Applications and Performance of Precision ID GlobalFiler NGS STR, Identity, and Ancestry Panels in Forensic Genetics
Abstract
:1. Introduction
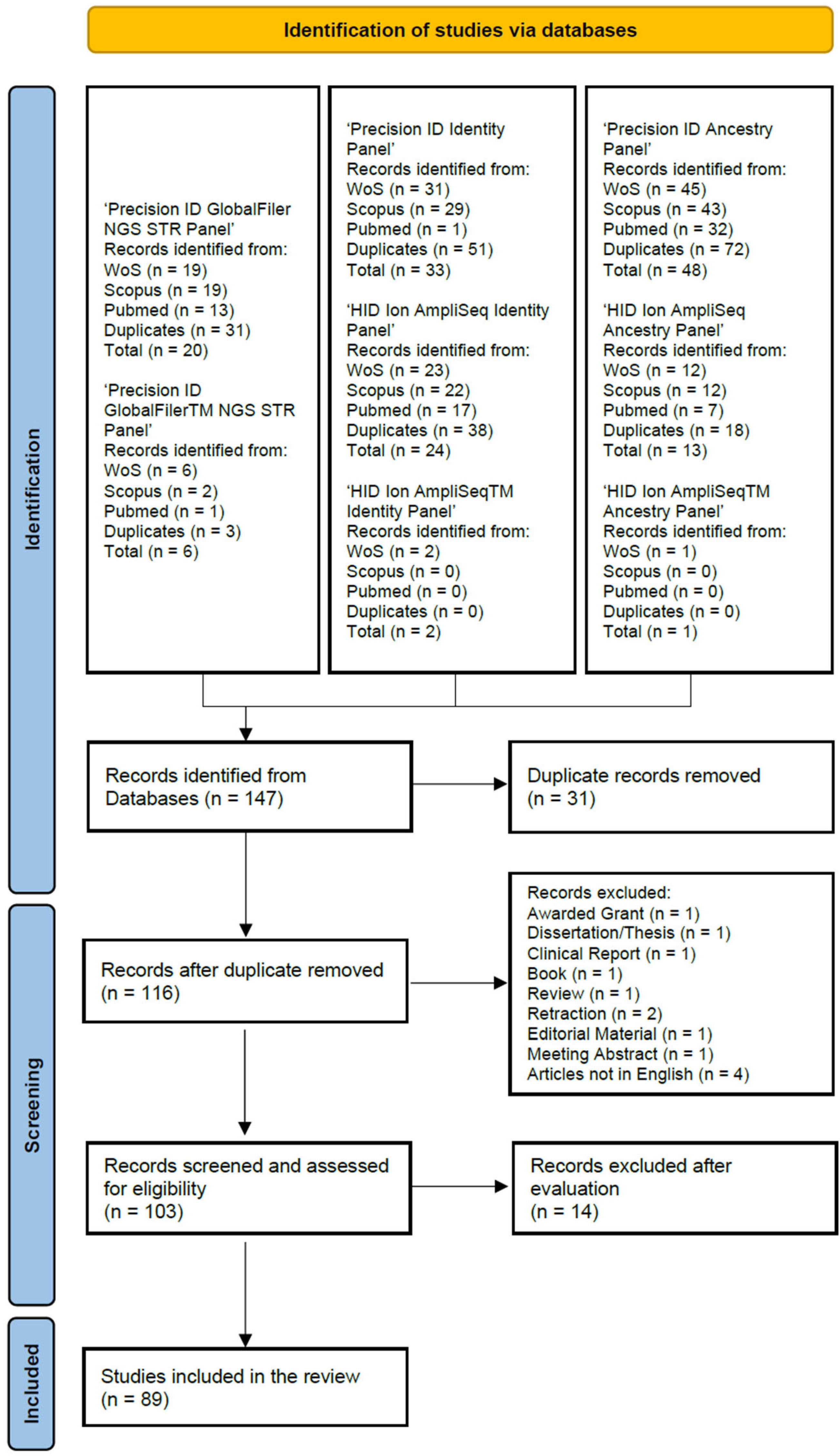
2. Materials and Methods
3. Results
4. Discussion
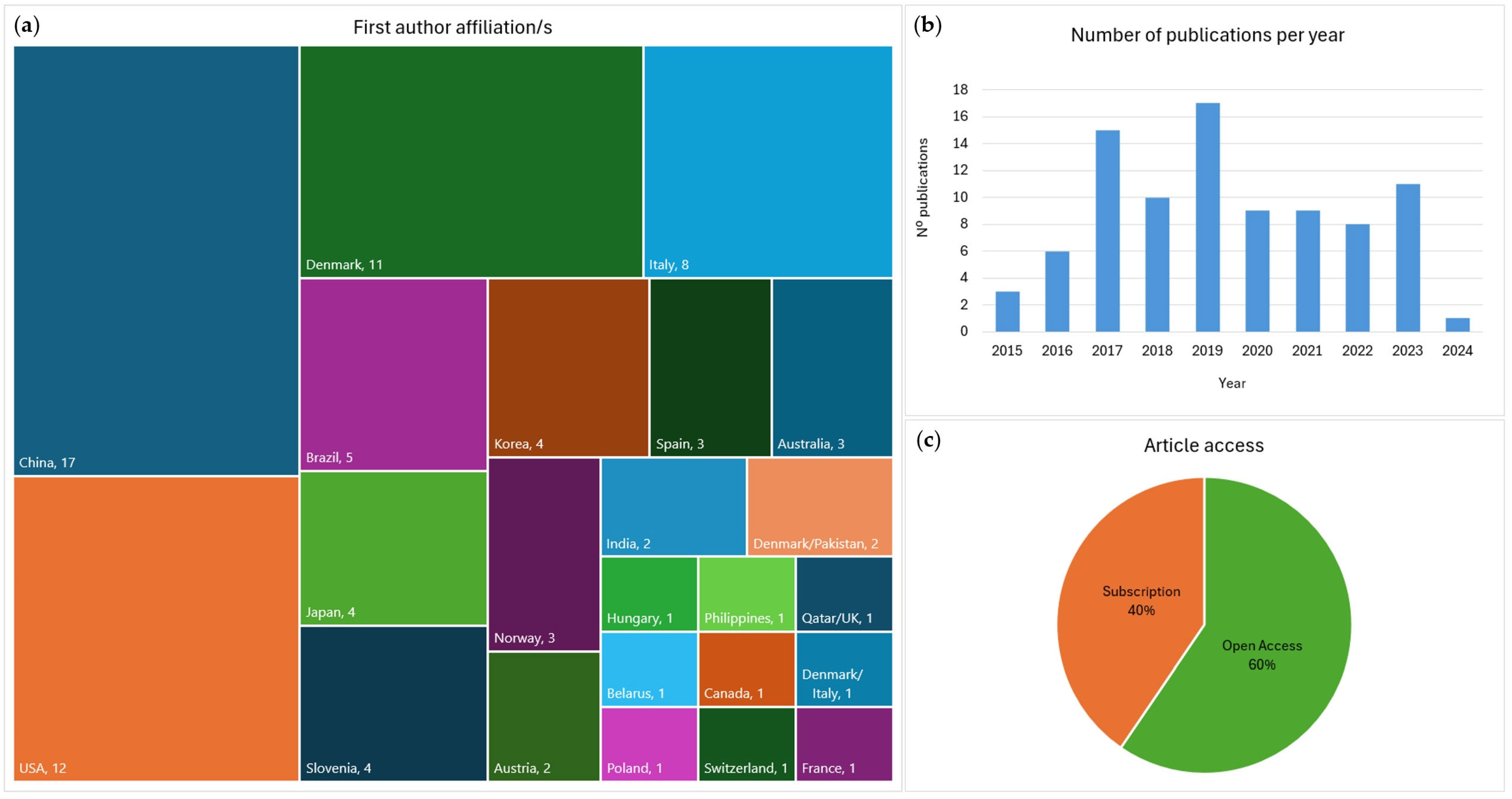
4.1. Contributions by Country
4.2. Contributions by Year
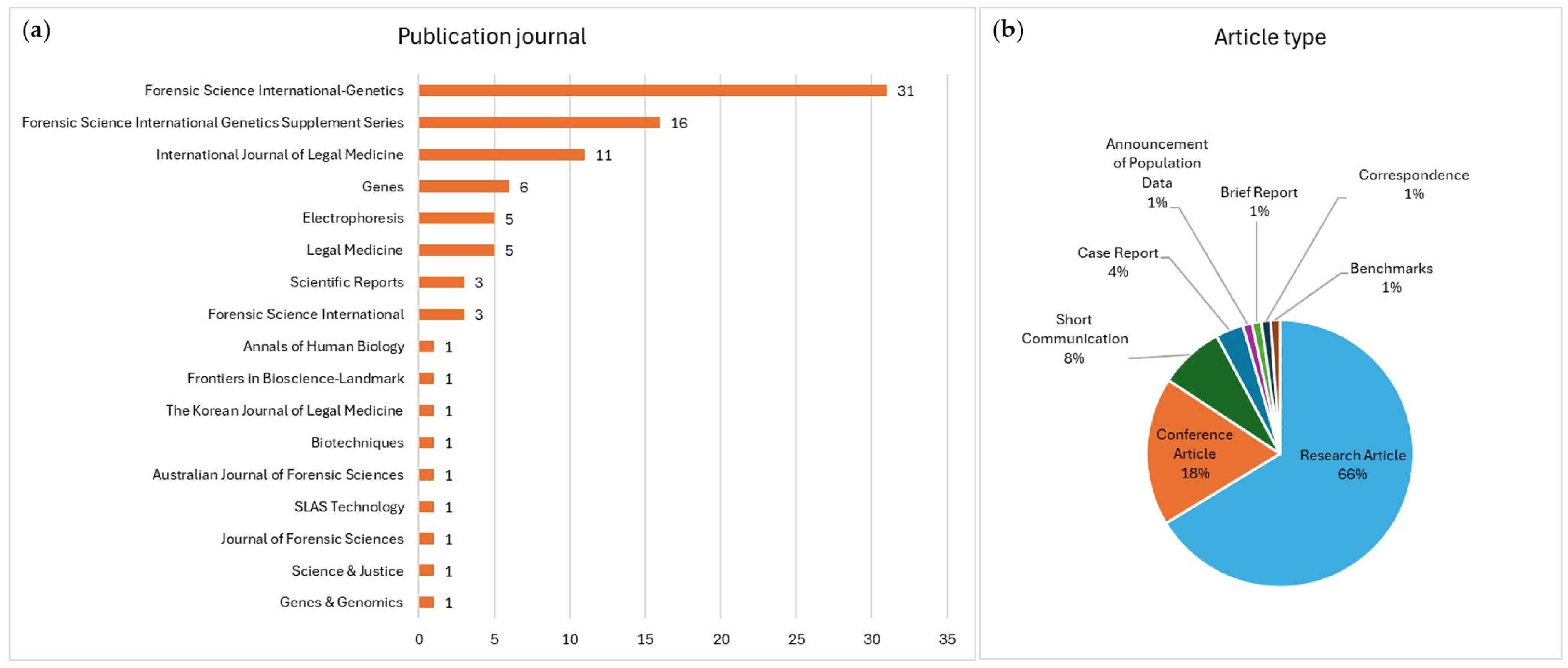
4.3. Articles Type, Journal, and Access
4.4. Articles Focus and Current Knowledge
4.4.1. Precision ID GlobalFiler NGS STR Panel
4.4.2. Precision ID Identity Panel
4.4.3. Precision ID Ancestry Panel
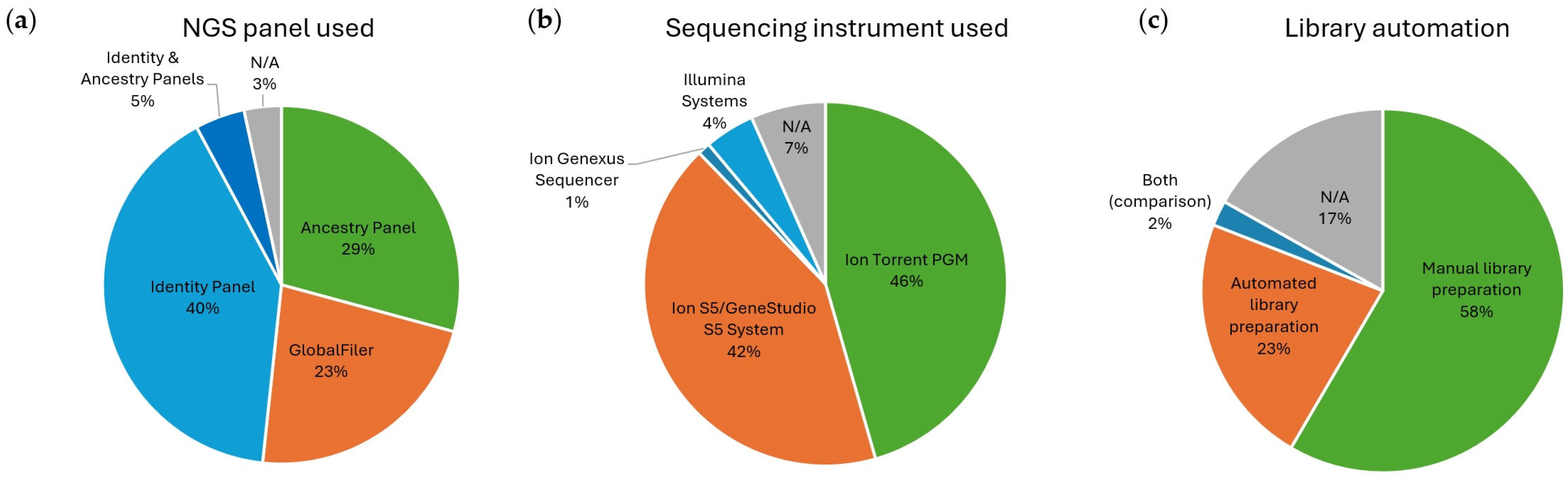
4.5. Sequencing Instrument and Automation
4.6. Limitations
4.7. Future Perspectives
4.8. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Knijff, P. From next generation sequencing to now generation sequencing in forensics. Forensic Sci. Int. Genet. 2019, 38, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Nwawuba Stanley, U.; Mohammed Khadija, A.; Bukola, A.T.; Omusi Precious, I.; Ayevbuomwan Davidson, E. Forensic DNA Profiling: Autosomal Short Tandem Repeat as a Prominent Marker in Crime Investigation. Malays. J. Med. Sci. 2020, 27, 22–35. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, S.H.; Abd El-Hafez, A.F. Paternity testing and forensic DNA typing by multiplex STR analysis using ABI PRISM 310 Genetic Analyzer. J. Genet. Eng. Biotechnol. 2012, 10, 101–112. [Google Scholar] [CrossRef]
- Phillips, C.; Fernandez-Formoso, L.; Gelabert-Besada, M.; Garcia-Magariños, M.; Santos, C.; Fondevila, M.; Carracedo, Á.; Lareu, M.V. Development of a novel forensic STR multiplex for ancestry analysis and extended identity testing. Electrophoresis 2013, 34, 1151–1162. [Google Scholar] [CrossRef]
- Carboni, I.; Iozzi, S.; Nutini, A.L.; Torricelli, F.; Ricci, U. Improving complex kinship analyses with additional STR loci. Electrophoresis 2014, 35, 3145–3151. [Google Scholar] [CrossRef]
- Alonso, A.; Barrio, P.A.; Müller, P.; Köcher, S.; Berger, B.; Martin, P.; Bodner, M.; Willuweit, S.; Parson, W.; Roewer, L.; et al. Current state-of-art of STR sequencing in forensic genetics. Electrophoresis 2018, 39, 2655–2668. [Google Scholar] [CrossRef]
- Daniel, R.; Walsh, S.J. The Continuing Evolution of Forensic DNA Profiling-From STRS to SNPS. Aust. J. Forensic Sci. 2006, 38, 59–74. [Google Scholar] [CrossRef]
- Butler, J.M.; Coble, M.D.; Vallone, P.M. STRs vs. SNPs: Thoughts on the future of forensic DNA testing. Forensic Sci. Med. Pat. 2007, 3, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Budowle, B.; van Daal, A. Forensically relevant SNP classes. BioTechniques 2008, 44, 603–610. [Google Scholar] [CrossRef]
- Oldoni, F.; Kidd, K.K.; Podini, D. Microhaplotypes in forensic genetics. Forensic Sci. Int. Genet. 2019, 38, 54–69. [Google Scholar] [CrossRef]
- Alonso, A.; Müller, P.; Roewer, L.; Willuweit, S.; Budowle, B.; Parson, W. European survey on forensic applications of massively parallel sequencing. Forensic Sci. Int. Genet. 2017, 29, e23–e25. [Google Scholar] [CrossRef]
- Gross, T.E.; Fleckhaus, J.; Schneider, P.M. Progress in the implementation of massively parallel sequencing for forensic genetics: Results of a European-wide survey among professional users. Int. J. Legal Med. 2021, 135, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Carratto, T.M.T.; Moraes, V.M.S.; Recalde, T.S.F.; Oliveira, M.L.G.d.; Teixeira Mendes-Junior, C. Applications of massively parallel sequencing in forensic genetics. Genet. Mol. Biol. 2022, 45, e20220077. [Google Scholar] [CrossRef]
