Molecular Characterizations of FAM13A and Its Functional Role in Inhibiting the Differentiation of Goat Intramuscular Adipocytes through RIG-I Receptor Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Cell Culture
2.2. CDS and 3′UTR Amplification of FAM13A, and Transfection
2.3. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
2.4. Preparation of RNAseq Samples
2.5. QC, GO, KEGG Assay Analysis and qRT-PCR Validation
2.6. MTT Measurement
2.7. Oil Red O Staining
2.8. Luciferase Reporter Assay
2.9. Statistical Analysis
3. Results
3.1. Cloning and Bioinformatics Analysis of the FAM13A Gene in Goats
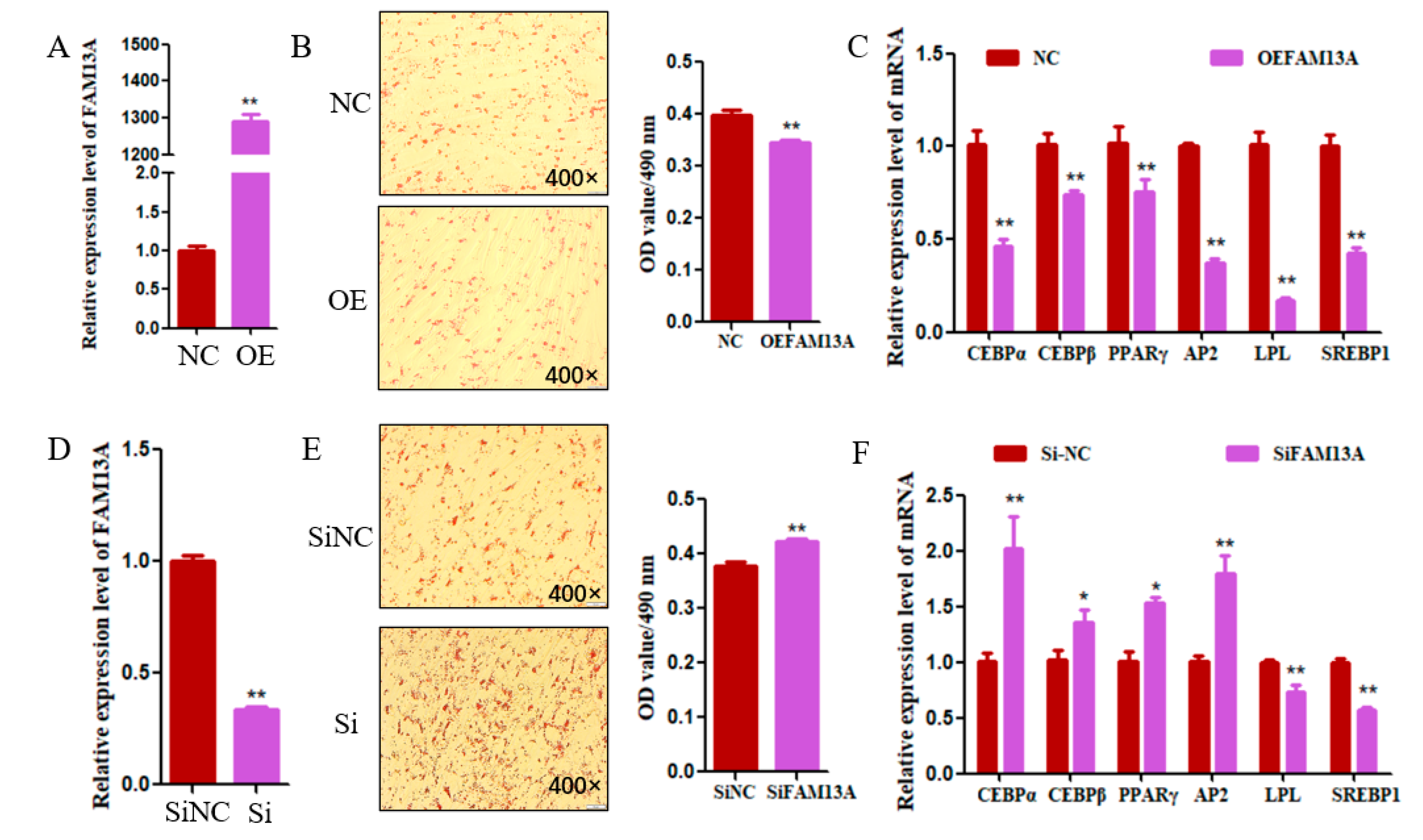
3.2. Goat FAM13A Inhibits Intramuscular Adipocyte Differentiation
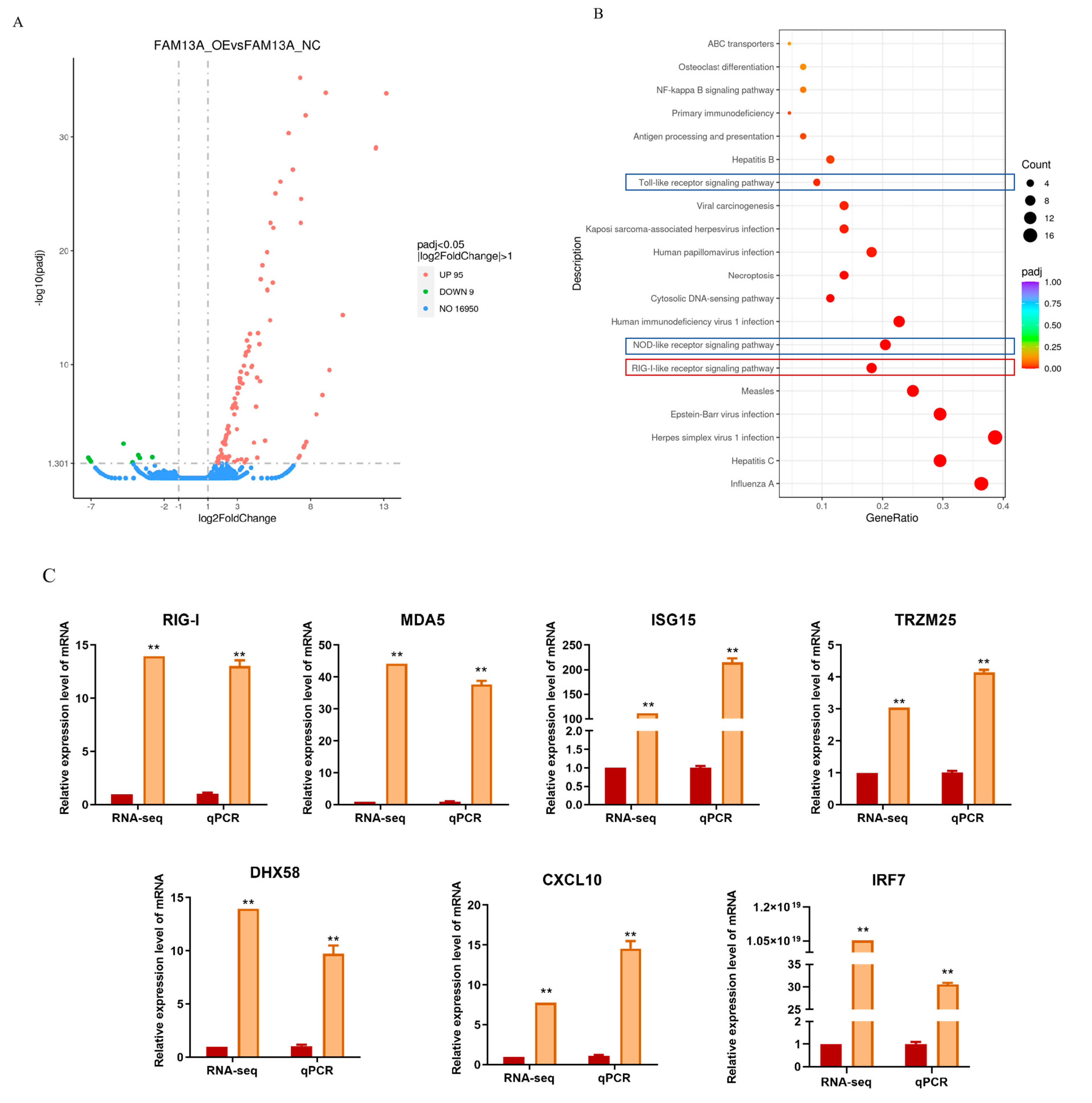
3.3. Overexpression of FAM13A Affects mRNA Transcriptional Profiles in Goat Adipocytes
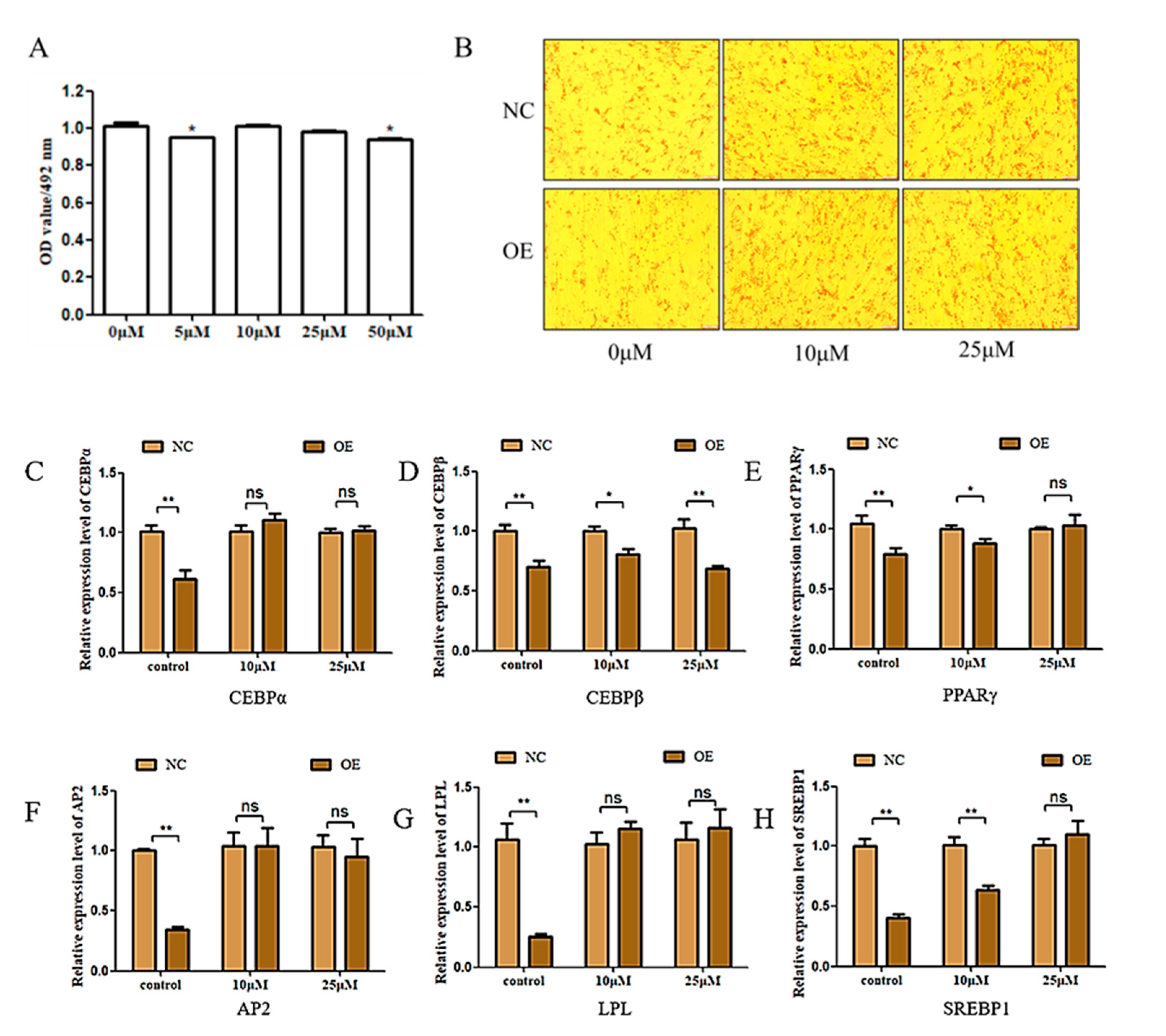
3.4. Overexpression of FAM13A Inhibits Goat Intramuscular Adipocyte Differentiation via the RIG-I-like Receptor Signaling Pathway
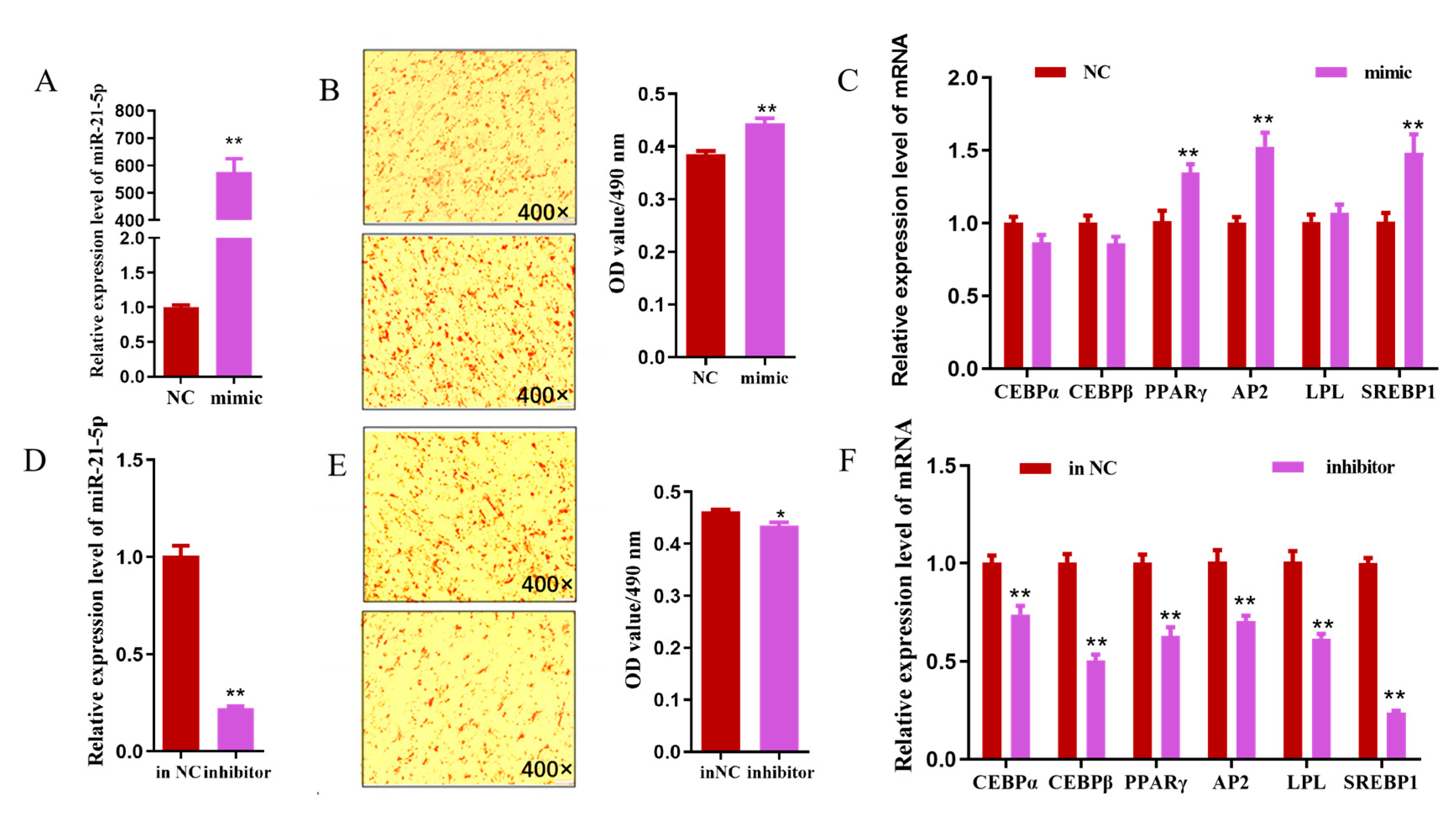
3.5. miR-21-5p Negatively Regulates the Expression of FAM13A
3.6. miR-21-5p Promotes the Differentiation of Goat Intramuscular Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, C.; Wei, T.; Liu, L.X.; Liu, J.Q.; Wang, C.X.; Yuan, Z.Y.; Ma, H.H.; Jin, H.G.; Zhang, L.C.; Cao, Y. Whole-Transcriptome Analysis of Preadipocyte and Adipocyte and Construction of Regulatory Networks to Investigate Lipid Metabolism in Sheep. Front. Genet. 