Histone Arginine Methylation as a Regulator of Gene Expression in the Dehydrating African Clawed Frog (Xenopus laevis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatments and Tissues
2.2. Total Protein Extraction
2.3. Electrophoresis
2.4. Immunoblotting
2.5. Data Quantification
3. Results
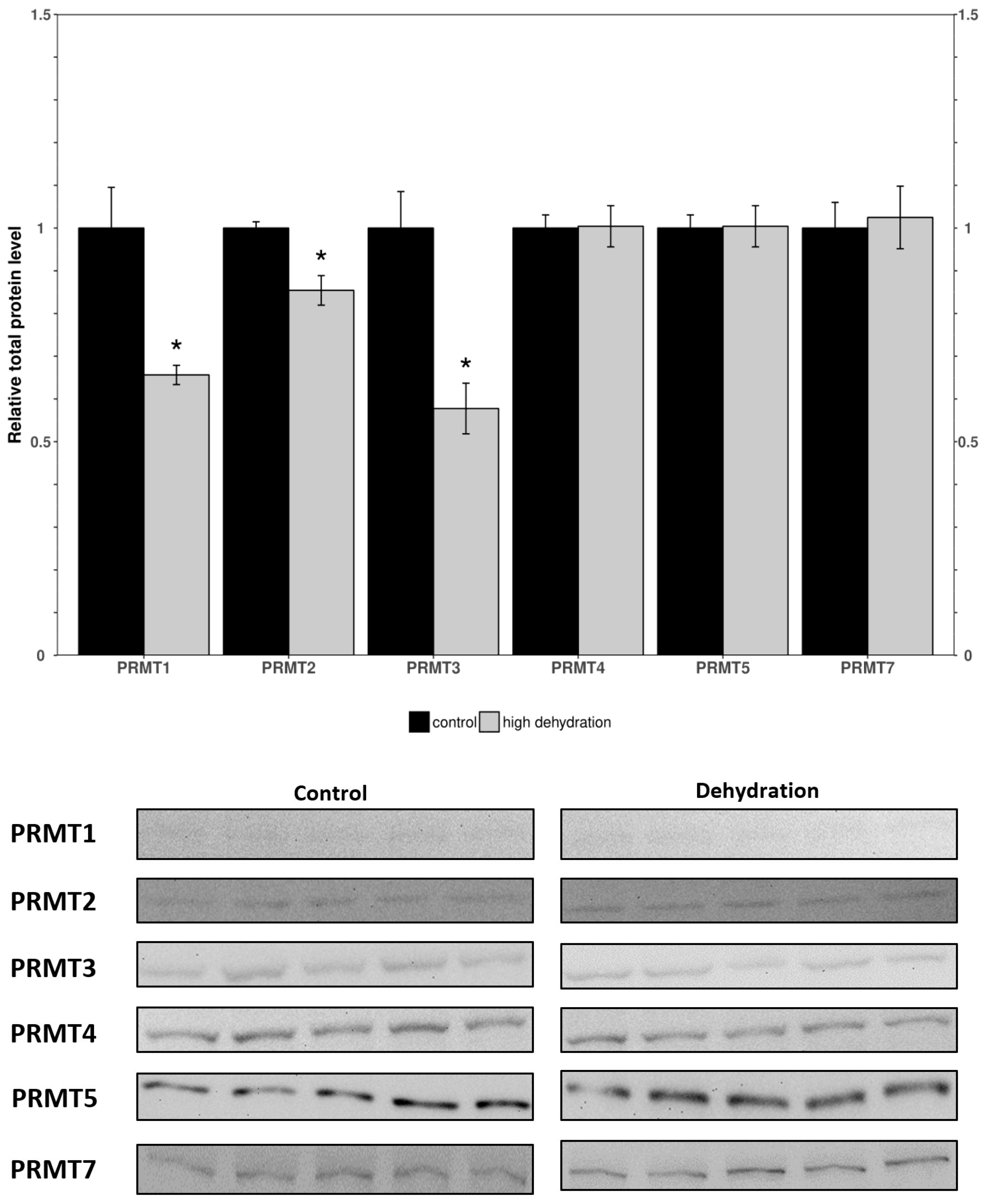
3.1. Protein Arginine Methyl Transferases
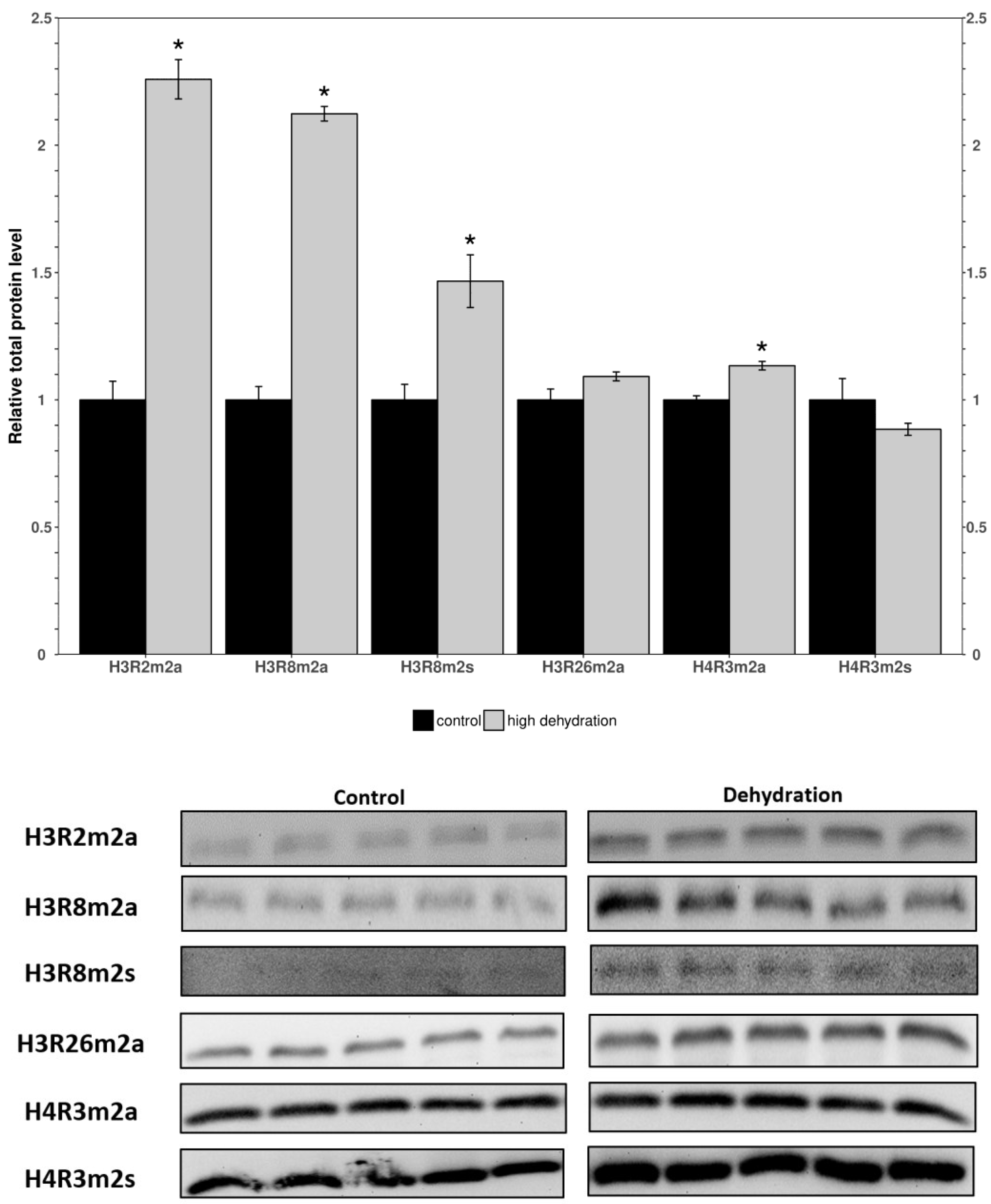
3.2. Methylated Histone Residues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Antibody | Company | Catalogue # |
|---|---|---|
| PRMT1 | Genetex | GTX128211 |
| PRMT2 | Santa Cruz | sc-393254 |
| PRMT3 | Genetex | GTX116478 |
| PRMT4 | Active Motif | AB_2793205 |
| PRMT5 | Abclonal | A2290 |
| PRMT6 | Abclonal | A7814 |
| PRMT7 | Abclonal | A12159 |
| PRMT8 | Novus | NBP1-55401 |
| H3R2m2a | MyBioSource | MBS9402172 |
| H3R8m2s | MyBioSource | MB59607605 |
| H3R8m2a | MyBioSource | MBS9409769 |
| H3R26m2a | Abclonal | A2375 |
| H4R3m2a | Abclonal | A2376 |
| H4R3m2s | MyBioSource | MBS126223 |
References
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2009, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Luu, B.E.; Zhang, Y.; Storey, K.B. The regulation of Akt and FoxO transcription factors during dehydration in the African clawed frog (Xenopus laevis). Cell Stress. Chaperones 2020, 6, 887–897. [Google Scholar] [CrossRef]
- Hillman, S.S. Some effects of dehydration on internal distributions of water and solutes in Xenopus laevis. Comp. Biochem. Physiol. Part A Physiol. 1978, 61, 303–307. [Google Scholar] [CrossRef]
- Loveridge, J.P. Strategies of Water Conservation in Southern African Frogs. Zool. Afr. 1976, 11, 319–333. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Aestivation: Signaling and hypometabolism. J. Exp. Biol. 2012, 215, 1425–1433. [Google Scholar] [CrossRef]
- Balinsky, J.B.; Choritz, E.L.; Coe, C.G.L.; Van Der Schans, G.S. Amino Acid Metabolism and Urea Synthesis in naturally aestivating Xenopus laevis. Comp. Biochem. Physiol. 1967, 22, 59–68. [Google Scholar] [CrossRef]
- Hillman, S.S. The roles of oxygen delivery and electrolyte levels in the dehydrational death of Xenopus laevis. J. Comp. Physiol. B 1978, 128, 169–175. [Google Scholar] [CrossRef]
- Hillman, S.S. Dehydrational Effects on Cardiovascular and Metabolic Capacity in Two Amphibians. Physiol. Zool. 1987, 60, 608–613. [Google Scholar] [CrossRef]
- Gatten, R.E., Jr. Activity Metabolism of Anuran Amphibians: Tolerance to Dehydration. Physiol. Zool. 1987, 60, 576–585. [Google Scholar] [CrossRef]
