Genomic and Pathogenic Characterization of Akanthomyces muscarius Isolated from Living Mite Infesting Hazelnut Big Buds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Culture Conditions
2.2. Total DNA Extraction
2.3. ONT Library Preparation and Genome Sequencing
2.4. De Novo Hybrid Assembly
2.5. Structural and Functional Annotation
2.6. AT-Rich Regions
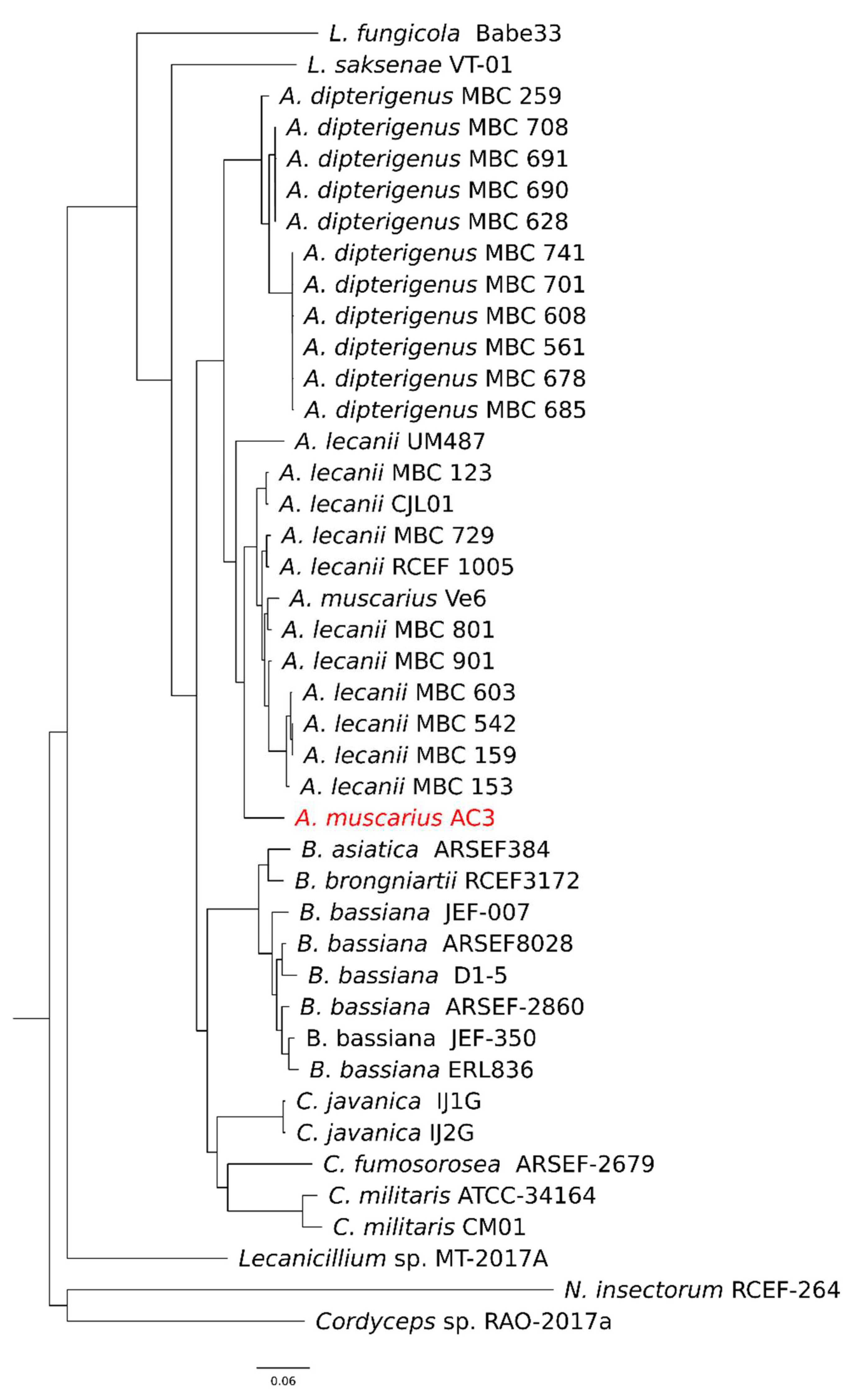
2.7. Comparative Genomics
2.8. Pathogenicity- and Virulence-Related Genes
3. Results
3.1. Genome Sequencing and De Novo Assembly
3.2. Structural and Functional Annotation
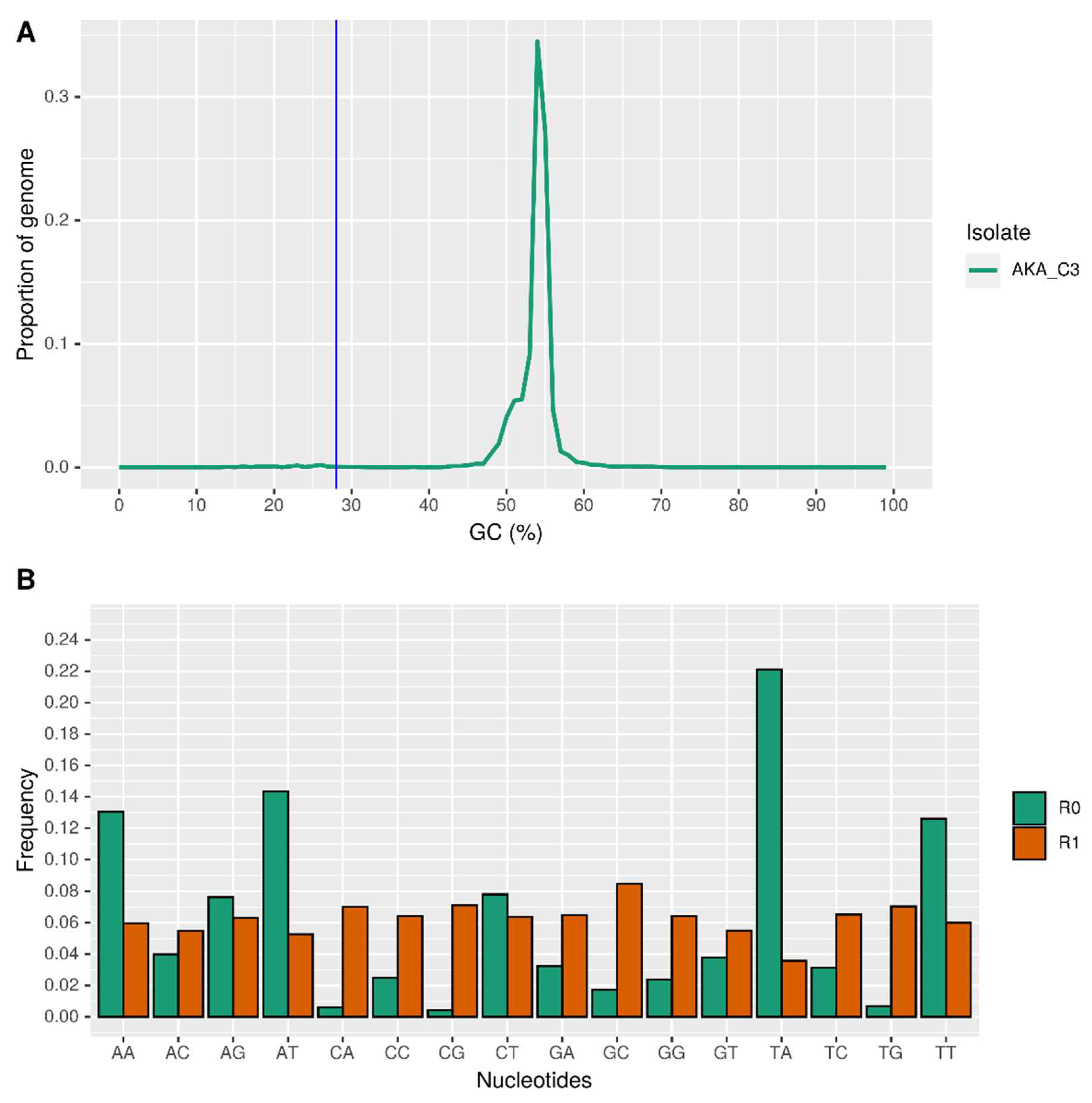
3.3. Repetitive Elements and AT-Rich Regions
3.4. Secondary Metabolites and Carbohydrate-Active Enzymes
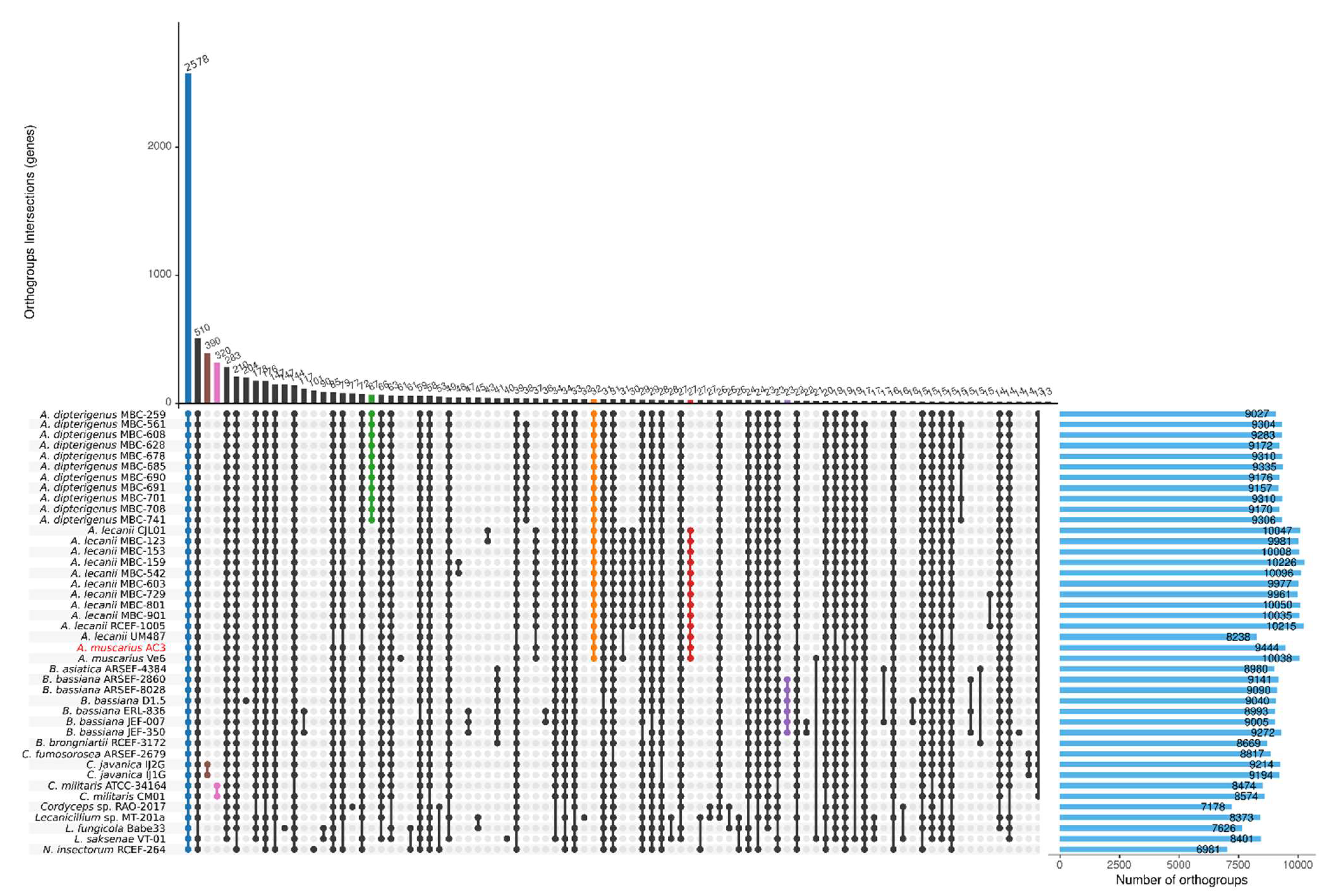
3.5. Orthogroups Inference
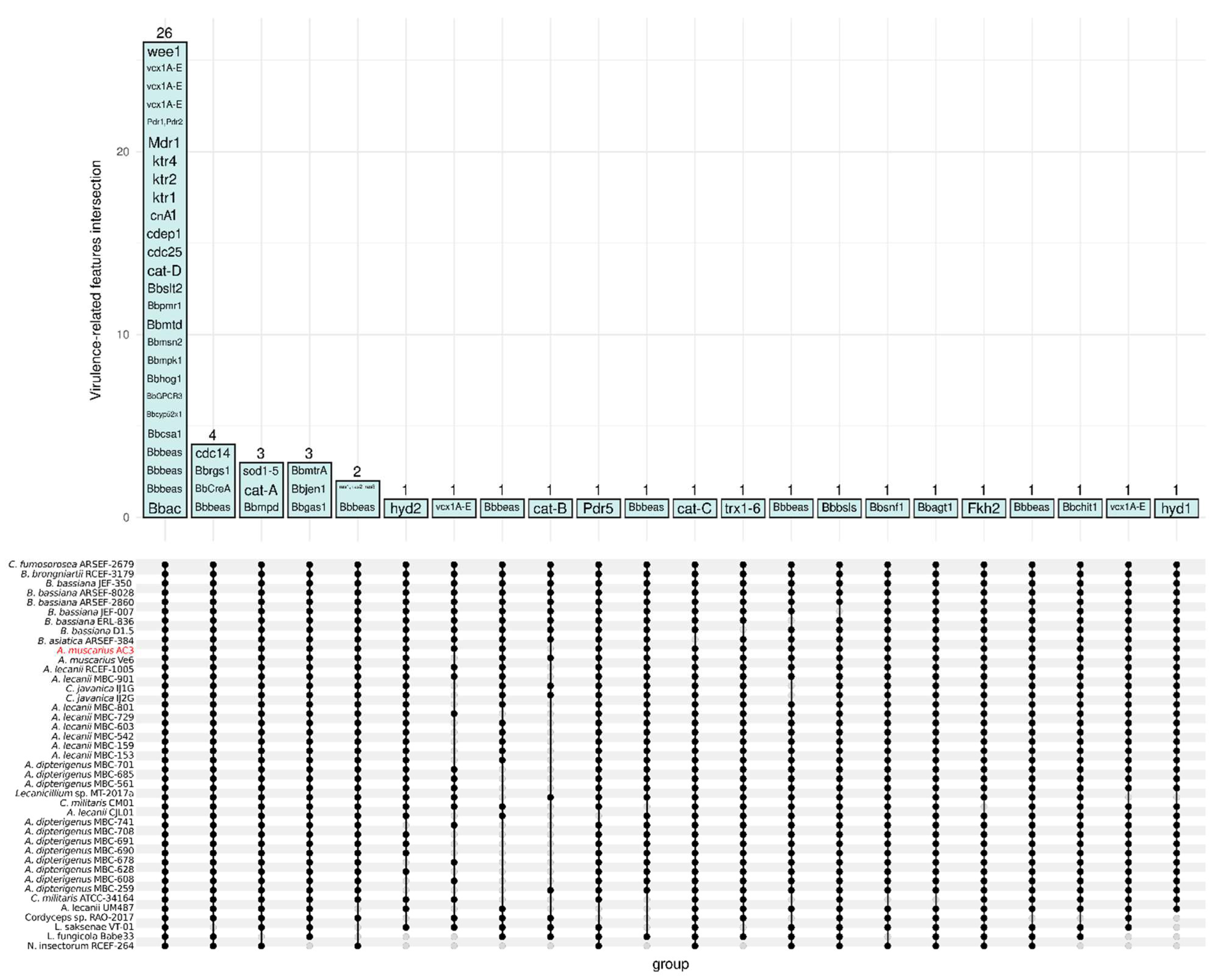
3.6. Entomopathogenic Fungus-Related Features
3.7. Evaluation of the Secretome Features with Virulence Function
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bono Rosselló, N.; Rossini, L.; Speranza, S.; Garone, E. Towards pest outbreak predictions: Are models supported by field monitoring the New Hope? Ecol. Inform. 2023, 78, 102310. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, E.; Pontini, M.; Saladini, M.A.; Alma, A. Effectiveness of Torymus sinensis: A successful long-term control of the asian chestnut gall wasp in Italy. J. Pest Sci. 2019, 92, 353–359. [Google Scholar] [CrossRef]
- Ibouh, K.; Oreste, M.; Bubici, G.; Tarasco, E.; Rossi Stacconi, M.V.; Ioriatti, C.; Verrastro, V.; Anfora, G.; Baser, N. Biological Control of Drosophila Suzukii: Efficacy of parasitoids, entomopathogenic fungi, nematodes and deterrents of oviposition in laboratory assays. Crop Prot. 2019, 125, 104897. [Google Scholar] [CrossRef]
- Di Sora, N.; Rossini, L.; Contarini, M.; Virla, E.G.; Speranza, S. Are the ladybugs Cryptolaemus montrouzieri and Exochomus quadripustulatus (Coleoptera: Coccinellidae) candidate predators of Toumeyella parvicornis (Hemiptera: Coccidae)? Pest Manag. Sci. 2024, 80, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W.; Culham, A. A Revision of Verticillium Sect. Prostrata I. Phylogenetic studies using ITS sequences. Nov. Hedwig. 2000, 71, 465–480. [Google Scholar] [CrossRef]
