Nanoparticle-Mediated Genetic Transformation in a Selaginella Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Arginine-Functionalized Nanohydroxyapatite Particles
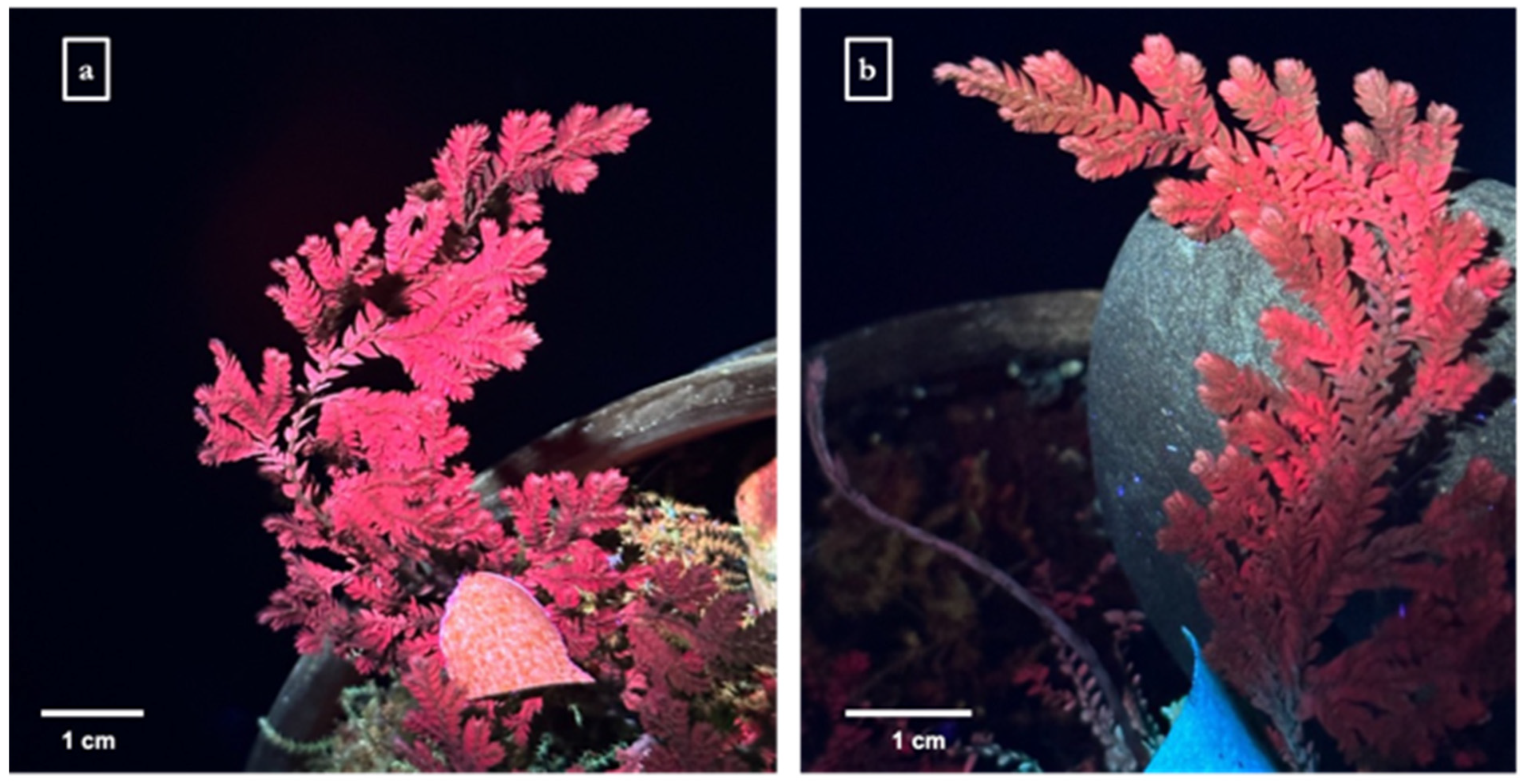
2.2. Plant Growth
2.3. The Cloning and Plasmid Isolation of the G3GFP-GUS Fusion Construct
2.4. Preparation of pGWB452-G3GFP::GUS|R-nHA Conjugate Solution
2.5. Transient Transformation of pGWB452-G3GFP::GUS|R-nHA Conjugates in S. moellendorffii
2.6. Determination of GFP or GUS Reporter Gene Expression
2.7. The Plasmid Isolation of the pAXY001 Expression Clone That Contains the eYGFPuv Gene
2.8. Preparation of pAXY001|R-nHA Conjugate Solution and Transient Transformation of pAXY001|R-nHA Conjugates into S. moellendorffii
2.9. Agrobacterium-Mediated Delivery of GUS Reporter Gene
2.10. Characterization of Conjugates
2.10.1. Transmission Electron Microscopy Imaging
2.10.2. Zeta Potential Measurements
3. Results
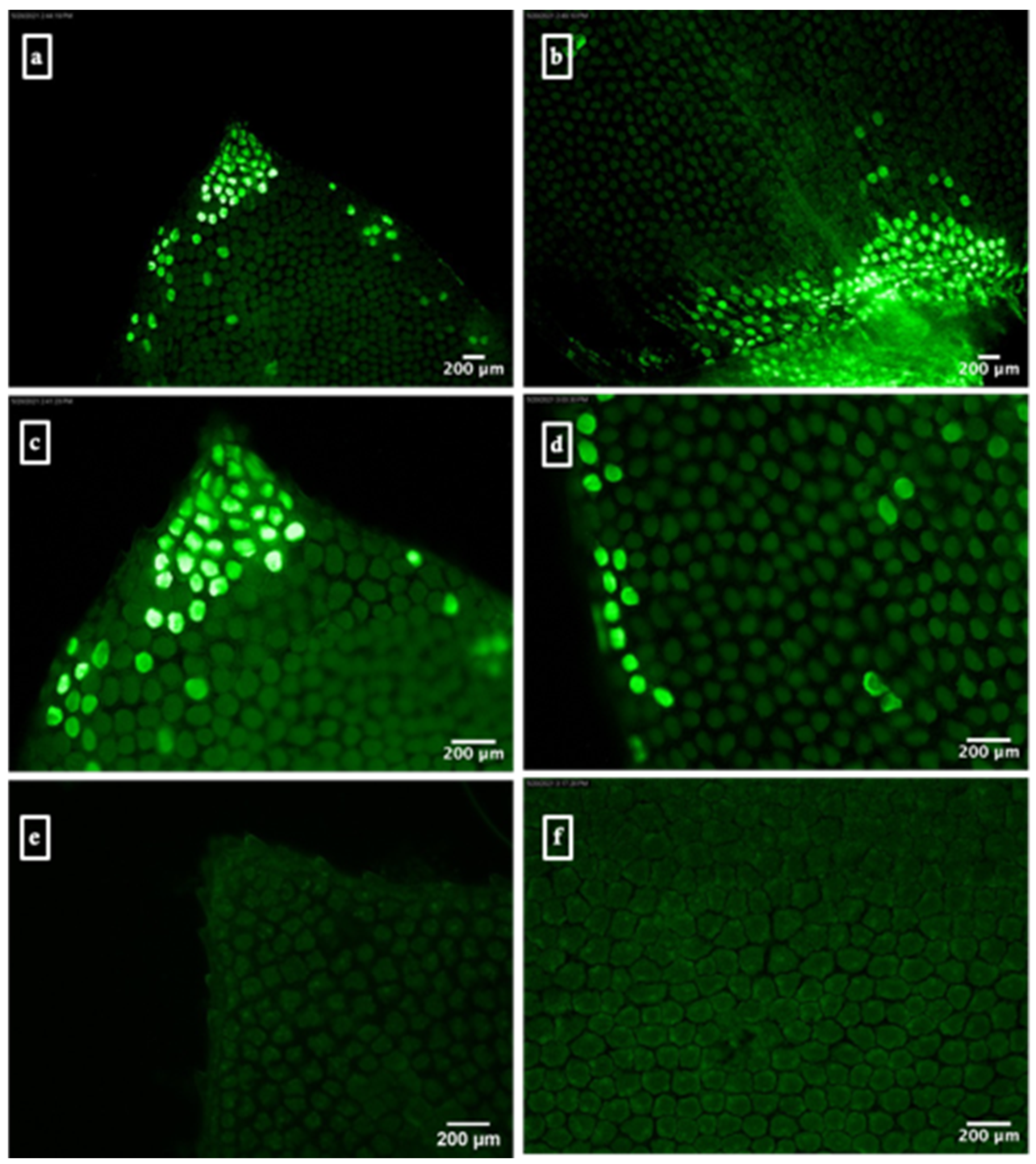
3.1. Arginine-Functionalized Nanohydroxyapatite Particle-Mediated Expression of GFP or GUS Reporter Genes in S. moellendorffii Sporophylls
3.2. Arginine-Functionalized Nanohydroxyapatite Particle-Mediated Expression of eYGFPuv Reporter Gene in S. moellendorffii Sporophylls
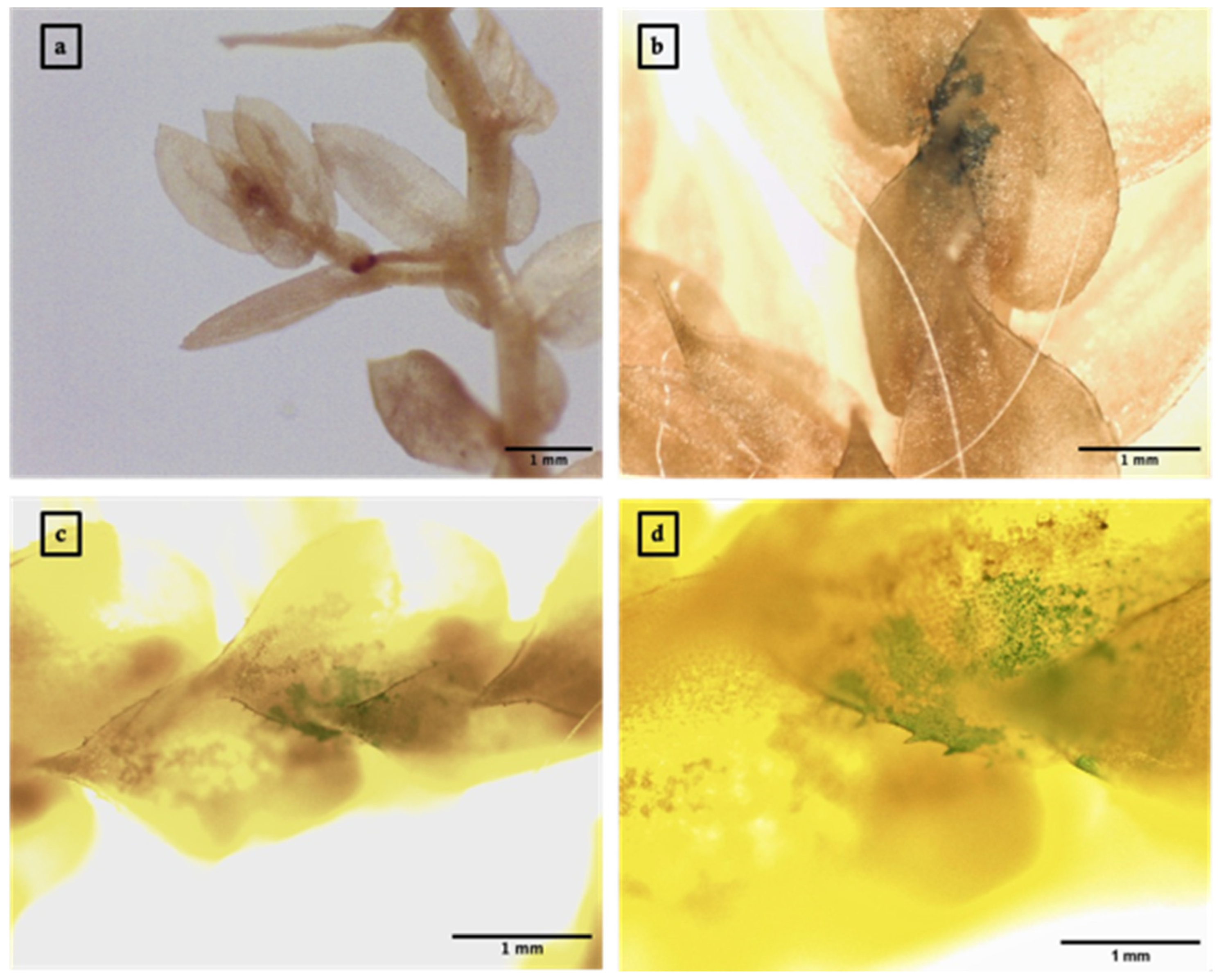