- MiSeq FGx Sequencing System. Available online: https://verogen.com/wp-content/uploads/2021/02/miseq-fgx-system-datasheet-vd2020057-a.pdf (accessed on 15 January 2024).
- ForenSeq™ DNA Signature Prep Kit. Available online: https://verogen.com/wp-content/uploads/2018/08/ForenSeq-prep-kit-data-sheet-VD2018002.pdf (accessed on 15 August 2024).
- Sharma, V.; van der Plaat, D.A.; Liu, Y.; Wurmbach, E. Analyzing degraded DNA and challenging samples using the ForenSeq™ DNA Signature Prep kit. Sci. Justice 2020, 60, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, Q.; Ma, G.; Chen, Z.; Liu, Q.; Fu, L.; Cong, B.; Li, S. Utility of ForenSeq™ DNA Signature Prep Kit in the research of pairwise 2nd-degree kinship identification. Int. J. Legal Med. 2019, 133, 1641–1650. [Google Scholar] [CrossRef]
- Hollard, C.; Ausset, L.; Chantrel, Y.; Jullien, S.; Clot, M.; Faivre, M.; Suzanne, É.; Pène, L.; Laurent, F. Automation and developmental validation of the ForenSeq™ DNA Signature Preparation kit for high-throughput analysis in forensic laboratories. Forensic Sci. Int. Genet. 2019, 40, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Pilli, E.; Tarallo, R.; La Riccia, P.; Berti, A.; Novelletto, A. Kinship assignment with the ForenSeq™ DNA Signature Prep Kit: Sources of error in simulated and real cases. Sci. Justice 2022, 62, 1–9. [Google Scholar] [CrossRef]
- Fattorini, P.; Previderé, C.; Carboni, I.; Marrubini, G.; Sorçaburu-Cigliero, S.; Grignani, P.; Bertoglio, B.; Vatta, P.; Ricci, U. Performance of the ForenSeqTM DNA Signature Prep kit on highly degraded samples. Electrophoresis 2017, 38, 1163–1174. [Google Scholar] [CrossRef]
- Gettings, K.B.; Borsuk, L.A.; Ballard, D.; Bodner, M.; Budowle, B.; Devesse, L.; King, J.; Parson, W.; Phillips, C.; Vallone, P.M. STRSeq: A catalog of sequence diversity at human identification Short Tandem Repeat loci. Forensic Sci. Int. Genet. 2017, 31, 111–117. [Google Scholar] [CrossRef]
- Precision ID GlobalFiler NGS STR Panel. Available online: https://assets.thermofisher.com/TFS-Assets/GSD/Flyers/precisionid-globalfiler-ngs-str-flyer.pdf (accessed on 16 January 2024).
- Ludeman, M.J.; Zhong, C.; Mulero, J.J.; Lagacé, R.E.; Hennessy, L.K.; Short, M.L.; Wang, D.Y. Developmental validation of GlobalFiler™ PCR amplification kit: A 6-dye multiplex assay designed for amplification of casework samples. Int. J. Legal Med. 2018, 132, 1555–1573. [Google Scholar] [CrossRef]
- Borsuk, L.A.; Gettings, K.B.; Steffen, C.R.; Kiesler, K.M.; Vallone, P.M. Sequencing of the highly polymorphic STR locus SE33. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e322–e323. [Google Scholar] [CrossRef]
- Converge Software. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FGSD%2FProduct-Bulletins%2Fconverge-software-productbulletin.pdf (accessed on 16 January 2024).
- Answers Revealed. Expand Your Forensics Workflow with the Precision ID NGS System. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FGSD%2Fbrochures%2Fprecisionid-ngs-system-brochure.pdf (accessed on 16 January 2024).