- Gams, W.; Zare, R. A Revision of Verticillium Sect. Prostrata. III. Genetic Classification. Nov. Hedwig. 2001, 72, 329–337. [Google Scholar] [CrossRef]
- Aiuchi, D.; Baba, Y.; Inami, K.; Shinya, R.; Tani, M.; Koike, M. Variation in growth at different temperatures and production and size of conidia in hybrid strains of Verticillium lecanii (Lecanicillium spp.) (Deuteromycotina: Hyphomycetes). Appl. Entomol. Zool. 2008, 43, 427–436. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A Phylogenetically-Based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Vinit, K.; Doilom, M.; Wanasinghe, D.N.; Bhat, D.J.; Brahmanage, R.S.; Jeewon, R.; Xiao, Y.; Hyde, K.D. Phylogenetic placement of Akanthomyces muscarius, a new endophyte record from Nypa fruticans in Thailand. Curr. Res. Environ. Appl. Mycol. 2018, 8, 404–417. [Google Scholar] [CrossRef]
- Meyer, S.L.; Huettel, R.N.; Sayre, R.M. Isolation of Fungi from Heterodera glycines and in vitro bioassays for their antagonism to Eggs. J. Nematol. 1990, 22, 532–537. [Google Scholar]
- Gan, Z.; Yang, J.; Tao, N.; Liang, L.; Mi, Q.; Li, J.; Zhang, K.-Q. Cloning of the gene Lecanicillium psalliotae chitinase lpchi1 and identification of its potential role in the biocontrol of root-knot nematode Meloidogyne incognita. Appl. Microbiol. Biotechnol. 2007, 76, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, N.; Kim, Y.-J.; Oh, K.-T.; Jung, W.-J.; Park, R.-D. The role of chitinase from Lecanicillium antillanum B-3 in parasitism to root-knot nematode Meloidogyne incognita Eggs. Biocontrol Sci. Technol. 2007, 17, 1047–1058. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A Revision of Verticillium Sect. Prostrata. IV. The Genera Lecanicillium and Simplicillium. Nov. Hedwig. 2001, 73, 1–50. [Google Scholar] [CrossRef]
- Kim, J.J.; Goettel, M.S.; Gillespie, D.R. Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca fuliginea. Biol. Control 2007, 40, 327–332. [Google Scholar] [CrossRef]
- Turco, S.; Brugneti, F.; Giubilei, I.; Silvestri, C.; Petrovic, M.; Drais, M.I.; Cristofori, V.; Speranza, S.; Mazzaglia, A.; Contarini, M.; et al. A bud’s life: Metabarcoding analysis to characterise hazelnut big buds microbiome biodiversity. Microbiol. Res. 2024, 127851. [Google Scholar] [CrossRef]