3.3. Agrobacterium-Mediated Transient Expression of GUS in S. moellendorffii Sporophylls
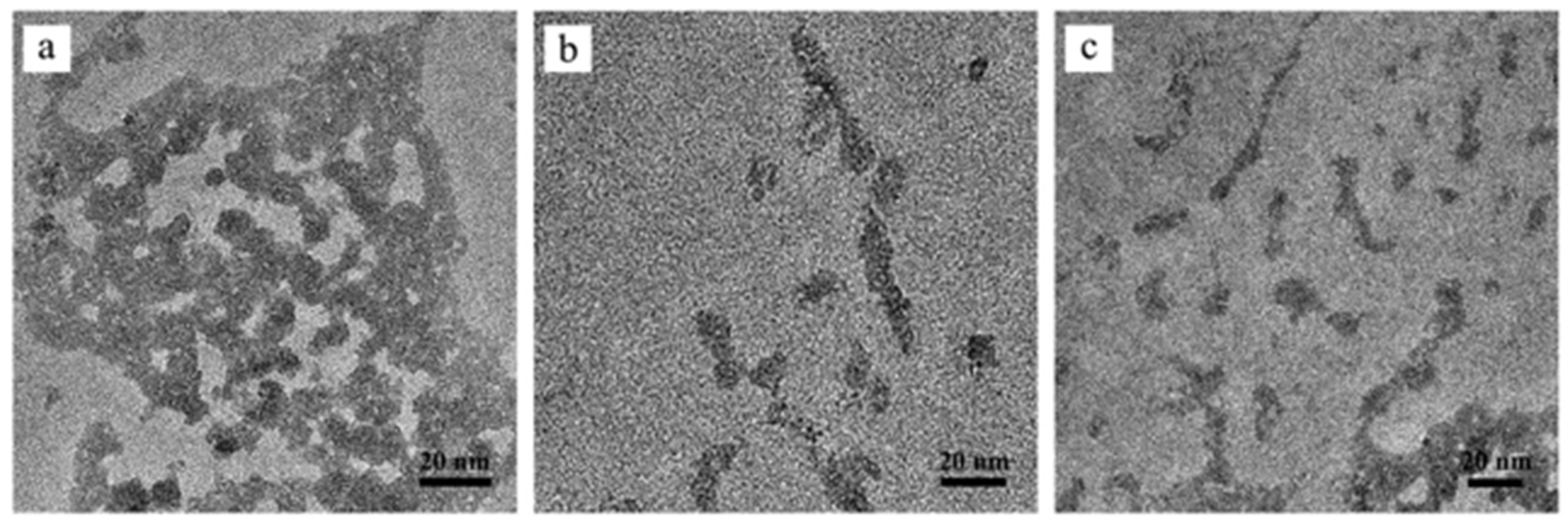
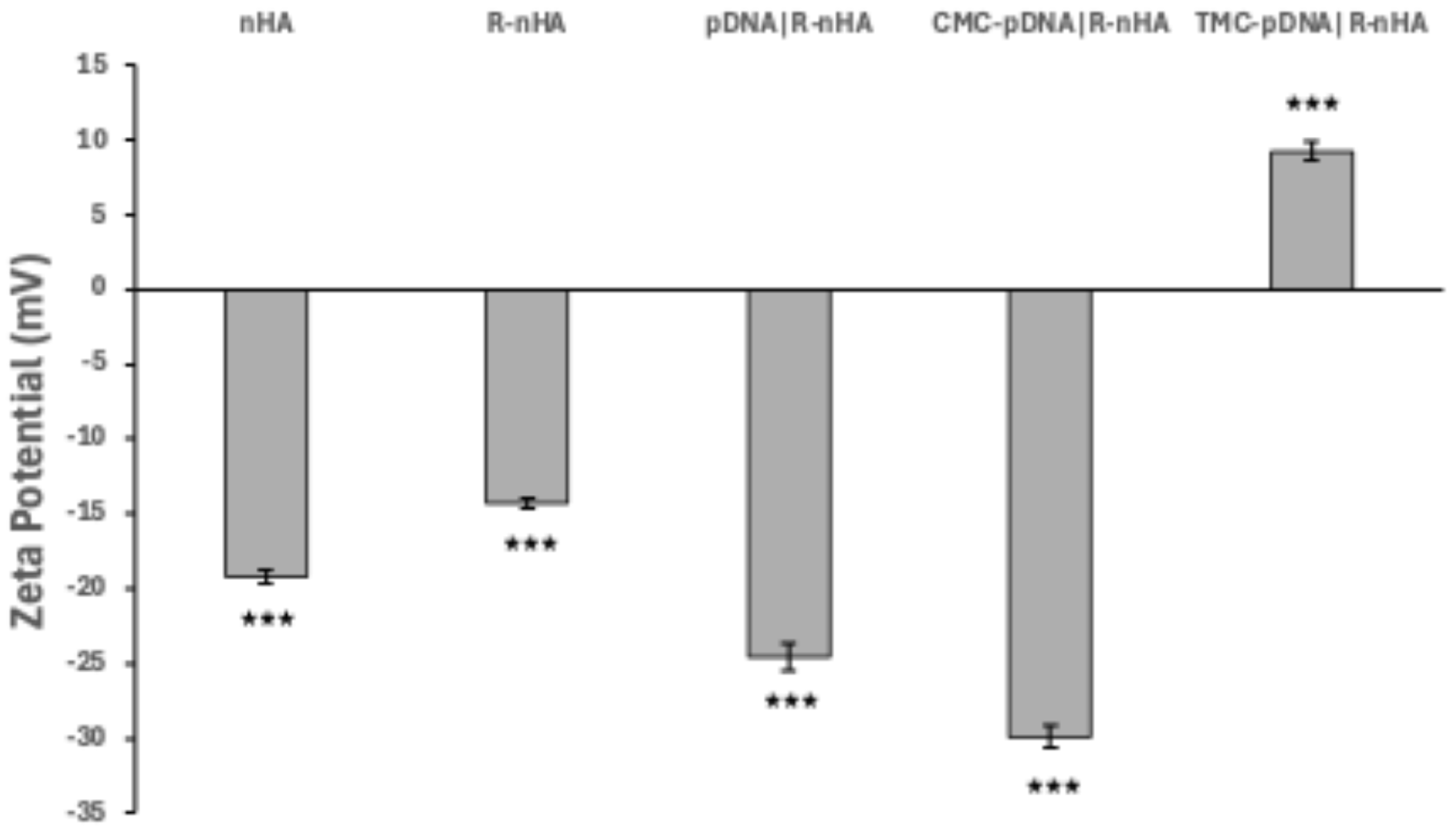
3.4. Characterization of Conjugates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, J.A. Selaginella and 400 Million Years of Separation. Annu. Rev. Plant Biol. 2009, 60, 223–238. [Google Scholar] [CrossRef]
- Yobi, A.; Wone, B.W.M.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Comparative metabolic profiling between desiccation-sensitive and desiccation-tolerant species of Selaginella reveals insights into the resurrection trait. Plant J. 2012, 72, 983–999. [Google Scholar] [CrossRef]
- Iturriaga, G.; Cushman, M.A.F.; Cushman, J.C. An EST catalogue from the resurrection plant Selaginella lepidophylla reveals abiotic stress-adaptive genes. Plant Sci. 2006, 170, 1173–1184. [Google Scholar] [CrossRef]
- VanBuren, R.; Wai, C.M.; Ou, S.; Pardo, J.; Bryant, D.; Jiang, N.; Mockler, T.C.; Edger, P.; Michael, T.P. Extreme haplotype variation in the desiccation-tolerant clubmoss Selaginella lepidophylla. Nat. Commun. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Yobi, A.; Wone, B.W.M.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol. Plant 2013, 6, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Zhang, H.; Shi, L.; Cao, F.; Guo, L.; Xie, Y.; Wang, T.; Yan, X.; Dai, S. Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J. Proteome Res. 2010, 9, 6561–6577. [Google Scholar] [CrossRef]
- Alejo-Jacuinde, G.; González-Morales, S.I.; Oropeza-Aburto, A.; Simpson, J.; Herrera-Estrella, L. Comparative transcriptome analysis suggests convergent evolution of desiccation tolerance in Selaginella species. BMC Plant Biol. 2020, 20, 468. [Google Scholar] [CrossRef]
- Deeba, F.; Pandey, A.K.; Pandey, V. Organ specific proteomic dissection of Selaginella bryopteris undergoing dehydration and rehydration. Front. Plant Sci. 2016, 7, 425. [Google Scholar] [CrossRef]
- Xu, Z.; Xin, T.; Bartels, D.; Li, Y.; Gu, W.; Yao, H.; Liu, S.; Yu, H.; Pu, X.; Zhou, J.; et al. Genome analysis of the ancient tracheophyte Selaginella tamariscina reveals evolutionary features relevant to the acquisition of desiccation tolerance. Mol. Plant 2018, 11, 983–994. [Google Scholar] [CrossRef]
- Cunningham, F.J.; Goh, N.S.; Demirer, G.S.; Matos, J.L.; Landry, M.P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018, 36, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Baltes, N.J.; Gil-Humanes, J.; Voytas, D.F. Genome engineering and agriculture: Opportunities and