- Childers, C.L.; Storey, K.B. Post-translational Regulation of Hexokinase Function and Protein Stability in the Aestivating Frog Xenopus laevis. Protein J. 2016, 35, 61–71. [Google Scholar] [CrossRef]
- Childers, C.L.; Storey, K.B. Purification and characterization of a urea sensitive lactate dehydrogenase from skeletal muscle of the African clawed frog, Xenopus laevis. J. Comp. Physiol. B 2019, 189, 271–281. [Google Scholar] [CrossRef]
- Malik, A.L.; Storey, K.B. Activation of extracellular signal-regulated kinases during dehydration in the African clawed frog, Xenopus laevis. J. Exp. Biol. 2009, 212, 2595–2603. [Google Scholar] [CrossRef]
- Dawson, N.J.; Biggar, Y.; Malik, A.I.; Storey, K.B. Comparative Biochemistry and Physiology, Part B Increased transcript levels and kinetic function of pyruvate kinase during severe dehydration in aestivating African clawed frogs, Xenopus laevis. Comp. Biochem. Physiol. Part B 2018, 224, 245–252. [Google Scholar] [CrossRef]
- Katzenback, B.A.; Dawson, N.J.; Storey, K.B. Purification and characterization of a urea sensitive lactate dehydrogenase from the liver of the African clawed frog, Xenopus laevis. J. Comp. Physiol. B 2014, 184, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hawkins, L.J.; Storey, K.B. DNA methylation and regulation of DNA methyltransferases in a freeze-tolerant vertebrate. Biochem. Cell Biol. 2020, 98, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.J.; Storey, K.B. Histone methylation in the freeze-tolerant wood frog (Rana sylvatica). J. Comp. Physiol. B 2018, 188, 113–125. [Google Scholar] [CrossRef]
- Hawkins, L.J.; Storey, K.B. MicroRNA expression in the heart of Xenopus laevis facilitates metabolic adaptation to dehydration. Genomics 2020, 112, 3525–3536. [Google Scholar] [CrossRef]
- Hawkins, L.J.; Wang, H.; Storey, K.B. Phosphoproteomic Analysis of Xenopus laevis Reveals Expression and Phosphorylation of Hypoxia-Inducible PFKFB3 during Dehydration Phosphoproteomic Analysis of Xenopus laevis Reveals Expression and Phosphorylation. Iscience 2020, 23, 101598. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.; Sterner, D.E.; Berger, S.L. Histone modifications: Now summoning sumoylation. Proc. Natl. Acad. Sci. USA 2003, 100, 13118–13120. [Google Scholar] [CrossRef]
- Berger, S.L. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 2002, 12, 142–148. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef]