- Askary, H.; Yarmand, H. Development of the entomopathogenic Hyphomycete Lecanicillium muscarium (Hyphomycetes: Moniliales) on Various Hosts. Eur. J. Entomol. 2007, 104, 67–72. [Google Scholar] [CrossRef]
- Hywel-Jones, N.L. Multiples of eight in Cordyceps ascospores. Mycol. Res. 2002, 106, 2–3. [Google Scholar] [CrossRef]
- Broumandnia, F.; Rajabpour, A.; Hamed Ghodoum Parizipour, M.; Yarahmadi, F. Morphological and molecular identification of four isolates of the entomopathogenic fungal genus Akanthomyces and their effects against Bemisia tabaci on cucumber. Bull. Entomol. Res. 2021, 111, 628–636. [Google Scholar] [CrossRef]
- Lopes, R.B.; Souza, T.A.D.; Mascarin, G.M.; Souza, D.A.; Bettiol, W.; Souza, H.R.; Faria, M. Akanthomyces Diversity in Brazil and their pathogenicity to plant-sucking insects. J. Invertebr. Pathol. 2023, 200, 107955. [Google Scholar] [CrossRef]
- Saidi, A.; Mebdoua, S.; Mecelem, D.; Al-Hoshani, N.; Sadrati, N.; Boufahja, F.; Bendif, H. Dual biocontrol potential of the entomopathogenic fungus Akanthomyces muscarius against Thaumetopoea pityocampa and plant pathogenic fungi. Saudi J. Biol. Sci. 2023, 30, 103719. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Askary, H.; Benhamou, N.; Brodeur, J. Ultrastructural and cytochemical characterization of aphid invasion by the Hyphomycete Verticillium lecanii. J. Invertebr. Pathol. 1999, 74, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Goettel, M.S.; Koike, M.; Kim, J.J.; Aiuchi, D.; Shinya, R.; Brodeur, J. Potential of Lecanicillium spp. for management of insects, nematodes and plant diseases. J. Invertebr. Pathol. 2008, 98, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Baksi, S.; Koris, A.; Vatai, G. Journey of enzymes in entomopathogenic fungi. Pacific Sci. Rev. A Nat. Sci. Eng. 2016, 18, 85–99. [Google Scholar] [CrossRef]
- Conclusion on the peer review of the pesticide risk assessment of the active substance Lecanicillium muscarium Strain Ve6 Notified as Verticillium lecanii. EFSA J. 2010, 8, 1446. [CrossRef]
- De Faria, M.R.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Nicoletti, R.; Becchimanzi, A. Endophytism of Lecanicillium and Akanthomyces. Agriculture 2020, 10, 205. [Google Scholar] [CrossRef]
- Askary, H.; Benhamou, N.; Brodeur, J. Ultrastructural and cytochemical investigations of the antagonistic effect of Verticillium lecanii on cucumber powdery mildew. Phytopathology 1997, 87, 359–368. [Google Scholar] [CrossRef]