2021, 12, 662143. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Hegarty, R.S.; Walker, P.J.; Pethick, D.W. Relationship between animal age, intramuscular fat, cooking loss, pH, shear force and eating quality of aged meat from sheep. Anim. Prod. Sci. 2006, 46, 879–884. [Google Scholar] [CrossRef]
- Pannier, L.; Gardner, G.E.; O’Reilly, R.A.; Pethick, D.W. Factors affecting lamb eating quality and the potential for their integration into an MSA sheepmeat grading model. Meat Sci. 2018, 144, 43–52. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Jeon, M.; Kim, Y.S. Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/β-catenin signaling. Biofactors 2016, 42, 49–59. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr. Metab. 2007, 4, 22. [Google Scholar] [CrossRef]
- Cohen, M.; Reichenstein, M.; Everts-van der Wind, A.; Heon-Lee, J.; Shani, M.; Lewin, H.A.; Weller, J.I.; Ron, M.; Seroussi, E. Cloning and characterization of FAM13A1—A gene near a milk protein QTL on BTA6: Evidence for population-wide linkage disequilibrium in Israeli Holsteins. Genomics 2004, 84, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.J.; Guo, F.; Qiao, D.; Du, F.; Naing, Z.Z.C.; Li, Y.; Pham, B.; Mikkelsen, T.S.; Cho, M.H.; Silverman, E.K.; et al. Identification of Functional Variants in the FAM13A Chronic Obstructive Pulmonary Disease Genome-Wide Association Study Locus by Massively Parallel Reporter Assays. Am. J. Respir. Crit. Care Med. 2019, 199, 52–61. [Google Scholar] [CrossRef]