- Pakstis, A.J.; Speed, W.C.; Fang, R.; Hyland, F.C.L.; Furtado, M.R.; Kidd, J.R.; Kidd, K.K. SNPs for a universal individual identification panel. Hum. Genet. 2010, 127, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Fang, R.; Ballard, D.; Fondevila, M.; Harrison, C.; Hyland, F.; Musgrave-Brown, E.; Proff, C.; Ramos-Luis, E.; Sobrino, B.; et al. SNPforID Consortium Evaluation of the Genplex SNP typing system and a 49plex forensic marker panel. Forensic Sci. Int. Genet. 2007, 1, 180–185. [Google Scholar] [CrossRef] [PubMed]
- de Knijff, P. On the Forensic Use of Y-Chromosome Polymorphisms. Genes 2022, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Furtado, M.R.; Fang, R.; Madbouly, A.; Maiers, M.; Middha, M.; Friedlaender, F.R.; Kidd, J.R. Progress toward an efficient panel of SNPs for ancestry inference. Forensic Sci. Int. Genet. 2014, 10, 23–32. [Google Scholar] [CrossRef]
- Nassir, R.; Kosoy, R.; Tian, C.; White, P.A.; Butler, L.M.; Silva, G.; Kittles, R.; Alarcon-Riquelme, M.; Gregersen, P.K.; Belmont, J.W.; et al. An ancestry informative marker set for determining continental origin: Validation and extension using human genome diversity panels. BMC Genet. 2009, 10, 39. [Google Scholar] [CrossRef]
- Apaga, D.L.T.; Dennis, S.E.; Salvador, J.M.; Calacal, G.C.; De Ungria, M.C.A. Comparison of Two Massively Parallel Sequencing Platforms using 83 Single Nucleotide Polymorphisms for Human Identification. Sci. Rep. 2017, 7, 398. [Google Scholar] [CrossRef]
- Elwick, K.; Zeng, X.; King, J.; Budowle, B.; Hughes-Stamm, S. Comparative tolerance of two massively parallel sequencing systems to common PCR inhibitors. Int. J. Legal Med. 2018, 132, 983–995. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, D.; Wang, H.; Jia, Z.; Liu, J.; Qian, X.; Li, C.; Hou, Y. Massively parallel sequencing of 32 forensic markers using the Precision ID GlobalFiler™ NGS STR Panel and the Ion PGM™ System. Forensic Sci. Int. Genet. 2017, 31, 126–134. [Google Scholar] [CrossRef]
- Müller, P.; Alonso, A.; Barrio, P.A.; Berger, B.; Bodner, M.; Martin, P.; Parson, W. Systematic evaluation of the early access applied biosystems precision ID Globalfiler mixture ID and Globalfiler NGS STR panels for the ion S5 system. Forensic Sci. Int. Genet. 2018, 36, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Qi, W.; Chen, C.; Zhang, J.; Yang, Z.; Song, W.; Zhang, S.; Li, C. Pilot study for forensic evaluations of the Precision ID GlobalFiler™ NGS STR Panel v2 with the Ion S5™ system. Forensic Sci. Int. Genet. 2019, 43, 102147. [Google Scholar] [CrossRef]
- Faccinetto, C.; Serventi, P.; Staiti, N.; Gentile, F.; Marino, A. Internal validation study of the next generation sequencing of Globalfiler™ PCR amplification kit for the Ion Torrent S5 sequencer. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 336–338. [Google Scholar] [CrossRef]
- Shyla, A.; Borovko, S.; Luhauniou, A.; Aranovich, S.; Zayerka, Y. Two loci ‘exclusion’ of true paternity is due to genetic disorder in a child. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 3–4. [Google Scholar] [CrossRef]
- Pajnič, I.Z.; Pogorelc, B.G.; Zupanc, T. Next generation sequencing technology in Second World War victim identification. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 123–125. [Google Scholar] [CrossRef]
- Fattorini, P.; Pogorelc, B.G.; Cilieri, S.S.; Zupanc, T.; Previderè, C.; Pajnič, I.Z. MPS reveals isometric PCR artefacts in degraded samples. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 368–369. [Google Scholar] [CrossRef]
- Oldoni, F.; Bader, D.; Fantinato, C.; Wootton, S.C.; Lagacé, R.; Hasegawa, R.; Chang, J.; Kidd, K.; Podini, D. A massively parallel sequencing assay of microhaplotypes for mixture deconvolution. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 522–524. [Google Scholar] [CrossRef]
- Barrio, P.A.; Martín, P.; Alonso, A.; Müller, P.; Bodner, M.; Berger, B.; Parson, W.; Budowle, B. Massively parallel sequence data of 31 autosomal STR loci from 496 Spanish individuals revealed concordance with CE-STR technology and enhanced discrimination power. Forensic Sci. Int. Genet. 2019, 42, 49–55. [Google Scholar] [CrossRef]
- Ragazzo, M.; Carboni, S.; Caputo, V.; Buttini, C.; Manzo, L.; Errichiello, V.; Puleri, G.; Giardina, E. Interpreting mixture profiles: Comparison between precision ID globalfiler™ NGS STR panel v2 and traditional methods. Genes 2020, 11, 591. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Liu, J.; Ye, J.; Hou, Y. Characterization of sequence variation at 30 autosomal STRs in Chinese Han and Tibetan populations. Electrophoresis 2020, 41, 194–201. [Google Scholar] [CrossRef]
- Zupanič Pajnič, I.; Obal, M.; Zupanc, T. Identifying victims of the largest Second World War family massacre in Slovenia. Forensic Sci. Int. 2020, 306, 110056. [Google Scholar] [CrossRef] [PubMed]
- Dash, H.R.; Vajpayee, K.; Srivastava, A.; Das, S. Prevalence and characterisation of size and sequence-based microvariant alleles at nine autosomal STR markers in the Central Indian population. Ann. Hum. Biol. 2021, 48, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, T.; Kiesler, K.M.; Fukagawa, T.; Watahiki, H.; Mita, Y.; Fujii, K.; Sekiguchi, K.; Vallone, P.M.; Mizuno, N. Massively parallel sequencing data of 31 autosomal STR loci obtained using the Precision ID GlobalFiler NGS STR Panel v2 for 82 Japanese population samples. Leg. Med. 2022, 58, 102082. [Google Scholar] [CrossRef]
- Ohuchi, T.; Guan, X.; Hirai, E.; Hashiyada, M.; Manabe, S.; Akane, A.; Adachi, N.; Tamaki, K.; Funayama, M. Allele frequencies of 31 autosomal short tandem repeat (auSTR) loci obtained using the Precision ID GlobalFiler™ NGS STR Panel v2 in 322 individuals from the Japanese population. Leg. Med. 2022, 59, 102151. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lang, Y.; Long, G.; Liu, Z.; Jing, G.; Zhou, Y.; Zhang, B.; Yu, S. Ion Torrent ™ Genexus ™ Integrated Sequencer and ForeNGS Analysis Software—An automatic NGS-STR workflow from DNA to profile for forensic science. Forensic Sci. Int. Genet. 2022, 61, 102753. [Google Scholar] [CrossRef] [PubMed]
- Zupanič Pajnič, I.; Previderè, C.; Zupanc, T.; Zanon, M.; Fattorini, P. Isometric artifacts from polymerase chain reaction-massively parallel sequencing analysis of short tandem repeat loci: An emerging issue from a new technology? Electrophoresis 2022, 43, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.I.B.; Fridman, C. Analysis of isoalleles and flanking SNPs of STR markers by NGS to distinguish monozygotic twins. Forensic Sci. Int. Genet. Suppl. Ser. 2022, 8, 285–287. [Google Scholar] [CrossRef]
- Kocsis, B.; Mátrai, N.; Egyed, B. Forensic Implications of the Discrepancies Caused between NGS and CE Results by New Microvariant Allele at Penta E Microsatellite. Genes 2023, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Wurmbach, E. Systematic evaluation of the Precision ID GlobalFiler™ NGS STR panel v2 using single-source samples of various quantity and quality and mixed DNA samples. Forensic Sci. Int. Genet. 2024, 69, 102995. [Google Scholar] [CrossRef]
- Eduardoff, M.; Santos, C.; de la Puente, M.; Gross, T.E.; Fondevila, M.; Strobl, C.; Sobrino, B.; Ballard, D.; Schneider, P.M.; Carracedo, A.; et al. Inter-laboratory evaluation of SNP-based forensic identification by massively parallel sequencing using the Ion PGM™. Forensic Sci. Int. Genet. 2015, 17, 110–121. [Google Scholar] [CrossRef]
- Gill, P.; Haned, H.; Eduardoff, M.; Santos, C.; Phillips, C.; Parson, W. The open-source software LRmix can be used to analyse SNP mixtures. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, E50–E51. [Google Scholar] [CrossRef]
- Guo, F.; Zhou, Y.; Song, H.; Zhao, J.; Shen, H.; Zhao, B.; Liu, F.; Jiang, X. Next generation sequencing of SNPs using the HID-Ion AmpliSeq™ Identity Panel on the Ion Torrent PGM™ platform. Forensic Sci. Int. Genet. 2016, 25, 73–84. [Google Scholar] [CrossRef]
- Ochiai, E.; Minaguchi, K.; Nambiar, P.; Kakimoto, Y.; Satoh, F.; Nakatome, M.; Miyashita, K.; Osawa, M. Evaluation of Y chromosomal SNP haplogrouping in the HID-Ion AmpliSeg™ Identity Panel. Leg. Med. 2016, 22, 58–61. [Google Scholar] [CrossRef]
- Buchard, A.; Kampmann, M.; Poulsen, L.; Borsting, C.; Morling, N. ISO 17025 validation of a next-generation sequencing assay for relationship testing. Electrophoresis 2016, 37, 2822–2831. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M.; Buchard, A.; Borsting, C.; Morling, N. High-throughput sequencing of forensic genetic samples using punches of FTA cards with buccal swabs. BioTechniques 2016, 61, 149–151. [Google Scholar] [CrossRef]
- Pilli, E.; Agostino, A.; Vergani, D.; Salata, E.; Ciuna, I.; Berti, A.; Caramelli, D.; Lambiase, S. Human identification by lice: A Next Generation Sequencing challenge. Forensic Sci. Int. 2016, 266, E71–E78. [Google Scholar] [CrossRef] [PubMed]
- Meiklejohn, K.A.; Robertson, J.M. Evaluation of the Precision ID Identity Panel for the Ion Torrent™ PGM™ sequencer. Forensic Sci. Int. Genet. 2017, 31, 48–56. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, S.; de Oliveira, S.J.; Kampmann, M.; Borsting, C.; Morling, N. Comparison of manual and automated AmpliSeq™ workflows in the typing of a Somali population with the Precision ID Identity Panel. Forensic Sci. Int. Genet. 2017, 31, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.; Soto, A.; Yurrebaso, I. Allele frequencies and other forensic parameters of the HID-Ion AmpliSeq™ Identity Panel markers in Basques using the Ion Torrent PGM™ platform. Forensic Sci. Int. Genet. 2017, 28, E8–E10. [Google Scholar] [CrossRef]
- Juras, A.; Chylenski, M.; Krenz-Niedbala, M.; Malmstrom, H.; Ehler, E.; Pospieszny, L.; Lukasik, S.; Bednarczyk, J.; Piontek, J.; Jakobsson, M.; et al. Investigating kinship of Neolithic post-LBK human remains from Krusza Zamkowa, Poland using ancient DNA. Forensic Sci. Int. Genet. 2017, 26, 30–39. [Google Scholar] [CrossRef]
- Bleka, O.; Eduardoff, M.; Santos, C.; Phillips, C.; Parson, W.; Gill, P. Using EuroForMix to analyse complex SNP mixtures, up to six contributors. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, E277–E279. [Google Scholar] [CrossRef]
- Bleka, O.; Eduardoff, M.; Santos, C.; Phillips, C.; Parson, W.; Gill, P. Open source software EuroForMix can be used to analyse complex SNP mixtures. Forensic Sci. Int. Genet. 2017, 31, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, J.H.; Kim, C.J.; Kim, M.Y.; Kim, K.W.; Hwang, D.; Lee, S.D. SNP-Based Fetal DNA Detection in Maternal Serum Using the HID-Ion AmpliSeq TM Identity Panel. Korean J. Leg. Med. 2017, 41, 41–45. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; He, G.; Zhao, X.; Wang, M.; Luo, T.; Li, C.; Hou, Y. Massively parallel sequencing of 124 SNPs included in the precision ID identity panel in three East Asian minority ethnicities. Forensic Sci. Int. Genet. 2018, 35, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, C.; Li, H.; Wu, R.; Li, H.; Tang, Z.; Zhen, C.; Ge, J.; Peng, D.; Wang, Y.; et al. SNP typing using the HID-Ion AmpliSeq™ Identity Panel in a southern Chinese population. Int. J. Legal Med. 2018, 132, 997–1006. [Google Scholar] [CrossRef]
- Sun, L.; Fu, L.; Liu, Q.; Zhou, J.; Ma, C.; Cong, B.; Li, S. Population data using Precision ID Identity Panel in a Chinese Han population from Hebei Province. Forensic Sci. Int. Genet. 2019, 42, E27–E29. [Google Scholar] [CrossRef]
- Christiansen, S.L.; Jakobsen, B.; Borsting, C.; Udengaard, H.; Buchard, A.; Kampmann, M.; Grondahl, M.L.; Morling, N. Non-invasive prenatal paternity testing using a standard forensic genetic massively parallel sequencing assay for amplification of human identification SNPs. Int. J. Legal Med. 2019, 133, 1361–1368. [Google Scholar] [CrossRef]
- Turchi, C.; Onofria, V.; Melchionda, F.; Bini, C.; Previdere, C.; Carnevali, E.; Robino, C.; Sorcaburu-Ciglieri, S.; Marrubini, G.; Pelotti, S.; et al. Dealing with low amounts of degraded DNA: Evaluation of SNP typing of challenging forensic samples by using massive parallel sequencing. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 83–84. [Google Scholar] [CrossRef]
- Bottino, C.G.; Silva, R.; Moura-Neto, R.S. Analysis of 124 SNP loci included in HID Ampliseq identity panel in a small population of Rio de Janeiro, Brazil. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 243–244. [Google Scholar] [CrossRef]
- Avila, E.; Felkl, A.B.; Graebin, P.; Nunes, C.P.; Alho, C.S. Forensic characterization of Brazilian regional populations through massive parallel sequencing of 124 SNPs included in HID ion Ampliseq Identity Panel. Forensic Sci. Int. Genet. 2019, 40, 74–84. [Google Scholar] [CrossRef]
- Avila, E.; Cavalheiro, C.P.; Felkl, A.B.; Graebin, P.; Kahmann, A.; Alho, C.S. Brazilian forensic casework analysis through MPS applications: Statistical weight-of-evidence and biological nature of criminal samples as an influence factor in quality metrics. Forensic Sci. Int. 2019, 303, 109938. [Google Scholar] [CrossRef] [PubMed]