- Kawai, A.; Kusunoki, K.; Aiuchi, D.; Koike, M.; Tani, M. Biological control of Verticillium black spot of japanese radish using Bacillus spp. and genotypic differentiation of selected antifungal Bacillus strains with antibiotic marker. Res. Bull. Obihiro. 2006, 27, 109–119. [Google Scholar]
- Koike, M.; Yoshida, S.; Abe, N.; Asano, K. Microbial Pesticide Inhibiting the Outbreak of Plant Disease Damage. U.S. Patent Application No. 11/568,369, 13 July 2007. [Google Scholar]
- Mazzaglia, A.; Turco, S.; D’Attilia, C.; Contarini, M.; Cristofori, V.; Speranza, S.; Drais, M.I. First Report of Akanthomyces muscarius associated with hazelnut gall mite. Acta Hortic. 2023, 1379, 365–371. [Google Scholar] [CrossRef]
- Turco, S.; Mazzaglia, A.; Drais, M.I.; Bastianelli, G.; Gonthier, P.; Vannini, A.; Morales-Rodríguez, C. Hybrid de novo genome assembly and comparative genomics of three different isolates of Gnomoniopsis castaneae. Sci. Rep. 2023, 13, 3356. [Google Scholar] [CrossRef] [PubMed]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 14 November 2023).
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k -mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.V.; Salzberg, S.L. The Genome polishing tool POLCA makes fast and accurate corrections in genome assemblies. PLoS Comput. Biol. 2020, 16, e1007981. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Yandell, M. MAKER2: An annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinform. 2011, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. DbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Oliver, R.P.; Hane, J.K. OcculterCut: A comprehensive survey of at-rich regions in fungal genomes. Genome Biol. Evol. 2016, 8, 2044–2064. [Google Scholar] [CrossRef] [PubMed]
- Hane, J.K.; Oliver, R.P. RIPCAL: A Tool for alignment-based analysis of Repeat-Induced Point Mutations in fungal genomic sequences. BMC Bioinform. 2008, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Valero-Jiménez, C.A.; Wiegers, H.; Zwaan, B.J.; Koenraadt, C.J.M.; van Kan, J.A.L. Genes involved in virulence of the entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol. 2016, 133, 41–49. [Google Scholar] [CrossRef]