- Corvol, H.; Hodges, C.A.; Drumm, M.L.; Guillot, L. Moving beyond genetics: Is FAM13A a major biological contributor in lung physiology and chronic lung diseases? J. Med. Genet. 2014, 51, 646–649. [Google Scholar] [CrossRef]
- Ziółkowska-Suchanek, I.; Mosor, M.; Podralska, M.; Iżykowska, K.; Gabryel, P.; Dyszkiewicz, W.; Słomski, R.; Nowak, J. FAM13A as a Novel Hypoxia-Induced Gene in Non-Small Cell Lung Cancer. J. Cancer 2017, 8, 3933–3938. [Google Scholar] [CrossRef] [PubMed]
- Lundbäck, V.; Kulyte, A.; Strawbridge, R.J.; Ryden, M.; Arner, P.; Marcus, C.; Dahlman, I. FAM13A and POM121C are candidate genes for fasting insulin: Functional follow-up analysis of a genome-wide association study. Diabetologia 2018, 61, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhang, Y.; Ding, R.; Wang, X.; Zhang, S.; Li, X. miR-30a-5p induces the adipogenic differentiation of bone marrow mesenchymal stem cells by targeting FAM13A/Wnt/β-catenin signaling in aplastic anemia. Mol. Med. Rep. 2022, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, G.; Raza, S.H.A.; Wang, X.; Li, B.; Zhang, W.; Zan, L. FAM13A promotes proliferation of bovine preadipocytes by targeting Hypoxia-Inducible factor-1 signaling pathway. Adipocyte 2021, 10, 546–557. [Google Scholar] [CrossRef]