challenges. Prog. Mol. Biol. Transl. Sci. 2017, 149, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.; Brutnell, T.P.; Citovsky, V.; Conrad, L.; Gelvin, S.B.; Jackson, D.; Kausch, A.P.; et al. Advancing crop transformation in the era of genome editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
- Barampuram, S.; Zhang, Z.Y. Recent advances in plant transformation. In Plant Biotechnology and Agriculture; Altman, A., Hasegawa, P.M., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 133–159. [Google Scholar] [CrossRef]
- Rakoczy-Trojanowska, M. Alternative methods of plant transformation—A short review. Cell Mol. Biol. Lett. 2002, 7, 849–858. [Google Scholar]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.-J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, F.-J.; Kopittke, P.M. Engineering crops without genome integration using nanotechnology. Trends Plant Sci. 2019, 24, 574–577. [Google Scholar] [CrossRef]
- Izuegbunam, C.L.; Wijewantha, N.; Wone, B.; Ariyarathne, M.A.; Sereda, G.; Wone, B.W.M. A nano-biomimetic transformation system enables in planta expression of a reporter gene in mature plants and seeds. Nanoscale Adv. 2021, 3, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Priyam, A.; Das, R.K.; Schultz, A.; Singh, P.P. A new method for biological synthesis of agriculturally relevant nanohydroxyapatite with elucidated effects on soil bacteria. Sci. Rep. 2019, 9, 15083. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Tan, J.; Zhu, S.; Zhou, K. Arginine functionalized hydroxyapatite nanoparticles and its bioactivity for gene delivery. Trans. Nonferrous Met. Soc. China 2015, 25, 490–496. [Google Scholar] [CrossRef]
- Toldi, O.; Tuba, Z.; Scott, P. Vegetative desiccation tolerance: Is it a goldmine for bioengineering crops? Plant Sci. 2009, 176, 187–199. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kurose, T.; Hino, T.; Tanaka, K.; Kawamukai, M.; Niwa, Y.; Toyooka, K.; Matsuoka, K.; Jinbo, T.; Kimura, T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- Lim, S.D.; Yim, W.C.; Liu, D.; Hu, R.; Yang, X.; Cushman, J.C. A Vitis vinifera basic helix-loop-helix transcription factor enhances plant cell size, vegetative biomass and reproductive yield. Plant Biotechnol. J. 2018, 16, 1595–1615. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lu, H.; Tang, D.; Hassan, M.M.; Li, Y.; Chen, J.-G.; Tuskan, G.A.; Yang, X. Expanding the application of a UV-visible reporter for transient gene expression and stable transformation in plants. Hortic. Res. 2021, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Thagun, C.; Horii, Y.; Mori, M.; Fujita, S.; Ohtani, M.; Tsuchiya, K.; Kodama, Y.; Odahara, M.; Numata, K. Non-transgenic gene modulation via spray delivery of nucleic acid/peptide complexes into plant nuclei and chloroplasts. ACS Nano 2022, 16, 3506–3521. [Google Scholar] [CrossRef]
- Wang, K. Agrobacterium Protocols, 2nd ed.; Humana Press Inc.: New York, NY, USA, 2006. [Google Scholar]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780. [Google Scholar] [CrossRef]
- Ahmed, S.; Gao, X.; Jahan, M.d.A.; Adams, M.; Wu, N.; Kovinich, N. Nanoparticle-based genetic transformation of Cannabis sativa. J. Biotechnol. 2021, 326, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Novel one step transformation method for Escherichia coli and Staphylococcus aureus using arginine-glucose functionalized hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2019, 96, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Wu, Y.-Y.; Lin, Y.-H.; Sonaje, K.; Ho, Y.-C.; Chen, C.-T.; Juang, J.-H.; Sung, H.-W. Oral delivery of peptide drugs using nanoparticles self-assembled by poly(γ-glutamic acid) and a chitosan derivative functionalized by trimethylation. Bioconjug. Chem. 2008, 19, 1248–1255. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S. Topical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention, and anti-cataract effect. Int. J. Pharm. 2009, 372, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, C.; Bartels, D. Desiccation tolerance in resurrection plants: New insights from transcriptome, proteome and metabolome analysis. Front. Plant Sci. 2013, 4, 482. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Little, D.P.; Stevenson, D.W.; Bauer, D.; Moloney, C.; Stützel, T. An overview of the morphology, anatomy, and life cycle of a new model species: The lycophyte Selaginella apoda (L.) Spring. Int. J. Plant Sci. 2010, 171, 693–712. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyarathne, M.A.; Wone, B.; Wijewantha, N.; Wone, B.W.M. Nanoparticle-Mediated Genetic Transformation in a Selaginella Species. Genes 2024, 15, 1091. https://doi.org/10.3390/genes15081091
Ariyarathne MA, Wone B, Wijewantha N, Wone BWM. Nanoparticle-Mediated Genetic Transformation in a Selaginella Species. Genes. 2024; 15(8):1091. https://doi.org/10.3390/genes15081091
Chicago/Turabian StyleAriyarathne, Madhavi A., Beate Wone, Nisitha Wijewantha, and Bernard W. M. Wone. 2024. "Nanoparticle-Mediated Genetic Transformation in a Selaginella Species" Genes 15, no. 8: 1091. https://doi.org/10.3390/genes15081091