- Urban, M.; Cuzick, A.; Seager, J.; Wood, V.; Rutherford, K.; Venkatesh, S.Y.; De Silva, N.; Martinez, M.C.; Pedro, H.; Yates, A.D.; et al. PHI-Base: The Pathogen–Host Interactions Database. Nucleic Acids Res. 2020, 48, D613–D620. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N. EffectorP 3.0: Prediction of apoplastic and cytoplasmic effectors in fungi and oomycetes. Mol. Plant-Microbe Interact. 2022, 35, 146–156. [Google Scholar] [CrossRef]
- Rouxel, T.; Grandaubert, J.; Hane, J.K.; Hoede, C.; van de Wouw, A.P.; Couloux, A.; Dominguez, V.; Anthouard, V.; Bally, P.; Bourras, S.; et al. Effector diversification within compartments of the Leptosphaeria maculans Genome affected by Repeat-Induced Point Mutations. Nat. Commun. 2011, 2, 202. [Google Scholar] [CrossRef]
- Freitag, M.; Williams, R.L.; Kothe, G.O.; Selker, E.U. A Cytosine methyltransferase homologue is essential for Repeat-Induced Point Mutation in Neurospora crassa. Proc. Natl. Acad. Sci. USA 2002, 99, 8802–8807. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Barreiro-Hurle, J.; Rodriguez-Cerezo, E. Pesticide reduction amidst food and feed security concerns in Europe. Nat. Food 2023, 4, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, D.R.; Yadav, A. Insect pest management through integrated pest tactics. Adv. Trends Agric. Entomol. 2023, 1, 89–110. [Google Scholar]
- Erdos, Z.; Studholme, D.J.; Raymond, B.; Sharma, M.D. De novo Ggenome assembly of Akanthomyces muscarius, a biocontrol agent of insect agricultural pests. Access Microbiol. 2023, 5, 000568-v3. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of Age: Ten Years of Next-Generation Sequencing Technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- van Dijk, E.L.; Jaszczyszyn, Y.; Naquin, D.; Thermes, C. The Third Revolution in Sequencing Technology. Trends Genet. 2018, 34, 666–681. [Google Scholar] [CrossRef]
- Turco, S.; Grottoli, A.; Drais, M.I.; De Spirito, C.; Faino, L.; Reverberi, M.; Cristofori, V.; Mazzaglia, A. Draft genome sequence of a new Fusarium isolate belonging to Fusarium tricinctum species complex collected from hazelnut in central Italy. Front. Plant Sci. 2021, 12, 788584. [Google Scholar] [CrossRef]
