A Multivariable Mendelian Randomization Study of Systolic and Diastolic Blood Pressure, Lipid Profile, and Heart Failure Subtypes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.; Gaggin, H.K.; Dec, G.W. ACC/AHA Versus ESC Guidelines on Heart Failure: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 2756–2768. [Google Scholar] [CrossRef]
- Maeder, M.T.; Buser, M.; Brenner, R.; Rickli, H. Heart failure with preserved ejection fraction (HFpEF). Ther. Umsch. 2018, 75, 161–169. [Google Scholar] [CrossRef]
- Lindgren, M.P.; PirouziFard, M.; Smith, J.G.; Sundquist, J.; Sundquist, K.; Zoller, B. A Swedish Nationwide Adoption Study of the Heritability of Heart Failure. JAMA Cardiol. 2018, 3, 703–710. [Google Scholar] [CrossRef]
- Joseph, J.; Liu, C.; Hui, Q.; Aragam, K.; Wang, Z.; Charest, B.; Huffman, J.E.; Keaton, J.M.; Edwards, T.L.; Demissie, S.; et al. Genetic architecture of heart failure with preserved versus reduced ejection fraction. Nat. Commun. 2022, 13, 7753. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Ning, H.; Carnethon, M.R.; Allen, N.B.; Wilkins, J.T.; Lloyd-Jones, D.M.; Khan, S.S. Race-and Sex-Specific Population Attributable Fractions of Incident Heart Failure: A Population-Based Cohort Study from the Lifetime Risk Pooling Project. Circ. Heart Fail. 2021, 14, e008113. [Google Scholar] [CrossRef]
- Velagaleti, R.S.; Massaro, J.; Vasan, R.S.; Robins, S.J.; Kannel, W.B.; Levy, D. Relations of lipid concentrations to heart failure incidence: The Framingham Heart Study. Circulation 2009, 120, 2345–2351. [Google Scholar] [CrossRef]
- Gaziano, L.; Cho, K.; Djousse, L.; Schubert, P.; Galloway, A.; Ho, Y.L.; Kurgansky, K.; Gagnon, D.R.; Russo, J.P.; Di Angelantonio, E.; et al. Risk factors and prediction models for incident heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021, 8, 4893–4903. [Google Scholar] [CrossRef]
- Molsberry, R.J.; Rethy, L.; Wang, M.C.; Mehta, R.C.; Lloyd-Jones, D.M.; Ning, H.; Lewis, C.E.; Yancy, C.W.; Shah, S.J.; Khan, S.S. Risk-Based Intensive Blood Pressure Lowering and Prevention of Heart Failure: A SPRINT Post Hoc Analysis. Hypertension 2021, 78, 1742–1749. [Google Scholar] [CrossRef]