- Lin, X.; Liou, Y.H.; Li, Y.; Gong, L.; Li, Y.; Hao, Y.; Pham, B.; Xu, S.; Jiang, Z.; Li, L.; et al. FAM13A Represses AMPK Activity and Regulates Hepatic Glucose and Lipid Metabolism. iScience 2020, 23, 100928. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, S.; Wang, Y.; Zhu, J.; Lin, Y. Fibroblast growth factor 10 (FGF10) promotes the adipogenesis of intramuscular preadipocytes in goat. Mol. Biol. Rep. 2018, 45, 1881–1888. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Zhu, J.; Huang, K.; Wang, Y. MiR-26b-5p regulates the preadipocyte differentiation by targeting FGF21 in goats. Vitr. Cell Dev. Biol. Anim. 2021, 57, 257–263. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef]
- Engin, A. Fat Cell and Fatty Acid Turnover in Obesity. Adv. Exp. Med. Biol. 2017, 960, 135–160. [Google Scholar] [CrossRef]
- Ma, L.; Shi, W.; Ma, X.; Zou, M.; Chen, W.; Li, W.; Zou, R.; Chen, X. Comprehensive analysis of differential immunocyte infiltration and the potential ceRNA networks during epicardial adipose tissue development in congenital heart disease. J. Transl. Med. 2020, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, S.H.; Kim, M.; Moon, J.S.; Kim, G.W.; Jung, H.G.; Kim, I.H.; Oh, J.E.; Jung, H.E.; Lee, H.K.; et al. Vibrio vulnificus quorum-sensing molecule cyclo(Phe-Pro) inhibits RIG-I-mediated antiviral innate immunity. Nat. Commun. 2018, 9, 1606. [Google Scholar] [CrossRef]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Schlee, M. Master sensors of pathogenic RNA–RIG-I like receptors. Immunobiology 2013, 218, 1322–1335. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, H.E.