- Turchi, C.; Previdere, C.; Bini, C.; Carnevali, E.; Grignani, P.; Manfredi, A.; Melchionda, F.; Onofri, V.; Pelotti, S.; Robino, C.; et al. Assessment of the Precision ID Identity Panel kit on challenging forensic samples. Forensic Sci. Int. Genet. 2020, 49, 102400. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, T.M.; McAtee, P.D.; McCormick, M.N.; Lakhtakia, A.; Roy, R. Massively parallel sequencing and STR analysis from partial bloody fingerprints enhanced with columnar thin films. Forensic Sci. Int. Genet. 2020, 49, 102369. [Google Scholar] [CrossRef]
- Gray, S.L.; Tiedge, T.M.; Butkus, J.M.; Earp, T.J.; Lindner, S.E.; Roy, R. Determination of human identity from Anopheles stephensi mosquito blood meals using direct amplification and massively parallel sequencing. Forensic Sci. Int. Genet. 2020, 48, 102347. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Q.; Li, S.; Ma, G.; Wang, Z.; Ma, C.; Cong, B.; Fu, L. A new strategy to confirm the identity of tumour tissues using single-nucleotide polymorphisms and next-generation sequencing. Int. J. Legal Med. 2020, 134, 399–409. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, X.; Ma, K.; Li, H.; Cao, Y.; Cao, Y.; Liu, W. Separation of SNP profiles from DNA mixtures with two contributors via massively parallel sequencing technology. Aust. J. Forensic Sci. 2020, 52, 537–546. [Google Scholar] [CrossRef]
- Tiedge, T.M.; Nagachar, N.; Wendt, F.R.; Lakhtakia, A.; Roy, R. High-throughput DNA sequencing of environmentally insulted latent fingerprints after visualization with nanoscale columnar-thin-film technique. Sci. Justice 2021, 61, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Dash, H.R.; Avila, E.; Jena, S.R.; Kaitholia, K.; Agarwal, R.; Alho, C.S.; Srivastava, A.; Singh, A.K. Forensic characterization of 124 SNPs in the central Indian population using precision ID Identity Panel through next-generation sequencing. Int. J. Legal Med. 2022, 136, 465–473. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, P.; Xing, Y. A New Computational Deconvolution Algorithm for the Analysis of Forensic DNA Mixtures with SNP Markers. Genes 2022, 13, 884. [Google Scholar] [CrossRef]
- Yang, S.; Lee, J.E.; Lee, H.Y. Forensic genetic analysis of single-nucleotide polymorphisms and microhaplotypes in Koreans through next-generation sequencing using precision ID identity panel. Genes Genom. 2023, 45, 1281–1293. [Google Scholar] [CrossRef]
- Joo, S.M.; Kwon, Y.; Moon, M.H.; Shin, K. Genetic investigation of 124 SNPs in a Myanmar population using the Precision ID Identity Panel and the Illumina MiSeq. Leg. Med. 2023, 63, 102256. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Q.; Yang, J.; Zhu, J.; Liu, J.; Wu, R.; Sun, H. Comparison of three massively parallel sequencing platforms for single nucleotide polymorphism (SNP) genotyping in forensic genetics. Int. J. Legal Med. 2023, 137, 1361–1372. [Google Scholar] [CrossRef]
- Pajnic, I.Z.; Leskovar, T.; Cresnar, M. Improving kinship probability in analysis of ancient skeletons using identity SNPs and MPS technology. Int. J. Legal Med. 2023, 137, 1007–1015. [Google Scholar] [CrossRef]
- Fattorini, P.; Previdere, C.; Livieri, T.; Zupanc, T.; Pajnic, I.Z. SNP analysis of challenging bone DNA samples using the HID-Ion AmpliSeq™ Identity Panel: Facts and artefacts. Int. J. Legal Med. 2023, 137, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, K.M.; Gettings, K.B.; Vallone, P.M. A Strategy for Characterization of Single Nucleotide Polymorphisms in a Reference Material. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, E363–E364. [Google Scholar] [CrossRef]
- Tasker, E.; LaRue, B.; Beherec, C.; Gangitano, D.; Hughes-Stamm, S. Analysis of DNA from post-blast pipe bomb fragments for identification and determination of ancestry. Forensic Sci. Int. Genet. 2017, 28, 195–202. [Google Scholar] [CrossRef]
- Scheible, M.K.R.; Timpano, E.K.; Boggs, L.M.; Meiklejohn, K.A. An alternate workflow for preparing Precision ID Ancestry and Identity Panel libraries for Illumina sequencing. Int. J. Legal Med. 2021, 135, 1717–1726. [Google Scholar] [CrossRef]
- Meiklejohn, K.A.; Scheible, M.K.R.; Boggs, L.M.; Dunn, R.R.; Ricke, D.O. Using FastID to analyze complex SNP mixtures from indoor dust. J. Forensic Sci. 2023, 68, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Themudo, G.E.; Mogensen, H.S.; Borsting, C.; Morling, N. Frequencies of HID-ion ampliseq ancestry panel markers among greenlanders. Forensic Sci. Int. Genet. 2016, 24, 60–64. [Google Scholar] [CrossRef]
- Garcia, O.; Ajuriagerra, J.A.; Alday, A.; Alonso, S.; Perez, J.A.; Soto, A.; Uriarte, I.; Yurrebaso, I. w Frequencies of the precision ID ancestry panel markers in Basques using the Ion Torrent PGM™ platform. Forensic Sci. Int. Genet. 2017, 31, E1–E4. [Google Scholar] [CrossRef]
- Truelsen, D.M.; Farzad, M.S.; Mogensen, H.S.; Pereira, V.; Tvedebrink, T.; Borsting, C.; Morling, N. Typing of two Middle Eastern populations with the Precision ID Ancestry Panel. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, E301–E302. [Google Scholar] [CrossRef]
- Pereira, V.; Mogensen, H.S.; Borsting, C.; Morling, N. Evaluation of the Precision ID Ancestry Panel for crime case work: A SNP typing assay developed for typing of 165 ancestral informative markers. Forensic Sci. Int. Genet. 2017, 28, 138–145. [Google Scholar] [CrossRef]
- Santangelo, R.; Gonzalez-Andrade, F.; Borsting, C.; Torroni, A.; Pereira, V.; Morling, N. Analysis of ancestry informative markers in three main ethnic groups from Ecuador supports a trihybrid origin of Ecuadorians. Forensic Sci. Int. Genet. 2017, 31, 29–33. [Google Scholar] [CrossRef]
- Hollard, C.; Keyser, C.; Delabarde, T.; Gonzalez, A.; Lamego, C.V.; Zvenigorosky, V.; Ludes, B. Case report: On the use of the HID-Ion AmpliSeq™ Ancestry Panel in a real forensic case. Int. J. Legal Med. 2017, 131, 351–358. [Google Scholar] [CrossRef]
- Wang, Z.; He, G.; Luo, T.; Zhao, X.; Liu, J.; Wang, M.; Zhou, D.; Chen, X.; Li, C.; Hou, Y. Massively parallel sequencing of 165 ancestry informative SNPs in two Chinese Tibetan-Burmese minority ethnicities. Forensic Sci. Int. Genet. 2018, 34, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Pereira, V.; Borsting, C.; Yamamoto, T.; Tvedebrink, T.; Hara, M.; Takada, A.; Saito, K.; Morling, N. Analysis of mainland Japanese and Okinawan Japanese populations using the precision ID Ancestry Panel. Forensic Sci. Int. Genet. 2018, 33, 106–109. [Google Scholar] [CrossRef]
- Jin, S.; Chase, M.; Henry, M.; Alderson, G.; Morrow, J.M.; Malik, S.; Ballard, D.; McGrory, J.; Fernandopulle, N.; Millman, J.; et al. Implementing a biogeographic ancestry inference service for forensic casework. Electrophoresis 2018, 39, 2757–2765. [Google Scholar] [CrossRef]
- He, G.; Wang, Z.; Wang, M.; Luo, T.; Liu, J.; Zhou, Y.; Gao, B.; Hou, Y. Forensic ancestry analysis in two Chinese minority populations using massively parallel sequencing of 165 ancestry-informative SNPs. Electrophoresis 2018, 39, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Al-Asfi, M.; McNevin, D.; Mehta, B.; Power, D.; Gahan, M.E.; Daniel, R. Assessment of the Precision ID Ancestry panel. Int. J. Legal Med. 2018, 132, 1581–1594. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, S.; Kim, M.; Shin, D.H.; Rakha, A.; Shinde, V.; Lee, S.D. Genetic resolution of applied biosystems™ precision ID Ancestry panel for seven Asian populations. Leg. Med. 2018, 34, 41–47. [Google Scholar] [CrossRef]
- Young, J.M.; Martin, B.; Kanokwongnuwut, P.; Linacre, A. Detection of forensic identification and intelligence SNP data from latent DNA using three commercial MPS panels. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 864–865. [Google Scholar] [CrossRef]
- Daniels-Higginbotham, J.; Gorden, E.M.; Farmer, S.K.; Spatola, B.; Damann, F.; Bellantoni, N.; Gagnon, K.S.; de la Puente, M.; Xavier, C.; Walsh, S.; et al. DNA Testing Reveals the Putative Identity of JB55, a 19th Century Vampire Buried in Griswold, Connecticut. Genes 2019, 10, 636. [Google Scholar] [CrossRef]
- Shan, M.A.; Refn, M.; Morling, N.; Borsting, C.; Pereira, V. Genetic Portrait of the Punjabi Population from Pakistan using the Precision Id Ancestry Panel. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 87–89. [Google Scholar] [CrossRef]
- Al-Dosari, W.R.; Al-Binali, I.A.; Pydi, S.S.; Goodwin, W. Analysis of forensic casework samples by Precision ID Ancestry panel—Manual and automated Ampliseq workflow. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 816–817. [Google Scholar] [CrossRef]
- Mogensen, H.S.; Tvedebrink, T.; Borsting, C.; Pereira, V.; Morling, N. Ancestry prediction efficiency of the software GenoGeographer using a z-score method and the ancestry informative markers in the Precision ID Ancestry Panel. Forensic Sci. Int. Genet. 2020, 44, 102154. [Google Scholar] [CrossRef] [PubMed]
- Cooley, A.M.; Meiklejohn, K.A.; Damaso, N.; Robertson, J.M.; Dawson Cruz, T. Performance Comparison of Massively Parallel Sequencing (MPS) Instruments Using Single-Nucleotide Polymorphism (SNP) Panels for Ancestry. Slas Technol. 2021, 26, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, D.; Tvedebrink, T.; Mogensen, H.S.; Farzad, M.S.; Shan, M.A.; Morling, N.; Pereira, V.; Borsting, C. Assessment of the effectiveness of the EUROFORGEN NAME and Precision ID Ancestry panel markers for ancestry investigations. Sci. Rep. 2021, 11, 18595. [Google Scholar] [CrossRef]
- He, G.; Liu, J.; Wang, M.; Zou, X.; Ming, T.; Zhu, S.; Yeh, H.; Wang, C.; Wang, Z.; Hou, Y. Massively parallel sequencing of 165 ancestry-informative SNPs and forensic biogeographical ancestry inference in three southern Chinese Sinitic/Tai-Kadai populations. Forensic Sci. Int. Genet. 2021, 52, 102475. [Google Scholar] [CrossRef]
- Shan, M.A.; Meyer, O.S.; Refn, M.; Morling, N.; Andersen, J.D.; Borsting, C. Analysis of Skin Pigmentation and Genetic Ancestry in Three Subpopulations from Pakistan: Punjabi, Pashtun, and Baloch. Genes 2021, 12, 733. [Google Scholar] [CrossRef]
- Young, J.M.; Power, D.; Kanokwongnuwut, P.; Linacre, A. Ancestry and phenotype predictions from touch DNA using massively parallel sequencing. Int. J. Legal Med. 2021, 135, 81–89. [Google Scholar] [CrossRef]
- Mogensen, H.S.; Tvedebrink, T.; Pereira, V.; Eriksen, P.S.; Morling, N. Update of aims population data and test with the genogeographer admixture module. Forensic Sci. Int. Genet. Suppl. Ser. 2022, 8, 15–16. [Google Scholar] [CrossRef]
- Cui, W.; Chen, M.; Yao, H.; Yang, Q.; Liu, L.; Bai, X.; Chen, L.; Zhu, B. Forensic Characterization and Genetic Portrait of the Gannan Tibetan Ethnic Group via 165 AI-SNP Loci. Front. Biosci. 2023, 28, 114. [Google Scholar] [CrossRef]
- Felkl, A.B.; Avila, E.; Gastaldo, A.Z.; Lindholz, C.G.; Dorn, M.; Alho, C.S. Ancestry resolution of South Brazilians by forensic 165 ancestry-informative SNPs panel. Forensic Sci. Int. Genet. 2023, 64, 102838. [Google Scholar] [CrossRef] [PubMed]
- Koksal, Z.; Meyer, O.L.; Andersen, J.D.; Gusmao, L.; Mogensen, H.S.; Pereira, V.; Borsting, C. Pitfalls and challenges with population assignments of individuals from admixed populations: Applying Genogeographer on Brazilian individuals. Forensic Sci. Int. Genet. 2023, 67, 102934. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Liu, C.; Zheng, J.; Han, X.; Du, W.; Huang, Y.; Li, C.; Wang, X.; Tong, D.; Ou, X.; et al. Genome-wide screen for universal individual identification SNPs based on the HapMap and 1000 Genomes databases. Sci. Rep. 2018, 8, 5553. [Google Scholar] [CrossRef] [PubMed]
- Ragazzo, M.; Puleri, G.; Errichiello, V.; Manzo, L.; Luzzi, L.; Potenza, S.; Strafella, C.; Peconi, C.; Nicastro, F.; Caputo, V.; et al. Evaluation of OpenArray™ as a Genotyping Method for Forensic DNA Phenotyping and Human Identification. Genes 2021, 12, 221. [Google Scholar] [CrossRef]
- Resutik, P.; Aeschbacher, S.; Kruetzen, M.; Kratzer, A.; Haas, C.; Phillips, C.; Arora, N. Comparative evaluation of the MAPlex, Precision ID Ancestry Panel, and VISAGE Basic Tool for biogeographical ancestry inference. Forensic Sci. Int. Genet. 2023, 64, 102850. [Google Scholar] [CrossRef] [PubMed]
- Jesubright, J.J.; Saravanan, P. A scientometric analysis of global forensic science research publications. Libr. Philos. Pract. 2014, 1024, 1–18. [Google Scholar]
- Stasi, A.; Mir, T.u.G.; Pellegrino, A.; Wani, A.K.; Shukla, S. Forty years of research and development on forensic genetics: A bibliometric analysis. Forensic Sci. Int. Genet. 2023, 63, 102826. [Google Scholar] [CrossRef]
- Björk, B.; Solomon, D. Open access versus subscription journals: A comparison of scientific impact. BMC Med. 2012, 10, 73. [Google Scholar] [CrossRef]
- What is the ISFG? Available online: https://www.isfg.org/About (accessed on 13 June 2024).