- Preiss, D.; Campbell, R.T.; Murray, H.M.; Ford, I.; Packard, C.J.; Sattar, N.; Rahimi, K.; Colhoun, H.M.; Waters, D.D.; LaRosa, J.C.; et al. The effect of statin therapy on heart failure events: A collaborative meta-analysis of unpublished data from major randomized trials. Eur. Heart J. 2015, 36, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Rodriguez-Artalejo, F.; De Backer, G.; Dallongeville, J.; Medina, J.; Guallar, E.; Perk, J.; Banegas, J.R.; Tubach, F.; Roy, C.; et al. The association between blood pressure and lipid levels in Europe: European Study on Cardiovascular Risk Prevention and Management in Usual Daily Practice. J. Hypertens. 2016, 34, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Concato, J.; Brophy, M.; Fiore, L.; Pyarajan, S.; Breeling, J.; Whitbourne, S.; Deen, J.; Shannon, C.; Humphries, D.; et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 2016, 70, 214–223. [Google Scholar] [CrossRef]
- Pulley, J.; Clayton, E.; Bernard, G.R.; Roden, D.M.; Masys, D.R. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin. Transl. Sci. 2010, 3, 42–48. [Google Scholar] [CrossRef]
- Magi, R.; Morris, A.P. GWAMA: Software for genome-wide association meta-analysis. BMC Bioinform. 2010, 11, 288. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, D.; Peloso, G.M.; Pereira, A.C.; Dashti, H.; Giambartolomei, C.; Wheeler, E.; Aung, N.; Ferolito, B.R.; Pietzner, M.; Farber-Eger, E.H.; et al. Genome-wide association analysis and Mendelian randomization proteomics identify drug targets for heart failure. Nat. Commun. 2023, 14, 3826. [Google Scholar] [CrossRef]
- Shah, S.; Henry, A.; Roselli, C.; Lin, H.; Sveinbjornsson, G.; Fatemifar, G.; Hedman, A.K.; Wilk, J.B.; Morley, M.P.; Chaffin, M.D.; et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat. Commun. 2020, 11, 163. [Google Scholar] [CrossRef]
- Henry, A.; Gordillo-Maranon, M.; Finan, C.; Schmidt, A.F.; Ferreira, J.P.; Karra, R.; Sundstrom, J.; Lind, L.; Arnlov, J.; Zannad, F.; et al. Therapeutic Targets for Heart Failure Identified Using Proteomics and Mendelian Randomization. Circulation 2022, 145, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- van Oort, S.; Beulens, J.W.J.; van Ballegooijen, A.J.; Handoko, M.L.; Larsson, S.C. Modifiable lifestyle factors and heart failure: A Mendelian randomization study. Am. Heart J. 2020, 227, 64–73. [Google Scholar] [CrossRef]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef]
