland, N.J. Retinoic acid represses invasion and stem cell phenotype by induction of the metastasis suppressors RARRES1 and LXN. Oncogenesis 2013, 2, e45. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, D.; Vigano, P.; Somigliana, E.; Vicentini, L.M.; Vignali, M.; Busacca, M.; Di Blasio, A.M. Endometrial stromal cells from women with endometriosis reveal peculiar migratory behavior in response to ovarian steroids. Fertil. Steril. 2010, 93, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Ornek, T.; Fadiel, A.; Tan, O.; Naftolin, F.; Arici, A. Regulation and activation of ezrin protein in endometriosis. Hum. Reprod. 2008, 23, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Yotova, I.; Quan, P.; Gaba, A.; Leditznig, N.; Pateisky, P.; Kurz, C.; Tschugguel, W. Raf-1 levels determine the migration rate of primary endometrial stromal cells of patients with endometriosis. J. Cell. Mol. Med. 2012, 16, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.H.E.; Macklon, N.S.; Post Uiterweer, E.D.; Brosens, J.J.; Gellersen, B. The motile and invasive capacity of human endometrial stromal cells: Implications for normal and impaired reproductive function. Hum. Reprod. 2013, 19, 542–557. [Google Scholar] [CrossRef]
- Gellersen, B.; Reimann, K.; Samalecos, A.; Aupers, S.; Bamberger, A.M. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum. Reprod. 2010, 25, 862–873. [Google Scholar] [CrossRef]
- Belliard, A.; Noël, A.; Foidart, J.-M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef]

| Gene Expression Analysis of Endometrial Tissues throughout the Menstrual Cycle | mRNA and Protein Expression Analyses of Paired Samples of Endometrium and Lesions | ||||
|---|---|---|---|---|---|
| ENDO (n = 34) a,b | Non-ENDO (n = 27) a,c | Healthy (n = 12) | Gene Expression (n = 12) | IHC (n = 9 d) | |
| Age (years, SD) p-value p-value | 31.2 ± 4.5 0.48 a 0.58 b | 32.1 ± 5.6 0.48 a 0.34 c | 30.4 ± 3.7 | 31.6 ± 4.9 | 34.3 ± 6.5 |
| BMI (kg/m2, SD) p-value p-value | 22.3 ± 3.5 0.29 a 0.62 b | 23.2 ± 3.7 0.29 a 0.80 c | 22.9 ± 4.6 | 23.1 ± 3.5 | 22.7 ± 3.7 |
| Infertility e (n, %) | 24 (70.6%) | 20 (75.1%) | NA | 6 (50%) | 4 (44%) |
| N of children, SD | 0.4 ± 0.7 | 0.5 ± 0.8 | 1.3 ± 0.5 | 0.7 ± 1.1 | 0.9 ± 1.4 |
| Endometriosis stage (%) | I–II (53%) III–IV (47%) | NA | NA | III–IV (100%) | I–II (56%) III–IV (44%) |
| Menstrual cycle phase (n) | P (7), ES (8), MS (10), LS (9) | P (5), ES (13), MS (7), LS (2) | ES (12), MS (12) | P (6), MS (6) | P (3), ES (3), LS (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarsenova, M.; Stepanjuk, A.; Saare, M.; Kasvandik, S.; Soplepmann, P.; Mikeltadze, I.; Götte, M.; Salumets, A.; Peters, M. Carboxypeptidase Inhibitor LXN Expression in Endometrial Tissue Is Menstrual Cycle Phase-Dependent and Is Upregulated in Endometriotic Lesions. Genes 2024, 15, 1086. https://doi.org/10.3390/genes15081086
Sarsenova M, Stepanjuk A, Saare M, Kasvandik S, Soplepmann P, Mikeltadze I, Götte M, Salumets A, Peters M. Carboxypeptidase Inhibitor LXN Expression in Endometrial Tissue Is Menstrual Cycle Phase-Dependent and Is Upregulated in Endometriotic Lesions. Genes. 2024; 15(8):1086. https://doi.org/10.3390/genes15081086
Chicago/Turabian StyleSarsenova, Meruert, Artjom Stepanjuk, Merli Saare, Sergo Kasvandik, Pille Soplepmann, Iveta Mikeltadze, Martin Götte, Andres Salumets, and Maire Peters. 2024. "Carboxypeptidase Inhibitor LXN Expression in Endometrial Tissue Is Menstrual Cycle Phase-Dependent and Is Upregulated in Endometriotic Lesions" Genes 15, no. 8: 1086. https://doi.org/10.3390/genes15081086
APA StyleSarsenova, M., Stepanjuk, A., Saare, M., Kasvandik, S., Soplepmann, P., Mikeltadze, I., Götte, M., Salumets, A., & Peters, M. (2024). Carboxypeptidase Inhibitor LXN Expression in Endometrial Tissue Is Menstrual Cycle Phase-Dependent and Is Upregulated in Endometriotic Lesions. Genes, 15(8), 1086. https://doi.org/10.3390/genes15081086