- Schimmer, R.; Geschuhn, K.K.; Vogler, A. Disrupting the subscription journals’ business model for the necessary large-scale transformation to open access. Max Planck Digital Library 2015. [Google Scholar] [CrossRef]
- Jäger, A.C.; Alvarez, M.L.; Davis, C.P.; Guzmán, E.; Han, Y.; Way, L.; Walichiewicz, P.; Silva, D.; Pham, N.; Caves, G.; et al. Developmental validation of the MiSeq FGx Forensic Genomics System for Targeted Next Generation Sequencing in Forensic DNA Casework and Database Laboratories. Forensic Sci. Int. Genet. 2017, 28, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, K.M.; Steffen, C.R.; Coble, M.D.; Vallone, P.M. Initial assessment of the Precision ID Globalfiler Mixture ID panel on the Ion Torrent S5XL DNA sequencer and Converge v2.0 software. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e94–e95. [Google Scholar] [CrossRef]
- Precision ID Panels. Available online: https://www.thermofisher.com/uk/en/home/industrial/forensics/human-identification/forensic-dna-analysis/dna-analysis/next-generation-sequencing-ngs-forensics/precision-id-panels.html (accessed on 15 June 2024).
- Delest, A.; Godfrin, D.; Chantrel, Y.; Ulus, A.; Vannier, J.; Faivre, M.; Hollard, C.; Laurent, F. Sequenced-based French population data from 169 unrelated individuals with Verogen’s ForenSeq DNA signature prep kit. Forensic Sci. Int. Genet. 2020, 47, 102304. [Google Scholar] [CrossRef]
- Guevara, E.K.; Palo, J.U.; King, J.L.; Buś, M.M.; Guillen, S.; Budowle, B.; Sajantila, A. Autosomal STR and SNP characterization of populations from the Northeastern Peruvian Andes with the ForenSeq™ DNA Signature Prep Kit. Forensic Sci. Int. Genet. 2021, 52, 102487. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Dong, X.; Zhen, X.; Xia, R.; Qu, Y.; Liu, S.; Zhang, S.; Li, C. Population genetic analyses of Eastern Chinese Han nationality using ForenSeq™ DNA Signature Prep Kit. Mol. Genet. Genom. 2024, 299, 1–9. [Google Scholar] [CrossRef]
- Hussing, C.; Bytyci, R.; Huber, C.; Morling, N.; Børsting, C. The Danish STR sequence database: Duplicate typing of 363 Danes with the ForenSeq™ DNA Signature Prep Kit. Int. J. Legal Med. 2019, 133, 325–334. [Google Scholar] [CrossRef]
- Aguilar-Velázquez, J.A.; Duran-Salazar, M.Á.; Córdoba-Mercado, M.F.; Coronado-Avila, C.E.; Salas-Salas, O.; Martinez-Cortés, G.; Casals, F.; Calafell, F.; Ramos-González, B.; Rangel-Villalobos, H. Characterization of 58 STRs and 94 SNPs with the ForenSeq™ DNA signature prep kit in Mexican-Mestizos from the Monterrey city (Northeast, Mexico). Mol. Biol. Rep. 2022, 49, 7601–7609. [Google Scholar] [CrossRef]
- Almohammed, E.; Iyengar, A.; Ballard, D.; Devesse, L.; Hadi, S. Evaluation of ForenSeq DNA signature kit for Qatari population. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e596–e598. [Google Scholar] [CrossRef]
- Westen, A.A.; Kraaijenbrink, T.; Robles de Medina, E.A.; Harteveld, J.; Willemse, P.; Zuniga, S.B.; van der Gaag, K.J.; Weiler, N.E.C.; Warnaar, J.; Kayser, M.; et al. Comparing six commercial autosomal STR kits in a large Dutch population sample. Forensic. Sci. Int. Genet. 2014, 10, 55–63. [Google Scholar] [CrossRef]
- Sharma, V.; Chow, H.Y.; Siegel, D.; Wurmbach, E. Qualitative and quantitative assessment of Illumina’s forensic STR and SNP kits on MiSeq FGx™. PLoS ONE 2017, 12, e0187932. [Google Scholar] [CrossRef]
- Zeng, X.; King, J.; Hermanson, S.; Patel, J.; Storts, D.R.; Budowle, B. An evaluation of the PowerSeq™ Auto System: A multiplex short tandem repeat marker kit compatible with massively parallel sequencing. Forensic. Sci. Int. Genet. 2015, 19, 172–179. [Google Scholar] [CrossRef]
- Churchill, J.D.; Schmedes, S.E.; King, J.L.; Budowle, B. Evaluation of the Illumina® Beta Version ForenSeq™ DNA Signature Prep Kit for use in genetic profiling. Forensic. Sci. Int. Genet. 2016, 20, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.; Winkler-Galicki, J.; Wesoły, J. Massive parallel sequencing in forensics: Advantages, issues, technicalities, and prospects. Int. J. Legal Med. 2020, 134, 1291–1303. [Google Scholar] [CrossRef]
- Alvarez-Cubero, M.J.; Saiz, M.; Martínez-García, B.; Sayalero, S.M.; Entrala, C.; Lorente, J.A.; Martinez-Gonzalez, L.J. Next generation sequencing: An application in forensic sciences? Ann. Hum. Biol. 2017, 44, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Z.; Liu, Q.; Liu, L.; Shao, L.; Zhang, M.; Ding, X.; Gao, Y.; Wang, S. Evaluation of the performance of Illumina’s ForenSeq™ system on serially degraded samples. Electrophoresis 2018, 39, 2674–2684. [Google Scholar] [CrossRef]
- Bragg, L.M.; Stone, G.; Butler, M.K.; Hugenholtz, P.; Tyson, G.W. Shining a Light on Dark Sequencing: Characterising Errors in Ion Torrent PGM Data. PLoS Comput. Biol. 2013, 9, e1003031. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Ijaz, U.Z.; D’Amore, R.; Hall, N.; Sloan, W.T.; Quince, C. Insight into biases and sequencing errors for amplicon sequencing with the Illumina MiSeq platform. Nucleic Acids Res. 2015, 43, e37. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, B.; Yan, J. Application of next-generation sequencing technology in forensic science. Genom. Proteom. Bioinf. 2014, 12, 190–197. [Google Scholar] [CrossRef]
- Du, Q.; Zhu, G.; Fu, G.; Zhang, X.; Fu, L.; Li, S.; Cong, B. A Genome-Wide Scan of DNA Methylation Markers for Distinguishing Monozygotic Twins. Twin Res. Hum. Genet. 2015, 18, 670–679. [Google Scholar] [CrossRef]
- Zgonjanin, D.; Alghafri, R.; Almheiri, R.; Antov, M.; Toljic, D.; Vukovic, R.; Petkovic, S. Mutation rate at 13 rapidly mutating Y-STR loci in the population of Serbia. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e377–e379. [Google Scholar] [CrossRef]
- Hwa, H.; Lin, C.; Yu, Y.; Linacre, A.; Lee, J.C. DNA identification of monozygotic twins. Forensic Sci. Int. Genet. 2024, 69, 102998. [Google Scholar] [CrossRef] [PubMed]
- Børsting, C.; Fordyce, S.L.; Olofsson, J.; Mogensen, H.S.; Morling, N. Evaluation of the Ion Torrent™ HID SNP 169-plex: A SNP typing assay developed for human identification by second generation sequencing. Forensic Sci. Int. Genet. 2014, 12, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Santos, C.; Phillips, C.; Fondevila, M.; van Oorschot, R.A.H.; Carracedo, Á.; Lareu, M.V.; McNevin, D. A SNaPshot of next generation sequencing for forensic SNP analysis. Forensic Sci. Int. Genet. 2015, 14, 50–60. [Google Scholar] [CrossRef]
- Avent, I.; Kinnane, A.G.; Jones, N.; Petermann, I.; Daniel, R.; Gahan, M.E.; McNevin, D. The QIAGEN 140-locus single-nucleotide polymorphism (SNP) panel for forensic identification using massively parallel sequencing (MPS): An evaluation and a direct-to-PCR trial. Int. J. Legal Med. 2019, 133, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.B.; King, J.L.; Warshauer, D.H.; Davis, C.P.; Ge, J.; Budowle, B. Single nucleotide polymorphism typing with massively parallel sequencing for human identification. Int. J. Legal Med. 2013, 127, 1079–1086. [Google Scholar] [CrossRef]
- Validation Guidelines for DNA Analysis Methods. Available online: https://www.swgdam.org/_files/ugd/4344b0_813b241e8944497e99b9c45b163b76bd.pdf (accessed on 31 January 2024).
- Vlachos, N.T.; Meiklejohn, K.A.; Robertson, J.M. An automated independent workflow for the analysis of massively parallel sequence data from forensic SNP assays. Electrophoresis 2018, 39, 2752–2756. [Google Scholar] [CrossRef]
- Visual SNP (v1.1) for MPS Data by STRait Razor 3.0. Available online: http://forensic.yonsei.ac.kr/VisualSNP/index.html (accessed on 24 June 2024).
- Børsting, C.; Morling, N. Next generation sequencing and its applications in forensic genetics. Forensic Sci. Int. Genet. 2015, 18, 78–89. [Google Scholar] [CrossRef]
- Yang, J.; Lin, D.; Deng, C.; Li, Z.; Pu, Y.; Yu, Y.; Li, K.; Li, D.; Chen, P.; Chen, F. The advances in DNA mixture interpretation. Forensic Sci. Int. 2019, 301, 101–106. [Google Scholar] [CrossRef]
- PowerPlex Fusion System Brochure. Available online: https://www.promega.co.uk/resources/pubhub/ebrochures/powerplex-fusion-system/ (accessed on 26 June 2024).