- Ofili, E.O.; Cohen, J.D.; St Vrain, J.A.; Pearson, A.; Martin, T.J.; Uy, N.D.; Castello, R.; Labovitz, A.J. Effect of treatment of isolated systolic hypertension on left ventricular mass. JAMA 1998, 279, 778–780. [Google Scholar] [CrossRef]
- Naing, P.; Forrester, D.; Kangaharan, N.; Muthumala, A.; Mon Myint, S.; Playford, D. Heart failure with preserved ejection fraction: A growing global epidemic. Aust. J. Gen. Pract. 2019, 48, 465–471. [Google Scholar] [CrossRef]
- Rzeznik, D.; Przewlocki, T.; Kablak-Ziembicka, A.; Kozanecki, A.; Roslawiecka, A.; Lach, J.; Tracz, W.; Podolec, P. Effect of renal artery revascularization on left ventricular hypertrophy, diastolic function, blood pressure, and the one-year outcome. J. Vasc. Surg. 2011, 53, 692–697. [Google Scholar] [CrossRef]
- Zeller, T.; Rastan, A.; Schwarzwalder, U.; Muller, C.; Frank, U.; Burgelin, K.; Sixt, S.; Schwarz, T.; Noory, E.; Neumann, F.J. Regression of left ventricular hypertrophy following stenting of renal artery stenosis. J. Endovasc. Ther. 2007, 14, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, Y.; Nishikimi, T.; Abe, H.; Yoshiwara, F.; Suzuki, T.; Ashizawa, A.; Nagata, S.; Kuramochi, M.; Matsuoka, H.; Omae, T. Comparison of changes in cardiac structure after treatment in secondary hypertension. Hypertension 1996, 27, 319–323. [Google Scholar] [CrossRef]
- Rowlands, D.B.; Glover, D.R.; Ireland, M.A.; McLeay, R.A.; Stallard, T.J.; Watson, R.D.; Littler, W.A. Assessment of left-ventricular mass and its response to antihypertensive treatment. Lancet 1982, 1, 467–470. [Google Scholar] [CrossRef]
- Devereux, R.B.; Wachtell, K.; Gerdts, E.; Boman, K.; Nieminen, M.S.; Papademetriou, V.; Rokkedal, J.; Harris, K.; Aurup, P.; Dahlof, B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004, 292, 2350–2356. [Google Scholar] [CrossRef]
- Soliman, E.Z.; Ambrosius, W.T.; Cushman, W.C.; Zhang, Z.M.; Bates, J.T.; Neyra, J.A.; Carson, T.Y.; Tamariz, L.; Ghazi, L.; Cho, M.E.; et al. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients with Hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation 2017, 136, 440–450. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart: Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Tran-Dinh, A.; Diallo, D.; Delbosc, S.; Varela-Perez, L.M.; Dang, Q.B.; Lapergue, B.; Burillo, E.; Michel, J.B.; Levoye, A.; Martin-Ventura, J.L.; et al. HDL and endothelial protection. Br. J. Pharmacol. 