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. RIG-I Deficiency Promotes Obesity-Induced Insulin Resistance. Pharmaceuticals 2021, 14, 1178. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yan, K.; Liu, P.; Li, N.; Liu, Z.; Zhu, W.; Chen, Y.; Han, D. Pattern recognition receptor-initiated innate antiviral response in mouse adipose cells. Immunol. Cell Biol. 2014, 92, 105–115. [Google Scholar] [CrossRef]
- Gack, M.U.; Kirchhofer, A.; Shin, Y.C.; Inn, K.S.; Liang, C.; Cui, S.; Myong, S.; Ha, T.; Hopfner, K.P.; Jung, J.U. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc. Natl. Acad. Sci. USA 2008, 105, 16743–16748. [Google Scholar] [CrossRef]
- Imaizumi, T.; Shimada, T.; Matsumiya, T.; Yoshida, H.; Watanabe, S.; Tsuruga, K.; Kawaguchi, S.; Murakami, M.; Joh, K.; Tanaka, H. Interferon-Stimulated Gene 15, a Type I Interferon-Dependent Transcript, Is Involved in a Negative Feedback Loop in Innate Immune Reactions in Human Mesangial Cells. Nephron 2016, 132, 144–152. [Google Scholar] [CrossRef]
- Karin, N.; Razon, H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, F.; Zhang, Y.; Zhang, X.; Chen, J.; Jiang, Y. PPARγ attenuates hepatic inflammation and oxidative stress of non-alcoholic steatohepatitis via modulating the miR-21-5p/SFRP5 pathway. Mol. Med. Rep. 2021, 24, 823. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem. Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lin, Y.; Zhao, N.; Wang, Y.; Li, Y. Hoxa5 Inhibits the Proliferation and Induces Adipogenic Differentiation of Subcutaneous Preadipocytes in Goats. Animals 2022, 12, 1859. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequences (5′-3′) |