- Elwick, K.; Bus, M.M.; King, J.L.; Chang, J.; Hughes-Stamm, S.; Budowle, B. Utility of the Ion S5™ and MiSeq FGx™ sequencing platforms to characterize challenging human remains. Leg. Med. 2019, 41, 101623. [Google Scholar] [CrossRef]
- Butler, J.M.; McCord, B.R.; Shen, Y. The development of reduced size STR amplicons as tools for analysis of degraded DNA. J. Forensic Sci. 2003, 48, JFS2003043. [Google Scholar] [CrossRef]
- Gettings, K.B.; Kiesler, K.M.; Vallone, P.M. Performance of a next generation sequencing SNP assay on degraded DNA. Forensic Sci. Int. Genet. 2015, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Ong, B. The MassARRAY® System for Targeted SNP Genotyping. Methods Mol. Biol. 2017, 1492, 77–94. [Google Scholar] [CrossRef]
- Marine, R.L.; Magaña, L.C.; Castro, C.J.; Zhao, K.; Montmayeur, A.M.; Schmidt, A.; Diez-Valcarce, M.; Ng, T.F.F.; Vinjé, J.; Burns, C.C.; et al. Comparison of Illumina MiSeq and the Ion Torrent PGM and S5 platforms for whole-genome sequencing of picornaviruses and caliciviruses. J. Virol. Methods 2020, 280, 113865. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genet. 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Forensic genetic analysis of bio-geographical ancestry. Forensic Sci. Int. Genet. 2015, 18, 49–65. [Google Scholar] [CrossRef]
- Tvedebrink, T.; Eriksen, P.S.; Mogensen, H.S.; Morling, N. GenoGeographer—A tool for genogeographic inference. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e463–e465. [Google Scholar] [CrossRef]
- GenoGeographer. Available online: http://apps.math.aau.dk/aims/# (accessed on 22 July 2024).
- Tvedebrink, T.; Eriksen, P.S. Inference of admixed ancestry with Ancestry Informative Markers. Forensic Sci. Int. Genet. 2019, 42, 147–153. [Google Scholar] [CrossRef]
- Cavanaugh, S.E.; Bathrick, A.S. Direct PCR amplification of forensic touch and other challenging DNA samples: A review. Forensic Sci. Int. Genet. 2018, 32, 40–49. [Google Scholar] [CrossRef]
- Young, J.M.; Martin, B.; Linacre, A. Evaluation of the QIAGEN 140-SNP forensic identification multiplex from latent DNA using massively parallel sequencing. Aust. J. Forensic Sci. 2019, 51, S72–S75. [Google Scholar] [CrossRef]
- Ion Torrent Next-Generation Sequencing Instruments. Available online: https://www.thermofisher.com/uk/en/home/life-science/sequencing/next-generation-sequencing/ion-torrent-next-generation-sequencing-workflow/ion-torrent-next-generation-sequencing-run-sequence.html.html?SID=fr-iontorrent-2 (accessed on 28 July 2024).
- Hwang, S.M.; Lee, K.C.; Lee, M.S.; Park, K.U. Comparison of Ion Personal Genome Machine Platforms for the Detection of Variants in BRCA1 and BRCA2. Cancer Res. Treat. 2018, 50, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.F.; Kohl, T.A.; Kotrová, M.; Rönsch, K.; Paprotka, T.; Mohr, V.; Hutzenlaub, T.; Brüggemann, M.; Zengerle, R.; Niemann, S.; et al. Library preparation for next generation sequencing: A review of automation strategies. Biotechnol. Adv. 2020, 41, 107537. [Google Scholar] [CrossRef] [PubMed]
- Scudder, N.; McNevin, D.; Kelty, S.F.; Walsh, S.J.; Robertson, J. Massively parallel sequencing and the emergence of forensic genomics: Defining the policy and legal issues for law enforcement. Sci. Justice 2018, 58, 153–158. [Google Scholar] [CrossRef]
- Butler, J.M.; Willis, S. Interpol review of forensic biology and forensic DNA typing 2016–2019. Forensic. Sci. Int. Synerg. 2020, 2, 352–367. [Google Scholar] [CrossRef]
- Lewis, D. Unethical studies on Chinese minority groups are being retracted—But not fast enough, critics say. Nature 2024, 625, 650–654. [Google Scholar] [CrossRef]
- Normile, D. Genetics papers from China face ethical scrutiny. Science 2021, 373, 727–728. [Google Scholar] [CrossRef]
- Simayijiang, H.; Børsting, C.; Tvedebrink, T.; Morling, N. RETRACTED: Analysis of Uyghur and Kazakh populations using the Precision ID Ancestry Panel. Forensic Sci. Int. Genet. 2019, 43, 102144. [Google Scholar] [CrossRef] [PubMed]
- Tvedebrink, T.; Morling, N. Editorial Expression of Concern regarding “Analysis of Uyghur and Kazakh populations using the Precision ID Ancestry Panel” Volume 43, November 2019, 102144 by H. Simayijiang, C. Børstinga, T. Tvedebrink, N. Morling. Forensic Sci. Int. Genet. 2021, 52, 102460. [Google Scholar] [CrossRef]
- Ethical Considerations for Forensic Genetic Frequency Databases: First Report by the Forensic Databases Advisory Board (FDAB). Available online: https://www.isfg.org/files/2023_FDAB_First_Report.pdf (accessed on 6 August 2024).
- Claw, K.G.; Anderson, M.Z.; Begay, R.L.; Tsosie, K.S.; Fox, K.; Garrison, N.A.; Bader, A.C.; Bardill, J.; Bolnick, D.A.; Brooks, J.; et al. Summer internship for INdigenous peoples in Genomics (SING) Consortium A framework for enhancing ethical genomic research with Indigenous communities. Nat. Commun. 2018, 9, 2957. [Google Scholar] [CrossRef]
- Garrison, N.A.; Hudson, M.; Ballantyne, L.L.; Garba, I.; Martinez, A.; Taualii, M.; Arbour, L.; Caron, N.R.; Rainie, S.C. Genomic Research Through an Indigenous Lens: Understanding the Expectations. Annu. Rev. Genom. Hum. Genet. 2019, 20, 495–517. [Google Scholar] [CrossRef]
| Reference | Sequencing Panel | Sequencing Instrument | Main Points |
|---|---|---|---|
| Wang et al., 2017 [35] | Precision ID GlobalFiler NGS STR Panel | Ion Torrent PGM | Evaluation of the kit on Ion PGM. Population data for Chinese Han were obtained. Minor contributor of mixture could be detected in 19:1 mixture. Good results were obtained from multiple casework-type samples. |
| Müller et al., 2018 [36] | Early Access Precision ID GlobalFiler NGS STR Panel | Ion S5 System | Inter-laboratory evaluation (two laboratories) of prototype kits, Early Access Precision ID GlobalFiler Mixture ID and GlobalFiler NGS STR Panel. Higher sensitivity was revealed with manual library preparation (full profiles at 62 pg) compared to automated. |
| Tao et al., 2019 [37] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Evaluation of the kit on Ion S5. Population data for Uyghur (inner Asia) were obtained. Full profiles were generated down to 62.5 pg and from 1:3 mixtures. Discordances with GlobalFiler CE kits were observed at Penta D due to high interlocus imbalance. Urea concentrations higher than 1000 ng/μL inhibited profiling. |
| Faccinetto et al., 2019 [38] | Precision ID GlobalFiler NGS STR Panel v2 | Ion GeneStudio S5 System | Internal validation of the kit. Complete profiles were obtained with 12 pg of DNA. Partial profiles were generated from 1:80 mixtures. More stutters and noise artefacts were observed in the NGS kit compared to CE with the GlobalFiler kit, which also produced more reliable calls from mixtures. |
| Shyla et al., 2019 [39] | Precision ID GlobalFiler NGS STR Panel v2 | HID Ion GeneStudio S5 System | Application of the kit for a complex paternity case with three incompatibilities. Uniparental disomy from the mother was proven. |
| Pajnič, Pogorelc and Zupanc, 2019 [40] | Precision ID GlobalFiler NGS STR Panel (v2) | Ion S5 System | Human remains from World War 2 were processed for kinship analysis, on top of ESI 17 and NGM kits STR typing. The added information obtained with the GlobalFiler NGS kit proved useful. |
| Fattorini et al., 2019 [41] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Isometric artefacts were identified from analysis of heat-degraded samples. These should be evaluated carefully as they could be interpreted as mixtures. |
| Oldoni et al., 2019 [42] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | A microhaplotype panel for Ion S5 was compared to GlobalFiler NGS and CE kits for mixture deconvolution. The GlobalFiler NGS kit performed better than CE for mixtures, and the microhaplotype panel performed even better, with 50 pg sensitivity and full profiles attainable from three–five people mixtures. |
| Barrio et al., 2019 [43] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 XL System | Spanish population data were reported. Comparison of the GlobalFiler NGS results with the PowerPlex Fusion 6C STR kit showed high concordance. Increased discrimination power was obtained with sequence data. |
| Ragazzo et al., 2020 [44] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Evaluation of the kit for mixture interpretation from saliva and urine samples. The GlobalFiler CE kit performed better at detecting the minor contributor, but the NGS kit provided increased discrimination. |
| Wang et al., 2020 [45] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 XL System | Chinese Han and Tibetan population data were reported. Increased variation was revealed from sequence data compared to CE data. Discordances at Penta E and interlocus imbalance were highlighted. |
| Pajnič, Obal and Zupanc, 2020 [46] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Human remains from World War 2 were processed for kinship analysis, on top of autosomal STR and Y-STR kits. The added information obtained with the GlobalFiler NGS kit proved useful to increase posterior probability. |
| Dash et al., 2021 [47] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Central Indian population samples were evaluated for microvariant alleles, which appeared to be rare. |
| Kitayama et al., 2022 [48] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 XL System | Japanese population data were reported. Discrepancy at locus D2S441 was observed with CE data from the GlobalFiler STR kit. |
| Ohuchi et al., 2022 [49] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Japanese population data were obtained for database generation. Care in evaluation of sequence errors and stutters was advised. |
| Guo et al., 2022 [50] | Precision ID GlobalFiler NGS STR Panel v2 | Ion Genexus Sequencer | Evaluation of the kit on Ion Torrent Genexus, a new automated technology for library preparation and sequencing coupled with analysis software for quick and easy profile generation. Sensitivity down to 100 pg of DNA was shown, while the minor contributor was identified in a 1:4 mixture. |
| Pajnič et al., 2022 [51] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Isometric artefacts were identified from analysis of heat-degraded samples. Additionally, 5.9 artefacts per test were observed, 5.2% with higher coverage than the original allele. These artefacts are stochastic and represent a particular issue with Ion Torrent technology. |
| Fonseca and Fridman, 2022 [52] | Precision ID GlobalFiler NGS STR Panel v2 | Ion GeneStudio S5 System | The kit was used to analyze sequence variation between monozygotic twins for differentiation purposes. Two SNPs were identified in the flanking regions, which highlights the possibility of using this kit to differentiate identical twins. |
| Kocsis, Matrai and Egyed, 2023 [53] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Evaluation of discordances at locus Penta E between the GlobalFiler NGS STR kit, PowerPlex Fusion 6C, and PowerPlex 18D Systems CE kits. Sanger sequencing was performed. |
| Sharma and Wurmbach, 2024 [54] | Precision ID GlobalFiler NGS STR Panel v2 | Ion S5 System | Kit evaluation on Ion S5. Optimal recovery PCR method was used. Full profiles were obtained from 50 pg of DNA, and degraded samples with Degradation Index (DI) > 60. 1:30–1:20 mixtures were identified using read count. High locus imbalance was observed at locus Penta D. Most artefacts were detected at D12S391. |
| Eduardoff et al., 2015 [55] | HID-Ion AmpliSeq Identity Panel v2.2 | Ion Torrent PGM | Evaluation of the Identity kit between three laboratories; 25–100 pg of DNA were found to deliver acceptable results. |
| Gill et al., 2015 [56] | HID-Ion AmpliSeq Identity Panel v2.2 | Ion Torrent PGM | The LRmix program was used for deconvolution of two–three people mixtures. Two people mixtures gave good results, while three people cases had variable success. |
| Guo et al., 2016 [57] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Evaluation of the kit following the Scientific Working Group on DNA Analysis Methods (SWGDAM) guidelines. Data for Han Chinese were obtained. Cross-reactivity with primates was observed. Full deconvolution was obtained with 1:9 mixtures. |
| Ochiai et al., 2016 [58] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Only Y-chromosome SNPs were considered. Data for Japanese and Malay were produced. Missing results and minor misreading were highlighted. |