- Turco, S.; Drais, M.I.; Rossini, L.; Chaboteaux, E.; Rahi, Y.J.; Balestra, G.M.; Iacobellis, N.S.; Mazzaglia, A. Complete genome assembly of the levan-positive strain PVFi1 of Pseudomonas savastanoi pv. savastanoi isolated from olive knots in central Italy. Environ. Microbiol. Rep. 2022, 14, 274–285. [Google Scholar] [CrossRef]
- Turco, S.; Zuppante, L.; Drais, M.I.; Mazzaglia, A. Dressing like a pathogen: Comparative analysis of different Pseudomonas genomospecies wearing different features to infect Corylus avellana. J. Phytopathol. 2022, 170, 504–516. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, Y.X.; Kim, B.; Keyhani, N.O. Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol. Microbiol. 2011, 80, 811–826. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Fang, W.; Zhang, J.; Luo, Z.; Zhang, M.; Fan, Y.; Pei, Y. Mitogen-Activated protein kinase hog1 in the entomopathogenic fungus Beauveria bassiana regulates environmental stress responses and virulence to insects. Appl. Environ. Microbiol. 2009, 75, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Orozco, R.; Wijeratne, E.M.K.; Gunatilaka, A.A.L.; Stock, S.P.; Molnár, I. Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana. Chem. Biol. 2008, 15, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, Y.; Keyhani, N.O. Contribution of the Gas1 gene of the entomopathogenic fungus Beauveria bassiana, encoding a putative glycosylphosphatidylinositol-anchored β-1,3-glucanosyltransferase, to conidial thermotolerance and virulence. Appl. Environ. Microbiol. 2011, 77, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhang, Y.; Fang, W.; Luo, Z.; Zhou, Y.; Pei, Y. Carboxylate transporter gene jen1 from the entomopathogenic fungus Beauveria bassiana is involved in conidiation and virulence. Appl. Environ. Microbiol. 2010, 76, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Ying, S.; Feng, M. P-type Calcium ATPase functions as a core regulator of Beauveria bassiana growth, conidiation and responses to multiple stressful stimuli through cross-talk with signalling networks. Environ. Microbiol. 2013, 15, 967–979. [Google Scholar] [CrossRef]
- Contarini, M.; Rossini, L.; Di Sora, N.; de Lillo, E.; Speranza, S. Monitoring the bud mite pest in a hazelnut orchard of central italy: Do plant height and irrigation influence the infestation Level? Agronomy 2022, 12, 1982. [Google Scholar] [CrossRef]