|---|---|
| U6 | S: TGGAACGCTTCACGAATTTGCG A: GGAACGATACAGAGAAGATTAGC |
| C/EBPα | S: CCGTGGACAAGAACAGCAAC A: AGGCGGTCATTGTCACTGGT |
| C/EBPβ | S: CAAGAAGACGGTGGACAAGC A: AACAAGTTCCGCAGGGTG |
| PPARγ | S: AAGCGTCAGGGTTCCACTATG A: GAACCTGATGGCGTTATGAGAC |
| AP2 | S: TGAAGTCACTCCAGATGACAGG A: TGACACATTCCAGCACCAGC |
| LPL | S: TCCTGGAGTGACGGAATCTGT A: GACAGCCAGTCCACCACGAT |
| SREBP1 | S: AAGTGGTGGGCCTCTCTGA A: GCAGGGGTTTCTCGGACT |
| FAM13A | S: AACTGATGTCGGATGGAAGCAA A: CACGAGGAGAAGGCAGGGATAG |
| UXT | S: GCAAGTGGATTTGGGCTGTAAC A: ATGGAGTCCTTGGTGAGGTTGT |
| ISG15 | S: ATCAATGTGCCTGCTTTCCA A: GGGCTTCCCTTCAAAAGACAG |
| RIG-I | S: GAAGAACTTGGGACCGTAACTC A: CACGCTTTCTGAACTGCGATAA |
| MDA5 | S:GGAGATGACAGTGATGAGAGTGATG A: ATTATTCCTCGTGCTGACCCTTC |
| CXCL10 | S: TCTGCCTTATCCTTCTGACTCTGA A: TTATGCCTCTTTCCGTGTTCG |
| TRIM25 | S: GCCTGTGAAGAAGGTTGTGAAAG A: ACCTTGGCGTTGAGAGATGC |
| LGP2 | S: AGAGGCATTTAGAGACCGTGG A: CGCTCAGGGTTGTGATGGT |
| IRF7 | S: TGACACGCCCATCTTTGACT A: GCCCAGGTAGATGGTGTAGTG |
| FAM13A-3′UTR | S: TCAAGGTTTACGCCAGACTA A: TGGTTGCCAAGAGAAGGTGT |
| FAM13A | S: GAAGAAACAGTTGTGAGGGTC A: ATCCTCTGTAGATGAGTGCGT |
| NC | 5′ UUCUCCGAACGUGUCACGUTT 3′ 5′ ACGUGACACGUUCGGAGAATT 3′ |
| Inhibitor NC | 5′ CAGUACUUUUGUGUAGUACAA 3′ |
| miR-21-5p mimic | 5′ UAGCUUAUCAGACUGAUGUUGAC 3′ 5′ CAACAUCAGUCUGAUAAGCUAUU 3′ |
| miR-21-5p inhibitor | 5′ GUCAACAUCAGUCUGAUAAGCUA 3′ |