2013, 169, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Damman, K.; Verhaar, M.C.; Paulus, W.J.; Duncker, D.J.; Cheng, C.; van Heerebeek, L.; Hillege, H.L.; Lam, C.S.; Navis, G.; et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur. J. Heart Fail. 2016, 18, 588–598. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Tan, Y.D.; Xiao, P.; Guda, C. In-depth Mendelian randomization analysis of causal factors for coronary artery disease. Sci. Rep. 2020, 10, 9208. [Google Scholar] [CrossRef] [PubMed]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
| Exposure | Model | HFrEF | HFpEF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N GIVs | OR (95% CI) | p | Pleiotropy Test p | N GIVs | OR (95% CI) | p | Pleiotropy Test p | ||
| SBP (per 10 mmHg) | MR Egger | 75 | 0.68 (0.41, 1.13) | 0.14 | 0.03 | 74 | 1.17 (0.81, 1.69) | 0.41 | 1.00 |
| IVW | 1.17 (1.02, 1.34) | 0.03 | 1.17 (1.07, 1.28) | 9.24 × 10−4 | |||||
| MR Egger (MR-PRESSO) | 71 | 0.81 (0.52, 1.26) | 0.35 | 0.08 | 73 | 1.25 (0.88, 1.76) | 0.22 | 0.79 | |
| IVW (MR-PRESSO) | 1.19 (1.06, 1.34) | 3.31 × 10−3 | 1.19 (1.09, 1.3) | 1.12 × 10−4 | |||||
| DBP (per 10 mmHg) | MR Egger | 83 | 1.85 (0.99, 3.47) | 0.06 | 0.52 | 84 | 0.97 (0.55, 1.69) | 0.90 | 0.73 |
| IVW | 1.51 (1.29, 1.77) | 2.32 × 10−7 | 1.06 (0.92, 1.22) | 0.40 | |||||
| MR Egger (MR-PRESSO) | 80 | 1.88 (1.07, 3.32) | 0.03 | 0.39 | 84 | 0.97 (0.55, 1.69) | 0.90 | 0.73 | |
| IVW (MR-PRESSO) | 1.47 (1.28, 1.7) | 7.95 × 10−8 | 1.06 (0.92, 1.22) | 0.40 | |||||
| PP (per 10 mmHg) | MR Egger | 145 | 0.98 (0.72, 1.34) | 0.91 | 0.30 | 146 | 1.29 (1.01, 1.65) | 0.04 | 0.41 |
| IVW | 1.15 (1.03, 1.27) | 8.96 × 10−3 | 1.17 (1.08, 1.27) | 1.27 × 10−4 | |||||
| MR Egger (MR-PRESSO) | 141 | 1.02 (0.78, 1.34) | 0.86 | 0.23 | 142 | 1.37 (1.1, 1.7) | 5.95 × 10−3 | 0.15 | |
| IVW (MR-PRESSO) | 1.2 (1.09, 1.31) | 9.85 × 10−5 | 1.17 (1.09, 1.26) | 1.81 × 10−5 | |||||
| HDL-C (per 10 mg/dL) | MR Egger | 149 | 1.04 (0.89, 1.22) | 0.59 | 0.25 | 149 | 1.1 (0.96, 1.25) | 0.18 | 0.06 |
| IVW | 0.96 (0.89, 1.04) | 0.33 | 0.98 (0.92, 1.05) | 0.56 | |||||
| MR Egger (MR-PRESSO) | 144 | 1.07 (0.94, 1.22) | 0.33 | 0.10 | 148 | 1.1 (0.96, 1.25) | 0.16 | 0.07 | |
| IVW (MR-PRESSO) | 0.97 (0.91, 1.03) | 0.34 | 0.99 (0.93, 1.05) | 0.71 | |||||