| Summary Statistics | A. muscarius AC3 |
|---|---|
| Assembly Statistics | |
| # contigs | 39 |
| Largest contig | 7,812,308 |
| Total length | 36,760,018 |
| N50 | 3,276,623 |
| L50 | 3 |
| GC (%) | 53.44 |
| Mismatches | |
| # N’s per 100 kbp | 0 |
| # N’s | 0 |
| BUSCO completeness | |
| BUSCO completeness | 99.5% |
| Complete | 4472 |
| Single-copy | 4460 |
| Duplicated | 12 |
| Fragmented | 3 |
| Missing | 19 |
| Protein coding genes | |
| Protein coding genes | 10,901 |
| Predicted tRNAs | 133 |
| Proteins with signal peptide | 1077 |
| Predicted effector proteins | 300 |
| Predicted cytoplasmic effectors | 88 |
| Predicted apoplastic effectors | 212 |
| Orthogroup | Gene_Name | Function | Accession Number |
|---|---|---|---|
| OG0004073 | Bbac | cAMP signaling | EJP69082.1 |
| OG0005551 | Bbagt1 | α-glucose transporter | AFN69126.1 |
| OG0000595, OG0000996, OG0001347, OG0001550, OG0003256, OG0004772, OG0004773, OG0006500, OG0007415 | Bbbeas | Beauvericina toxin | ACI30659.1, ACI30658.1, ACI30657.1 ACI30656.1, ACI30655.1.ACI30654.1 ACI30653.1.ACI30652.1, ACI30651.1 |
| OG0005080 | Bbbsls | Bassianolide synthetase | ACR78148.1, ACR78134.1 |
| OG0006326 | Bbchit1 | chitinase | AAN41259.1 |
| OG0002032 | BbCreA | CreA Aspergillus | AXG50936.1 |
| OG0002053 | Bbcsa1 | Neuronal calcium sensor | AFG25510.1 |
| OG0000010 | Bbcyp52x1 | Predicted_cytochrome_P450 | ADK36660.1 |
| OG0001161 | Bbgas1 | Cell-wall anchored enzyme | ADP22300.1 |
| OG0000852 | BbGPCR3 | G-protein coupled receptor | XP_008594187.1 |
| OG0001208 | Bbhog1 | MAP kinase | AAS77871.1 |
| OG0002187 | Bbjen1 | Carboxylate transporter | AAO33825.1 |
| OG0003168 | Bbmpd | Mannitol-1-phosphate-dehydrogenase | ACU32784.1 |
| OG0003410 | Bbmpk1 | MAP kinase | AAQ24633.1 |
| OG0001461 | Bbmsn2 | Transcription factor | EJP70102.1 |
| OG0001442 | Bbmtd | Mannitol dehydrogenase | ADV16370.1 |
| OG0000103 | BbmtrA | Putative methyltransferase | EJP69472.1 |
| OG0000771 | Bbpmr1 | ATPase, signaling | AAY40175 |
| OG0005594 | Bbrgs1 | G-protein signaling | ABL61517.1 |
| OG0000669 | Bbslt2 | MAP kinase | AEU60018.1 |
| OG0005917 | Bbsnf1 | Protein kinase | XP_008597580.1 |
| OG0003206, OG0009475, OG0000753, OG0000172 | cat-A, cat-B, cat-C, cat-D | Catalase A, B C and catalase D | JX050138.1, JX050139.1.JX050141.1, JX050142, JX050140 |