- Hwang, J.W.; Cho, Y.; Bae, G.U.; Kim, S.N.; Kim, Y.K. Protein arginine methyltransferases: Promising targets for cancer therapy. Exp. Mol. Med. 2021, 53, 788–808. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bedford, M.T. Histone arginine methylation. FEBS Lett. 2011, 585, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Briggs, S.D.; Brame, C.J.; Caldwell, J.A.; Koh, S.S.; Ma, H.; Cook, R.G.; Shabanowitz, J.; Hunt, D.F.; Stallcup, M.R.; et al. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr. Biol. 2001, 11, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zhang, Y.; Xu, R.M. Structural basis for specific binding of polycomb chromodomain to histone H3 methylated at Lys 27. Genes. Dev. 2003, 17, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Schurter, B.T.; Koh, S.S.; Chen, D.; Bunick, G.J.; Harp, J.M.; Hanson, B.L.; HenschenEdman, A.; Mackay, D.R.; Stallcup, M.R.; Aswad, D.W. Methylation of histone H3 by coactivator-associated arginine methyltransferase 1. Biochemistry 2001, 40, 5747–5756. [Google Scholar] [CrossRef]
- Ma, H.; Baumann, C.T.; Li, H.; Strahl, B.D.; Rice, R.; Jelinek, M.A.; Aswad, D.W.; Allis, C.D.; Hager, G.L.; Stallcup, M.R. Hormone-dependent, CARM1-directed, arginine-specific methylation of histone H3 on a steroid-regulated promoter. Curr. Biol. 2001, 11, 1981–1985. [Google Scholar] [CrossRef]
- Daujat, S.; Bauer, U.M.; Shah, V.; Turner, B.; Berger, S.; Kouzarides, T. Crosstalk between CARM1 methylation and CBP acetylation on histone H3. Curr. Biol. 2002, 12, 2090–2097. [Google Scholar] [CrossRef]
- Casadio, F.; Lu, X.; Pollock, S.B.; LeRoy, G.; Garcia, B.A.; Muir, T.W.; Roeder, R.G.; Allis, C.D. H3R42me2a is a histone modification with positive transcriptional effects. Proc. Natl. Acad. Sci. USA 2013, 110, 14894–14899. [Google Scholar] [CrossRef]
- Zhao, Q.; Rank, G.; Tan, Y.T.; Li, H.; Moritz, R.L.; Simpson, R.J.; Cerruti, L.; Curtis, D.J.; Patel, D.J.; Allis, C.D.; et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat. Struct. Mol. Biol. 2009, 16, 304–311. [Google Scholar] [CrossRef]
- Dacwag, C.S.; Ohkawa, Y.; Pal, S.; Sif, S.; Imbalzano, A.N. The Protein Arginine Methyltransferase Prmt5 Is Required for Myogenesis because It Facilitates ATP-Dependent Chromatin Remodeling. Mol. Cell. Biol. 2007, 27, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.; Zielinska, A.E.; Shaaban, A.M.; Sanchez-Bailon, M.P.; Jarrold, J.; Clarke, T.L.; Zhang, J.; Francis, A.; Jones, L.J.; Smith, S.; et al. PRMT5 Is a Critical Regulator of Breast Cancer Stem Cell Function via Histone Methylation and FOXP1 Expression. Cell Rep. 2017, 21, 3498–3513. [Google Scholar] [CrossRef]