| Buchard et al., 2016 [59] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | The panel was validated in an accredited laboratory for relationship testing. Thresholds were set and a Python script for analysis was devised. A 1:24 mixture of two people was identified by the script. |
| Kampmann et al., 2016 [60] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Buccal swab samples on FTA cards were successfully processed. |
| Pilli et al., 2016 [61] | HID-Ion AmpliSeq Identity Panel v2.3 | Ion Torrent PGM | Human DNA was extracted and successfully typed from lice for both STRs (AmpFℓSTR NGM Select kit) and SNPs (Identity Panel). More successful results were obtained with NGS SNP testing. |
| Meiklejohn and Robertson, 2017 [62] | Precision ID Identity Panel | Ion Torrent PGM | Evaluation of the kit on Ion PGM. Two types of genotyping analysis software were used, the Ion Torrent HID SNP Genotyper and CLC Genomics Workbench, achieving 100% concordance. |
| van der Heijden et al., 2017 [63] | Precision ID Identity Panel | Ion Torrent PGM and Ion S5 System | Comparison between manual library preparation with Ion PGM sequencing, automated library preparation (Biomek 3000) with Ion PGM sequencing, and automated library preparation (Ion Chef) with Ion S5 sequencing. The Ion Chef/Ion S5 workflow gave the best results but was more expensive. Somali population data were generated. |
| Garcia, Soto and Yurrebaso, 2017 [64] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Forensic evaluation of the kit on Ion PGM. Population data for Basques were obtained. |
| Juras et al., 2017 [65] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Human remains from neolithic graves were processed for kinship analysis. Illumina mtDNA sequencing was also performed. The SNP NGS kit was inefficient with poorly preserved ancient samples and was only useful with well-preserved samples. |
| Apaga et al., 2017 [32] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Comparison of shared markers between ForenSeq kit on MiSeq and Identity Panel on Ion PGM. Different performances were highlighted. Some SNPs with observed discordances require attention. |
| Bleka et al., 2017b [66] | HID-Ion AmpliSeq Identity Panel v2.2 | Ion Torrent PGM | Two–three people mixtures were analyzed to evaluate deconvolution performance of the quantitative model EuroForMix compared to the qualitative model LRmix. The discrimination power appeared improved. |
| Bleka et al., 2017a [67] | HID-Ion AmpliSeq Identity Panel v2.2 | Ion Torrent PGM | Two–three people mixtures were analyzed to evaluate the deconvolution performance of the quantitative model EuroForMix compared to the qualitative model LRmix. The discrimination power appeared improved. |
| Cho et al., 2017 [68] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | DNA was successfully typed from cell-free DNA from pregnant mothers’ serum as a method to perform pre-natal DNA test. |
| Liu et al., 2018 [69] | Precision ID Identity Panel | Ion Torrent PGM | Tibetan, Uyghur, and Hui population data were generated. Y haplogroups were compared with worldwide populations. |
| Li et al., 2018 [70] | HID-Ion AmpliSeq Identity Panel v2.3 | Ion Torrent PGM | Southern Chinese (Han) population data and forensic parameters were evaluated. Poor allelic balance was shown for seven markers, and 0.82% of miscalled reads were found. Y haplogroups were also investigated. |
| Sun et al., 2019 [71] | Precision ID Identity Panel | Ion Torrent PGM | Chinese Han (Hebei province) population data and forensic parameters were evaluated. Y haplogroups were investigated and microhaplotypes identified. |
| Christiansen et al., 2019 [72] | Precision ID Identity Panel | Ion S5 System | Paternal SNPs were successfully typed from cell-free DNA from pregnant mothers’ plasma to perform pre-natal DNA test. False calls were found, indicating the need for at least duplicate testing as these false calls were not reproducible. Paternal SNP dropouts could be reduced by using smaller amplicons. |
| Turchi et al., 2019 [73] | Precision ID Identity Panel | Ion Torrent PGM | The panel was successful with challenging samples and degraded and low-quantity DNA. Informative profiles were obtained from 1.2 to 1 ng of DNA with DI > 9. Most challenging samples could benefit from the generation of consensus profile of two to three replicates, from using <25–26 PCR cycles, and applying 50 reads coverage threshold. |
| Bottino, Silva and Moura-Neto, 2019 [74] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | The article reports forensic evaluation and investigation of Y haplogroups for Brazilians. Only twelve individuals were typed, but they appeared to represent the expectations for the general Brazilian population. |
| Avila et al., 2019b [75] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Forensic evaluation and population data for Brazilians were produced. Y haplogroups were also investigated. The kit appeared suitable for both identity and kinship testing. Comparison with worldwide population was performed. Improvements in inter- and intralocus balance were highlighted as required. |
| Avila et al., 2019a [76] | HID-Ion AmpliSeq Identity Panel | Ion Torrent PGM | Brazilian casework samples were processed with the PowerPlex Fusion STR kit and Identity Panel SNP kit. The statistical strength of NGS results was higher than STRs. Epithelial cells generated lower-quality results. Manual review of NGS data appeared fundamental. |
| Turchi et al., 2020 [77] | Precision ID Identity Panel | Ion Torrent PGM | The kit was successful with challenging samples and degraded and low-quantity DNA. Good results were obtained down to 12 pg of degraded DNA. The most challenging samples could benefit from three replicates testing and 50 reads coverage threshold. |
| Tiedge et al., 2020 [78] | Precision ID Identity Panel | Ion S5 System | PowerPlex Fusion 6C STRs and Identity Panel SNPs were typed from prints developed using the columnar thin-film (CTF) method. No inhibition from CTF was shown for both methods, but more information was obtained from the NGS kit for low-quality/quantity DNA. A maximum of 27 PCR cycles was recommended. |
| Gray et al., 2020 [79] | Precision ID Identity Panel | Ion S5 System | STR (PowerPlex Fusion 6C) and SNP (Identity Panel) profiles were obtained from mosquitos after feeding on blood. All SNPs were obtained after 48 h from feeding (single source) or 24 h (mixtures), while all STRs were obtained after 24 h (single source) or 20 h (mixtures). However, STR was more efficient for mixture interpretation. |
| Sun et al., 2020 [80] | Precision ID Identity Panel | Ion Torrent PGM | Tumor tissue was profiled to identify the body source. More than 99% accuracy was obtained using 69–89 threshold when counting the number of loci with two alleles shared. |
| Chen et al., 2020 [81] | Precision ID Identity Panel | Ion Torrent PGM | A Perl-based pipeline was developed to deconvolute mixtures of two people. The method worked well even when mixed samples were first-degree relatives. The 1:19 mixtures showed poor results compared to 1:4 and 1:9, which were also worse than 1:1.5 and 1:4 results. |
| Tiedge et al., 2021 [82] | Precision ID Identity Panel | Ion S5 System | Fingerprints exposed to different environmental conditions and collected via CTF were typed. All samples yielded complete profiles and no inhibition from CTF was shown; 23 PCR cycles were used with 0.5–1 ng DNA and 29 for DNA input < 0.5 ng. |
| Dash et al., 2022 [83] | Precision ID Identity Panel | Ion GeneStudio S5 System | Central Indian population data were produced. Y-chromosome SNPs were investigated and interpopulation comparison was performed. |
| Yin, Zhang and Xing, 2022 [84] | Precision ID Identity Panel | HID Ion GeneStudio S5 System | R algorithm was devised and tested for mixture deconvolution. The deconvolution accuracy was high for balanced mixture ratios or unbalanced with known minor contributor, and the ratio was estimated correctly from 1:1 to 1:6 ranges. |
| Yang, Lee and Lee, 2023 [85] | Precision ID Identity Panel | HID Ion S5/Ion GeneStudio S5 | Korean population data were produced and compared with other populations. Visual SNP caller was used to obtain microhaplotypes. The use of 16 microhaplotypes improved the discrimination power. Y-haplogroups were investigated. Population databases for the use of this kit for forensic application are still lacking. |
| Joo et al., 2023 [86] | Precision ID Identity Panel | Illumina MiSeq System | Myanmar population data were produced and compared with other populations. The kit was used on the MiSeq instrument. Microhaplotypes were found using Visual SNP caller. |
| Li et al., 2023 [87] | Precision ID Identity Panel | Ion S5 XL System | ForenSeq DNA Signature Prep Kit on MiSeq FGx, Precision ID Identity Panel on Ion S5 XL, and MGIEasy Signature Identification Library Prep Kit on MGISEQ-2000 were sequenced and compared. All performed differently, with no platform performing consistently better for all parameters considered. Six SNPs had discordant calls. |
| Pajnič, Leskovar and Cresnar, 2023 [88] | Precision ID Identity Panel | Ion GeneStudio S5 System | The Identity Panel was used together with the Investigator EssplexPlus SE QS kit for full siblings’ prediction from Early Middle Ages skeletons. The prediction was inconclusive with STRs only but successful with SNPs. |
| Fattorini et al., 2023 [89] | Precision ID Identity Panel | Ion S5 System | World War 2 bone remains delivering no results on STR testing were processed with the Identity Panel. The average DNA quantity was 6.8 pg; 93.8% of libraries produced results for 63/90 autosomal markers; 40% of results did not match the donor or were mixed profiles, possibly due to contamination. |
| Kiesler, Gettings and Vallone, 2015 [90] | HID-Ion AmpliSeq Identity Panel v4.0 and Ancestry Panel v4.0 | Ion Torrent PGM | Evaluation of Identity and Ancestry Panels on NIST samples for the establishment of reference material. Some markers (0.25% for Identity and 2.02% for Ancestry Panel) showed coverage below recommended values of 300 X. Some markers in both panels showed allelic imbalance and strand bias (ratio of positive strand reads to negative strand reads). Discordant replicates were observed on two occasions in the Ancestry Panel. |
| Tasker et al., 2017 [91] | HID-Ion AmpliSeq Identity Panel and Ancestry Panel | Ion Torrent PGM | DNA was typed from touch/blood samples recovered from bomb fragments for ancestry inference. The InnoTyper 21 Kit for insertions/null markers was also used and appeared good for challenging samples. The success of the SNP NGS kits was variable. |
| Scheible et al., 2021 [92] | Precision ID Ancestry and Identity Panels | Illumina MiniSeq System | Workflow for Ancestry and Identity Panels was tested on Illumina MiniSeq; 93.9% of SNPs were successfully genotyped from positive controls, buccal swabs, and dust samples. |
| Meiklejohn et al., 2023 [93] | Precision ID Ancestry and Identity Panels | Illumina MiniSeq System | SNPs from dust samples were typed on the MiniSeq instrument. The FastID software was used for mixture deconvolution; 72% of the alleles were recovered, and 93% of known occupants were detected in at least one sample, while 54% had non-occupant alleles. |
| Themudo et al., 2016 [94] | HID-Ion AmpliSeq Ancestry Panel | Ion Torrent PGM | Ancestry Panel profiles were obtained from Greenlanders. The training set was used as reference for subsequent, more successful assignment of ancestry. |
| Garcia et al., 2017 [95] | Precision ID Ancestry Panel | Ion Torrent PGM | Forensic evaluation of the Ancestry Panel on Ion PGM. Population data for Basques were obtained and used for ancestry inference, which showed clustering with Europeans. |
| Truelsen et al., 2017 [96] | Precision ID Ancestry Panel | Ion Torrent PGM | Middle East populations (Turks and Iranians) were analyzed to investigate genetic differentiation using GenoGeographer program. Differentiation was not possible. |
| Pereira et al., 2017 [97] | Precision ID Ancestry Panel | Ion Torrent PGM | Evaluation of the panel with casework samples (blood and buccal swabs on FTA cards) from Somalia and Denmark was reported. Some markers consistently performed poorly. |
| Santangelo et al., 2017 [98] | Precision ID Ancestry Panel | Ion Torrent PGM | Ancestry and admixture investigation of three Ecuadorians ethnic groups (Kichwa, Mestizo, and Afro-Ecuadorian) was reported. |