| si-NC | 5′ UUCUCCGAACGUGUCACGUTT 3′ 5′ ACGUGACACGUUCGGAGAATT 3′ |
| FAM13A siRNA | 5′ CCGAGUAUAAGCACAUUAATT 3′ 5′ UUAAUGUGCUUAUACUCGGTT 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ran, L.; Li, Y.; Wang, Y.; Xiong, Y.; Wang, Y.; Xing, J.; Lin, Y. Molecular Characterizations of FAM13A and Its Functional Role in Inhibiting the Differentiation of Goat Intramuscular Adipocytes through RIG-I Receptor Signaling Pathway. Genes 2024, 15, 1143. https://doi.org/10.3390/genes15091143
Li X, Ran L, Li Y, Wang Y, Xiong Y, Wang Y, Xing J, Lin Y. Molecular Characterizations of FAM13A and Its Functional Role in Inhibiting the Differentiation of Goat Intramuscular Adipocytes through RIG-I Receptor Signaling Pathway. Genes. 2024; 15(9):1143. https://doi.org/10.3390/genes15091143
Chicago/Turabian StyleLi, Xuening, Li Ran, Yanyan Li, Yong Wang, Yan Xiong, Youli Wang, Jiani Xing, and Yaqiu Lin. 2024. "Molecular Characterizations of FAM13A and Its Functional Role in Inhibiting the Differentiation of Goat Intramuscular Adipocytes through RIG-I Receptor Signaling Pathway" Genes 15, no. 9: 1143. https://doi.org/10.3390/genes15091143
APA StyleLi, X., Ran, L., Li, Y., Wang, Y., Xiong, Y., Wang, Y., Xing, J., & Lin, Y. (2024). Molecular Characterizations of FAM13A and Its Functional Role in Inhibiting the Differentiation of Goat Intramuscular Adipocytes through RIG-I Receptor Signaling Pathway. Genes, 15(9), 1143. https://doi.org/10.3390/genes15091143