- Holbert, M.A.; Marmorstein, R. Structure and activity of enzymes that remove histone modifications. Curr. Opin. Struct. Biol. 2005, 15, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta-Gene Regul. Mech. 2013, 1829, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Walport, L.J.; Hopkinson, R.J.; Chowdhury, R.; Schiller, R.; Ge, W.; Kawamura, A.; Schofield, C.J. Arginine demethylation is catalysed by a subset of JmjC histone lysine demethylases. Nat. Commun. 2016, 7, 11974. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Xiaomeng, L.; Zheng, L.; Yangge, D.; Xuejiao, L.; Longwei, L.; Xiao, Z.; Yunsong, L.; Ping, Z.; Yongsheng, Z. Asymmetrical methyltransferase PRMT3 regulates human mesenchymal stem cell osteogenesis via miR-3648. Cell Death Dis. 2019, 10, 581. [Google Scholar] [CrossRef]
- Blythe, S.A.; Cha, S.W.; Tadjuidje, E.; Heasman, J.; Klein, P.S. β-catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev. Cell 2010, 19, 220–231. [Google Scholar] [CrossRef]
- Hyllus, D.; Stein, C.; Schnabel, K.; Schiltz, E.; Imhof, A.; Dou, Y.; Hsieh, J.; Bauer, U.M. PRMT6-mediated methylation of R2 in histone H3 antagonizes H3 K4 trimethylation. Genes. Dev. 2007, 21, 3369–3380. [Google Scholar] [CrossRef]
- Lee, J.; Sayegh, J.; Daniel, J.; Clarke, S.; Bedford, M.T. PRMT8, a new membranebound tissue-specific member of the protein arginine methyltransferase family. J. Biol. Chem. 2005, 280, 32890–32896. [Google Scholar] [CrossRef]
- Gayatri, S.; Bedford, M.T. Readers of Histone Methylarginine Marks. Biochem. Biophys. Acta 2014, 1839, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wang, G.G. Tudor: A versatile family of histone methylation “readers”. Trends Biochem. Sci. 2013, 38, 546–555. [Google Scholar] [CrossRef]
- Romspert, A.P. Osmoregulation of the african clawed frog. Xenopus laevis, in hypersaline media. Comp. Biochem. Physiol. Part A Physiol. 1976, 54, 207–210. [Google Scholar] [CrossRef]
- Eaton, S.L.; Roche, S.L.; Hurtado, L.L.; Oldknow, M.J.; Farquharson, K.J. Total Protein Analysis as a Reliable Loading Control for Quantitative Fluorescent Western Blotting. PLoS ONE 2013, 8, 72457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Storey, K.B. RBioplot: An easy-to-use R pipeline for automated statistical analysis and data visualization in molecular biology and biochemistry. PeerJ 2016, 4, e2436. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic Rate Depression and Biochemical Adaptation in Anaerobiosis, Hibernation and Estivation Kenneth B. Storey, and Janet M. Storey. Q. Rev. Biol. 1990, 65, 145–174. [Google Scholar] [CrossRef]