| OG0005553 | cdc14 | Dual specificity phosphatase | EJP63157.1 |
| OG0004028 | cdc25 | Regulates Cdk1 activity | EJP68939 |
| OG0000004 | cdep1 | Cuticle-degrading_proteinase_CDEP-1 | AAK70804.1 |
| OG0000261 | CnA1, CnA2, CnB, Crz1 | Calcineuring-mediated phosphatase | XP_008596022.1 |
| OG0000461 | Fkh2 | Transcription factor | EJP62625.1 |
| OG0007102, OG0006455 | Hyd1, hyd2 | Hydrophobin, hydrophobin | ABO38181 |
| ABP58683.1 | |||
| OG0002106, OG0002128, OG0002169 | ktr1, ktr2, ktr4 | a-1,2-mannosyltransferase | EJP63998.1, EJP62226.1, EJP69973.1 |
| OG0001805 | Mdr1 | ABC transporters | XP_008595100, XP_008602098.1, XP_008600979.1, XP_008594344.1, |
| OG0000167 | Pdr1, Pdr2 | ABC transporters | XP_008595100, XP_008602098.1, XP_008600979.1, XP_008594344.1, |
| OG0001832 | Pdr5 | ABC transporters | XP_008595100, XP_008602098.1, XP_008600979.1, XP_008594344.1, |
| OG0006835 | ras1, ras2, ras3 | GTPase, signal transduction | EJP70406.1 |
| OG0005188 | sod1-5 | Superoxide dismutases | EJP67024.1 |
| OG0006040 | trx1-6 | Thioredoxin antioxidants | ACT21200.1 |
| OG0002437, OG0003500, OG0004231, OG0004459, OG0008389 | vcx1A-E | Calcium/proton exchanger | EJP69434.1, EJP63266.1, EJP67299.1, EJP69455.1, EJP69457.1 |
| OG0001590 | wee1 | Regulates Cdk1 activity | EJP62459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turco, S.; Drais, M.I.; Rossini, L.; Di Sora, N.; Brugneti, F.; Speranza, S.; Contarini, M.; Mazzaglia, A. Genomic and Pathogenic Characterization of Akanthomyces muscarius Isolated from Living Mite Infesting Hazelnut Big Buds. Genes 2024, 15, 993. https://doi.org/10.3390/genes15080993
Turco S, Drais MI, Rossini L, Di Sora N, Brugneti F, Speranza S, Contarini M, Mazzaglia A. Genomic and Pathogenic Characterization of Akanthomyces muscarius Isolated from Living Mite Infesting Hazelnut Big Buds. Genes. 2024; 15(8):993. https://doi.org/10.3390/genes15080993
Chicago/Turabian StyleTurco, Silvia, Mounira Inas Drais, Luca Rossini, Nicolò Di Sora, Federico Brugneti, Stefano Speranza, Mario Contarini, and Angelo Mazzaglia. 2024. "Genomic and Pathogenic Characterization of Akanthomyces muscarius Isolated from Living Mite Infesting Hazelnut Big Buds" Genes 15, no. 8: 993. https://doi.org/10.3390/genes15080993