| LDL-C (per 10 mg/dL) | MR Egger | 142 | 1.1 (1.05, 1.14) | 4.08 × 10−5 | 0.09 | 142 | 1.05 (1.01, 1.09) | 0.01 | 0.38 |
| IVW | 1.06 (1.04, 1.09) | 1.96 × 10−6 | 1.04 (1.01, 1.06) | 2.49 × 10−3 | |||||
| MR Egger (MR-PRESSO) | 138 | 1.07 (1.03, 1.11) | 9.59 × 10−4 | 0.25 | 141 | 1.05 (1.01, 1.09) | 0.01 | 0.48 | |
| IVW (MR-PRESSO) | 1.05 (1.03, 1.07) | 4.13 × 10−5 | 1.04 (1.01, 1.06) | 1.28 × 10−3 | |||||
| Triglycerides (per 10%) | MR Egger | 151 | 0.99 (0.97, 1.02) | 0.55 | 0.02 | 151 | 0.99 (0.97, 1.01) | 0.52 | 0.03 |
| IVW | 1.02 (1, 1.03) | 0.04 | 1.01 (1, 1.03) | 0.09 | |||||
| MR Egger (MR-PRESSO) | 147 | 1 (0.97, 1.02) | 0.75 | 0.02 | 148 | 1 (0.98, 1.02) | 0.75 | 0.06 | |
| IVW (MR-PRESSO) | 1.02 (1, 1.03) | 0.01 | 1.01 (1, 1.03) | 0.06 | |||||
| Exposure | HFrEF | HFpEF | ||
|---|---|---|---|---|
| N GIVs | OR (95% CI) | N GIVs | OR (95% CI) | |
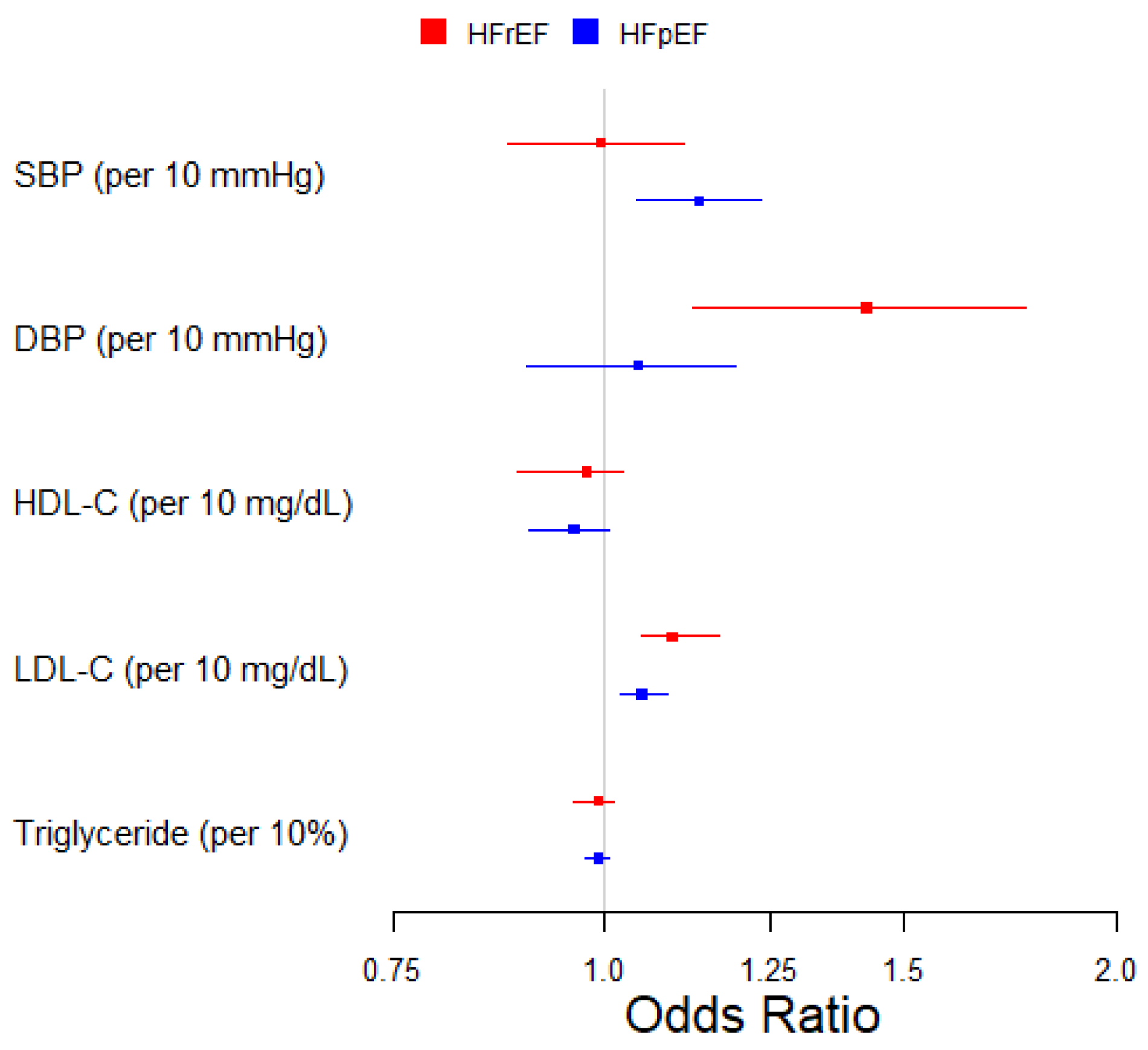
| SBP (per 10 mmHg) | 112 | 0.99 (0.88, 1.11) | 111 | 1.14 (1.04, 1.23) |
| DBP (per 10 mmHg) | 103 | 1.43 (1.13, 1.77) | 104 | 1.05 (0.90, 1.19) |
| HDL-C (per 10 mg/dL) | 175 | 0.98 (0.89, 1.02) | 172 | 0.96 (0.90, 1.01) |
| LDL-C (per 10 mg/dL) | 159 | 1.10 (1.05, 1.17) | 159 | 1.05 (1.02, 1.09) |
| Triglycerides (per 10%) | 175 | 0.99 (0.96, 1.01) | 175 | 0.99 (0.97, 1.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Hui, Q.; Wells, Q.S.; Farber-Eger, E.; Gaziano, J.M.; Wilson, P.W.F.; Quyyumi, A.A.; Vaccarino, V.; Hu, Y.-J.; Benkeser, D.; et al. A Multivariable Mendelian Randomization Study of Systolic and Diastolic Blood Pressure, Lipid Profile, and Heart Failure Subtypes. Genes 2024, 15, 1126. https://doi.org/10.3390/genes15091126
Liu C, Hui Q, Wells QS, Farber-Eger E, Gaziano JM, Wilson PWF, Quyyumi AA, Vaccarino V, Hu Y-J, Benkeser D, et al. A Multivariable Mendelian Randomization Study of Systolic and Diastolic Blood Pressure, Lipid Profile, and Heart Failure Subtypes. Genes. 2024; 15(9):1126. https://doi.org/10.3390/genes15091126
Chicago/Turabian StyleLiu, Chang, Qin Hui, Quinn S. Wells, Eric Farber-Eger, John Michael Gaziano, Peter W. F. Wilson, Arshed A. Quyyumi, Viola Vaccarino, Yi-Juan Hu, David Benkeser, and et al. 2024. "A Multivariable Mendelian Randomization Study of Systolic and Diastolic Blood Pressure, Lipid Profile, and Heart Failure Subtypes" Genes 15, no. 9: 1126. https://doi.org/10.3390/genes15091126