- Fabbrizio, E.; El Messaoudi, S.; Polanowska, J.; Paul, C.; Cook, J.R.; Lee, J.H.; Nère, V.; Rousset, M.; Pestka, S.; Le Cam, A.; et al. Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 2002, 3, 641–645. [Google Scholar] [CrossRef]
- Jain, K.; Clarke, S.G.; Hill, C.; Angeles, L.; States, U. PRMT7 as a unique member of the protein arginine methyltransferase family: A review. Arch. Biochem. Biophys. 2019, 665, 36–45. [Google Scholar] [CrossRef]
- Haghandish, N.; Baldwin, R.M.; Morettin, A.; Dawit, H.T.; Adhikary, H.; Masson, J.Y.; Mazroui, R.; Trinkle-Mulcahy, L.; Côté, J. PRMT7 methylates eukaryotic translation initiation factor 2α and regulates its role in stress granule formation. Mol. Biol. Cell 2019, 30, 778–793. [Google Scholar] [CrossRef]
- Szewczyk, M.M.; Ishikawa, Y.; Organ, S.; Sakai, N.; Li, F.; Halabelian, L.; Ackloo, S.; Couzens, A.L.; Eram, M.; Dilworth, D.; et al. Pharmacological inhibition of PRMT7 links arginine monomethylation to the 78 cellular stress response. Nat. Commun. 2020, 11, 2396. [Google Scholar] [CrossRef]
- Pal, S.; Vishwanath, S.N.; Erdjument-Bromage, H.; Tempst, P.; Sif, S. Human SWI/SNF-Associated PRMT5 Methylates Histone H3 Arginine 8 and Negatively Regulates Expression of ST7 and NM23 Tumor Suppressor Genes. Mol. Cell. Biol. 2004, 24, 9630–9645. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Hawkins, L.J.; Storey, K.B. Dynamic regulation of six histone H3 lysine (K) methyltransferases in response to prolonged anoxia exposure in a freshwater turtle. Gene 2018, 649, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hillman, S.S.; Sommerfeldt, R.W. Microsphere studies of amphibian systemic blood flow redistribution during dehydration, hypovolemia, and salt load. J. Exp. Zool. 1981, 218, 305–308. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.; Parent, M.; Storey, K.B. Histone Arginine Methylation as a Regulator of Gene Expression in the Dehydrating African Clawed Frog (Xenopus laevis). Genes 2024, 15, 1156. https://doi.org/10.3390/genes15091156
Rehman S, Parent M, Storey KB. Histone Arginine Methylation as a Regulator of Gene Expression in the Dehydrating African Clawed Frog (Xenopus laevis). Genes. 2024; 15(9):1156. https://doi.org/10.3390/genes15091156
Chicago/Turabian StyleRehman, Saif, Mackenzie Parent, and Kenneth B. Storey. 2024. "Histone Arginine Methylation as a Regulator of Gene Expression in the Dehydrating African Clawed Frog (Xenopus laevis)" Genes 15, no. 9: 1156. https://doi.org/10.3390/genes15091156