| Hollard et al., 2017 [99] | HID-Ion AmpliSeq Ancestry Panel | Ion Torrent PGM | Ancestry kit, Yfiler Plus, mtDNA HV1 sequencing, and Irisplex SNPs were used to analyze ancestry and phenotype from a carbonized corpse that failed CE STR testing. A combined approach appeared preferable. |
| Wang et al., 2018 [100] | Precision ID Ancestry Panel | Ion Torrent PGM | Forensic evaluation, population data, and ancestry inference for Chinese Tibeto-Burman were reported. The panel performed well and was suitable for both ancestry and identification. |
| Nakanishi et al., 2018 [101] | Precision ID Ancestry Panel | Ion Torrent PGM | Japanese populations from mainland and Okinawa were analyzed to evaluate the possibility to distinguish them via ancestry investigation. Differentiation was not possible. |
| Jin et al., 2018 [102] | Precision ID Ancestry Panel | Ion S5 System | Development of guidelines for the implementation of a biogeographic ancestry inference service based on Admixture Prediction produced in the Ion Torrent Suite for Precision ID Ancestry Panel. The panel appeared effective for ancestry prediction. |
| He et al., 2018 [103] | Precision ID Ancestry Panel | Ion Torrent PGM | Chinese populations (Uyghur and Hui) were analyzed to investigate population data and ancestry. Individuals could be differentiated both by ancestry and individually. A limitation was observed in distinguishing homogeneous populations. |
| Al-Asfi et al., 2018 [104] | Precision ID Ancestry Panel | Ion Torrent PGM | Evaluation of the kit on Ion PGM. Ancestry prediction was accurate at 125 pg and 30 pg using 21 and 25 PCR cycles, respectively. Partial profiles (85%) were obtained from 15 pg of DNA, while <6 pg produced less than 50% concordance; 1 ng was suggested as minimum input for high-confidence ancestry assignment. |
| Lee et al., 2018 [105] | Precision ID Ancestry Panel | Ion S5 XL System | Seven Asian populations (Southern Chinese, Beijing Chinese, Japanese, Koreans, Vietnamese, Nepalese, Indians, and Pakistani) were profiled, and ancestry was investigated. All Northeast (China, Japan, and Korea) and Southeast Asians (Vietnamese) were predicted as East Asians, while Southwest Asians (Nepal, India, and Pakistan) were predominantly assigned to South Asia. |
| Young et al., 2019 [106] | Precision ID Ancestry Panel | Ion GeneStudio S5 System | Ancestry Panel, 24 SNP HIrisplex System, and QIAGEN 140-SNP forensic identification multiplex kits were used on different touched items. Accurate calls were obtained from 70% of samples for all three panels combined. |
| Daniels-Higginbotham et al., 2019 [107] | Precision ID Ancestry Panel | Illumina MiSeq FGx System | 19th century skeletal remains were sequenced using MiSeq FGx to identify ancestry. AmpFℓSTR Yfiler PCR Amplification Kit and Y-typing of four important SNP variants were also used to upload results on the FamilyTreeDNA website. |
| Shan et al., 2019 [108] | Precision ID Ancestry Panel | N/A | Ancestry investigation from Punjabi Pakistani samples was reported. The GenoGeographer software was used. The population appeared admixed and non-distinguishable from South Central Asia and Middle East populations. |
| Al-Dosari et al., 2019 [109] | Precision ID Ancestry Panel | Ion Torrent PGM | Comparison between manual and automated library preparation to type casework samples. No significant differences were found, but the automated method was faster and avoided pipetting errors. A full profile was obtained from 0.12 ng of DNA. |
| Mogensen et al., 2020 [110] | Precision ID Ancestry Panel | N/A | The GenoGeographer software was assessed for ancestry assignment using publicly available data from multiple populations. Ancestry was not assigned to 22.4% of the individuals. Of the assigned individuals, 8.2% had discordant assignment, and 8.2% had ambiguous assignment. Prediction would be improved with more SNPs and more data in databases. |
| Cooley et al., 2021 [111] | Precision ID Ancestry Panel | Ion S5 System and Ion Torrent PGM | Comparison of Ion OneTouch 2/Ion PGM and Ion Chef/Ion S5 systems using forensic-type samples. The Ion Chef/Ion S5 method was faster and generated higher coverage and SNP quality, even if both systems predicted concordant ancestries. |
| Truelsen et al., 2021 [112] | Precision ID Ancestry Panel | Ion S5 System | Samples from 14 European, Middle Eastern, African, and Asian countries were sequenced with the Ancestry and EUROFORGEN panels to perform ancestry prediction with GenoGeographer. The Ancestry Panel was sufficient to distinguish North Africans from European and Middle Eastern, with improved separation when combining the two panels. Separation of Middle Eastern from Europeans and South-Central Asians was difficult even with both kits combined. |
| He et al., 2021 [113] | Precision ID Ancestry Panel | Ion S5 XL System | Population genetics, admixture, ancestry, and forensic parameters were evaluated in Southern Chinese Sinitic/Tai-Kadai individuals. |
| Shan et al., 2021 [114] | Precision ID Ancestry Panel | Ion S5 System | Pakistani individuals (Baloch, Pashtun, and Punjabi subpopulations) samples were processed with the Ancestry Panel and HuPi AmpliSeq Custom panel to evaluate both ancestry and skin pigmentation. Significantly different genetic distance was observed between the subgroups. Skin differentiation was also significantly different for one group compared to the other two. |
| Young et al., 2021 [115] | Precision ID Ancestry Panel | Ion GeneStudio S5 Plus System | The Ancestry Panel and HIrisplex System (Ion AmpliSeq HID Phenotyping Community Panel) were used for ancestry evaluation and skin pigmentation prediction applied to touch DNA samples with direct PCR. 90% of samples generated correct ancestry assignment at the major population level. Full SNP profiles were obtained from 23% of touch samples and partial profiles (>85%) from 32%. Moreover, 42% of samples obtained high confidence ancestry assignments. Correct ancestry was assigned when >70% SNPs were detected. |
| Mogensen et al., 2022 [116] | Precision ID Ancestry Panel | N/A | Data for Slovenia, Greece, Albania, and Eritrea were obtained and added to GenoGeographer as a reference population. The Admixture Module was tested on 3548 profiles; 95.5% were assigned to one or more groups (95.4% concordant), while 4.5% were not assigned. An additional 1486 profiles expected to belong to populations other than the reference were also tested; 70% of North and South America samples were rejected, while only 20% of Central, North, and Northeast Asia samples were rejected. The rejection rate was decreased by using the Admixture Module. |
| Cui et al., 2023 [117] | Precision ID Ancestry Panel | Ion S5 XL System | Gannan Tibetan population samples were analyzed, and forensic parameters and ancestry were evaluated. The kit appeared useful for ancestry prediction for continental populations but less accurate for subpopulations. Individual identification appeared suitable. |
| Felkl et al., 2023 [118] | HID-Ion AmpliSeq Ancestry Panel | Ion Torrent PGM | Forensic evaluation and ancestry prediction of South Brazilian heterogeneous population samples were performed. Use for individual identification and kinship appeared suitable. Ancestry analysis showed admixture and more homogeneous groups among the subtypes. |
| Koksal et al., 2023 [119] | Precision ID Ancestry Panel | Ion S5 System | Ancestry and admixture investigation of Brazilians using GenoGeographer was reported. Performance was low on the heterogeneous Brazilian population; 55% of assignments failed. Likelihood Ratio (LR) < 1000 was observed as a possible indicator of admixture. Higher Asian and African genetic contributions were observed in failed samples. |
| Huang et al., 2018 [120] | N/A | N/A | A panel of 117 universal SNPs with Minor Allele Frequency (MAF) > 0.39 in 37 populations was defined for identity testing. Combined Match Probability (CMP) was lower for this set rather than the HID-Ion AmpliSeq Identity Panel for the Chinese Han population. |
| Ragazzo et al., 2021 [121] | N/A | N/A | Evaluation of a custom OpenArray panel for phenotype prediction and identification including 60 SNPs from HIrisplex-s, Precision ID Identity SNP Panel, and ForenSeq DNA Signature Prep Kit. |
| Resutik et al., 2023 [122] | N/A | N/A | Ancestry prediction performance of MAPlex, Precision ID Ancestry Panel, and VISAGE Basic Tool panels was compared using publicly available datasets. Similar results were produced from all panels when using six broad continental regions on STRUCTURE. The most consistent performance in all regions was given by the VISAGE panel. |
| Continent | Country | Population | Publication/s |
|---|---|---|---|
| Asia | Afghanistan | Afghani | Truelsen et al., 2021 [112] |
| China | Beijing Chinese | Lee et al., 2018 [105] | |
| Chinese Han | Wang et al., 2017 [35], Guo et al., 2016 [57], Sun et al., 2019 [71], Wang et al., 2020 [45] | ||
| Chinese Hui | He et al., 2018 [103], Liu et al., 2018 [69] | ||
| Chinese Gannan Tibetan | Cui et al., 2023 [117] | ||
| Chinese Tibetans | Wang et al., 2020 [45], Liu et al., 2018 [69] | ||
| Chinese Tibeto-Burman | Wang et al., 2018 [100] | ||
| Chinese Uyghur | He et al., 2018 [103], Tao et al., 2019 [37], Liu et al., 2018 [69] | ||
| Southern Chinese | Li et al., 2018 [70], Lee et al., 2018 [105] | ||
| Southern Chinese Sinitic/Tai-Kadai | He et al., 2021 [113] | ||
| India | Central Indians | Dash et al., 2021 [47], Dash et al., 2022 [83] | |
| Indians | Lee et al., 2018 [105] | ||
| Iran | Iranians | Truelsen et al., 2017 [96], Truelsen et al., 2021 [112] | |
| Iraq | Iraqi | Truelsen et al., 2021 [112] | |
| Japan | Japanese | Kitayama et al., 2022 [48], Ohuchi et al., 2022 [49], Nakanishi et al., 2018 [101], Ochiai et al., 2016 [58], Lee et al., 2018 [105] | |
| Korea | Koreans | Yang, Lee and Lee, 2023 [85], Lee et al., 2018 [105] | |
| Malaysia | Malay | Ochiai et al., 2016 [58] | |
| Myanmar | Burmese | Joo et al., 2023 [86] | |
| Nepal | Nepalese | Lee et al., 2018 [105] | |
| Pakistan | Pakistani | Lee et al., 2018 [105], Truelsen et al., 2021 [112] | |
| Pakistani Baloch | Shan et al., 2021 [114] | ||
| Pakistani Pashtun | Shan et al., 2021 [114] | ||
| Pakistani Punjabi | Shan et al., 2019 [108], Shan et al., 2021 [114] | ||
| Syria | Syrians | Truelsen et al., 2021 [112] | |
| Turkey | Turks | Truelsen et al., 2017 [96], Truelsen et al., 2021 [112] | |
| Vietnam | Vietnamese | Lee et al., 2018 [105] | |
| Europe | Albania | Albanians | Truelsen et al., 2021 [112], Mogensen et al., 2022 [116] |
| Denmark | Danes | Truelsen et al., 2021 [112], Buchard et al., 2016 [59], Pereira et al., 2017 [97] | |
| Greece | Greeks | Truelsen et al., 2021 [112], Mogensen et al., 2022 [116] | |
| Portugal | Portuguese | Truelsen et al., 2021 [112] | |
| Slovenia | Slovenians | Truelsen et al., 2021 [112], Mogensen et al., 2022 [116] | |
| Spain | Basques | Garcia, Soto and Yurrebaso, 2017 [64], Garcia et al., 2017 [95] | |
| Spanish | Barrio et al., 2019 [43] | ||
| Africa | Eritrea | Eritreans | Truelsen et al., 2021 [112], Mogensen et al., 2022 [116] |
| Morocco | Moroccans | Truelsen et al., 2021 [112] | |
| Somalia | Somali | Truelsen et al., 2021 [112], Pereira et al., 2017 [97], van der Heijden et al., 2017 [63] | |
| South America | Brazil | Brazilians | Bottino, Silva and Moura-Neto, 2019 [74], Avila et al., 2019b [75], Koksal et al., 2023 [119] |
| South Brazilians | Felkl et al., 2023 [118] | ||
| Ecuador | Ecuadorians | Santangelo et al., 2017 [98] | |
| North America | Greenland | Greenlanders | Themudo et al., 2016 [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroza Matute, S.; Iyavoo, S. Applications and Performance of Precision ID GlobalFiler NGS STR, Identity, and Ancestry Panels in Forensic Genetics. Genes 2024, 15, 1133. https://doi.org/10.3390/genes15091133
Pedroza Matute S, Iyavoo S. Applications and Performance of Precision ID GlobalFiler NGS STR, Identity, and Ancestry Panels in Forensic Genetics. Genes. 2024; 15(9):1133. https://doi.org/10.3390/genes15091133
Chicago/Turabian StylePedroza Matute, Sharlize, and Sasitaran Iyavoo. 2024. "Applications and Performance of Precision ID GlobalFiler NGS STR, Identity, and Ancestry Panels in Forensic Genetics" Genes 15, no. 9: 1133. https://doi.org/10.3390/genes15091133







