Schistosoma japonicum sja-let-7 Inhibits the Growth of Hepatocellular Carcinoma Cells via Cross-Species Regulation of Col1α2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and DEGs Identification
2.2. Enrichment Analysis
2.3. Protein-Protein Interaction Network Construction
2.4. Construction of Liver Fibrosis Mice Model
2.5. Pan-Cancer View and Clinical Correlation Analysis of the Cancer Genome Atlas Program (TCGA) Platform
2.6. Analysis of Immune Cell Infiltration
2.7. Diagnostic Value Analysis
2.8. Cell Culture and Treatments
2.9. Quantitative Real-Time PCR
2.10. Cell Proliferation Assay
2.11. Colony Formation Assay
2.12. Wound Healing Assay
2.13. Statistical Analysis
3. Results
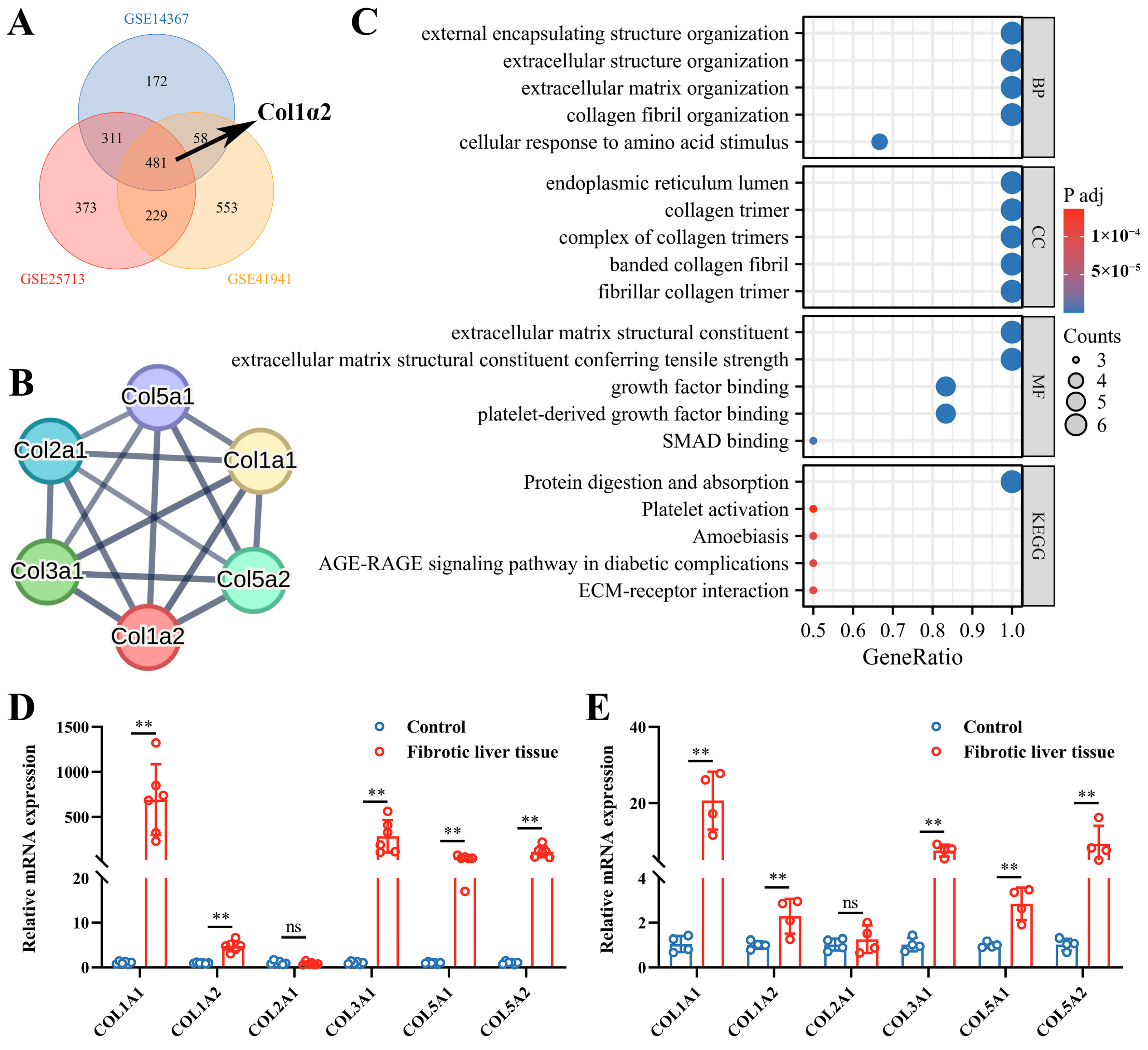
3.1. Col1α2 Expression and Its Functional Interactions in Fibrosis Datasets
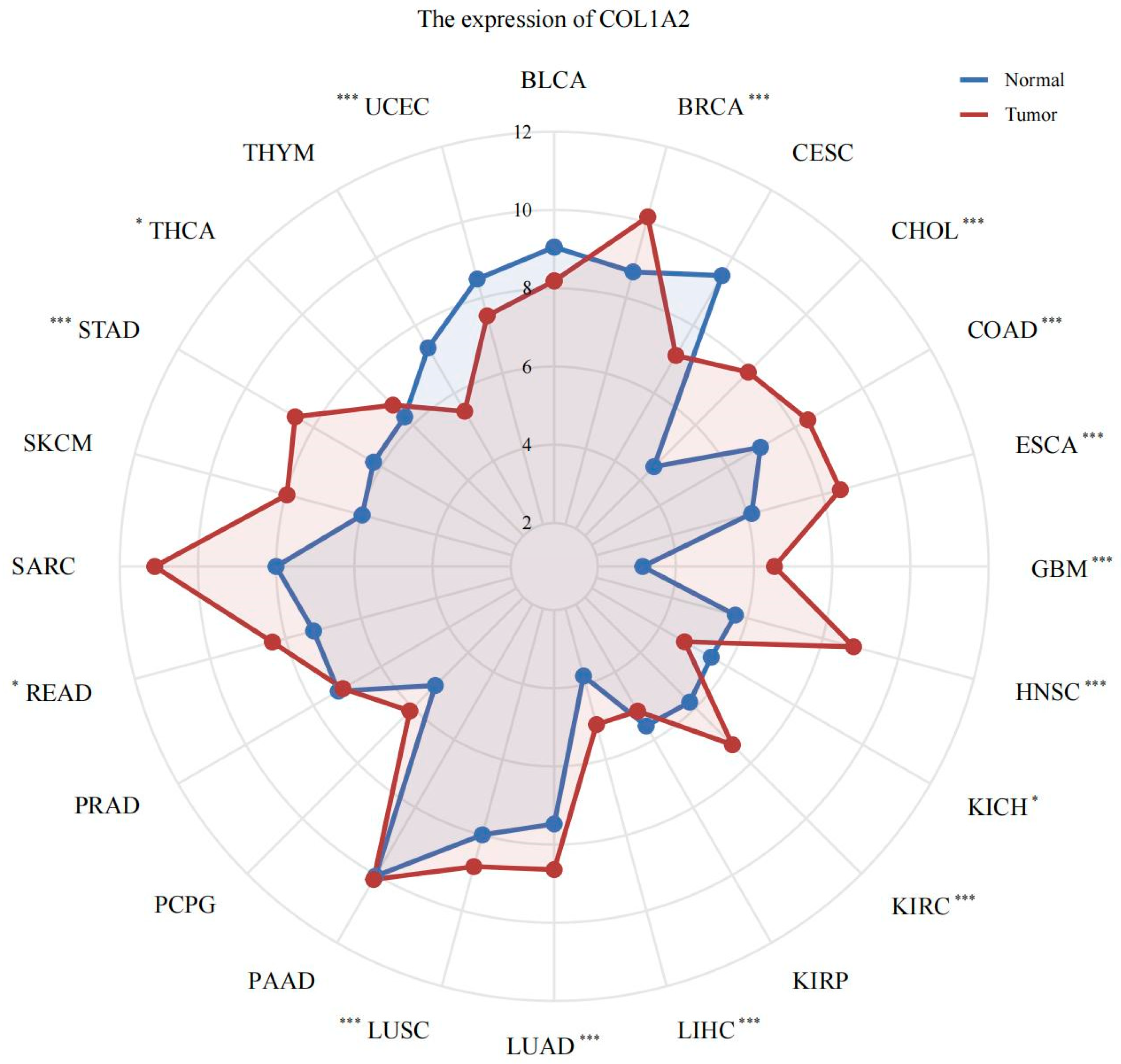
3.2. Pan-Cancer Analysis of col1α2 Expression
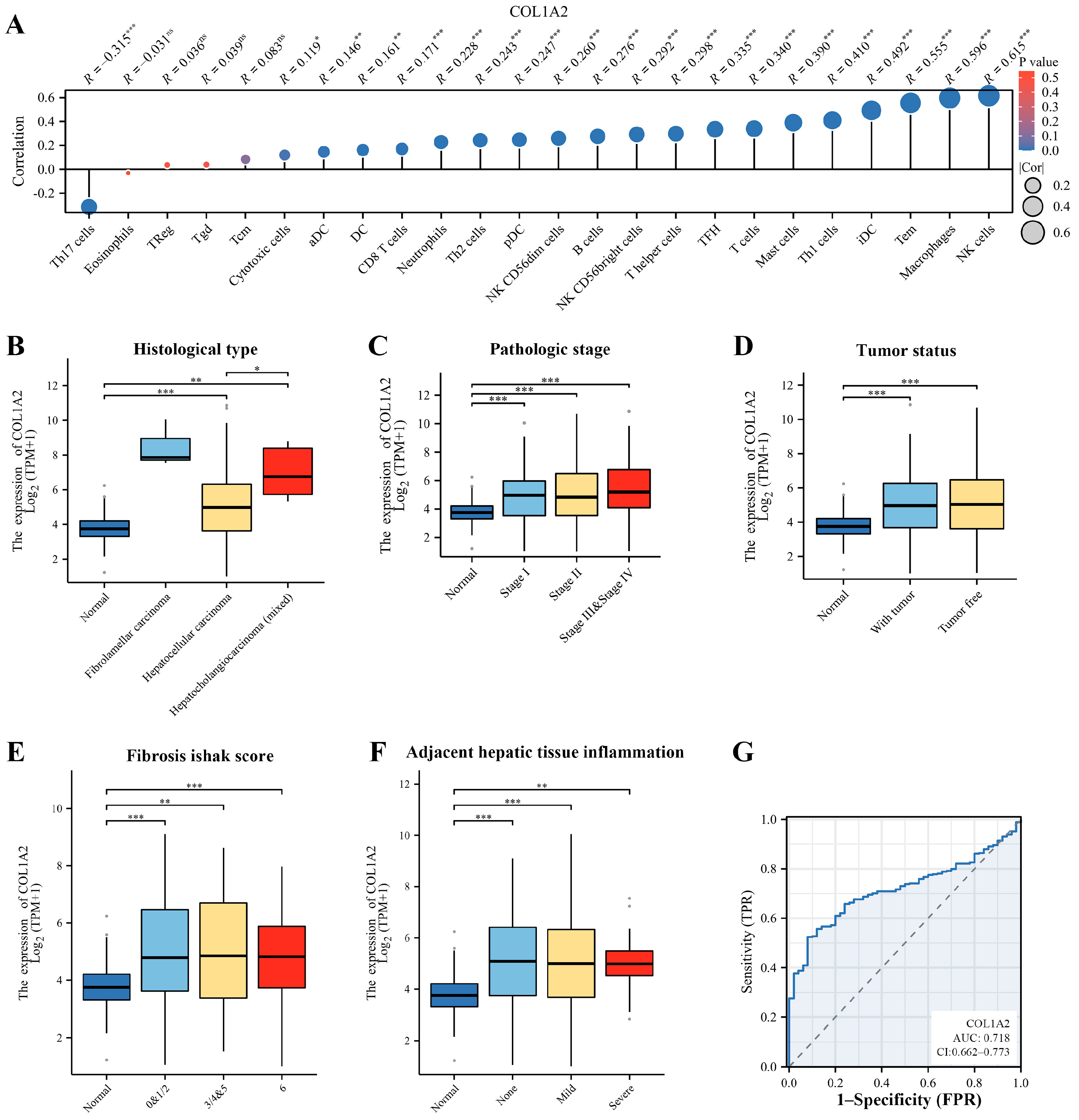
3.3. Immune Infiltration and Clinical Correlation Analysis of Col1α2 in HCC
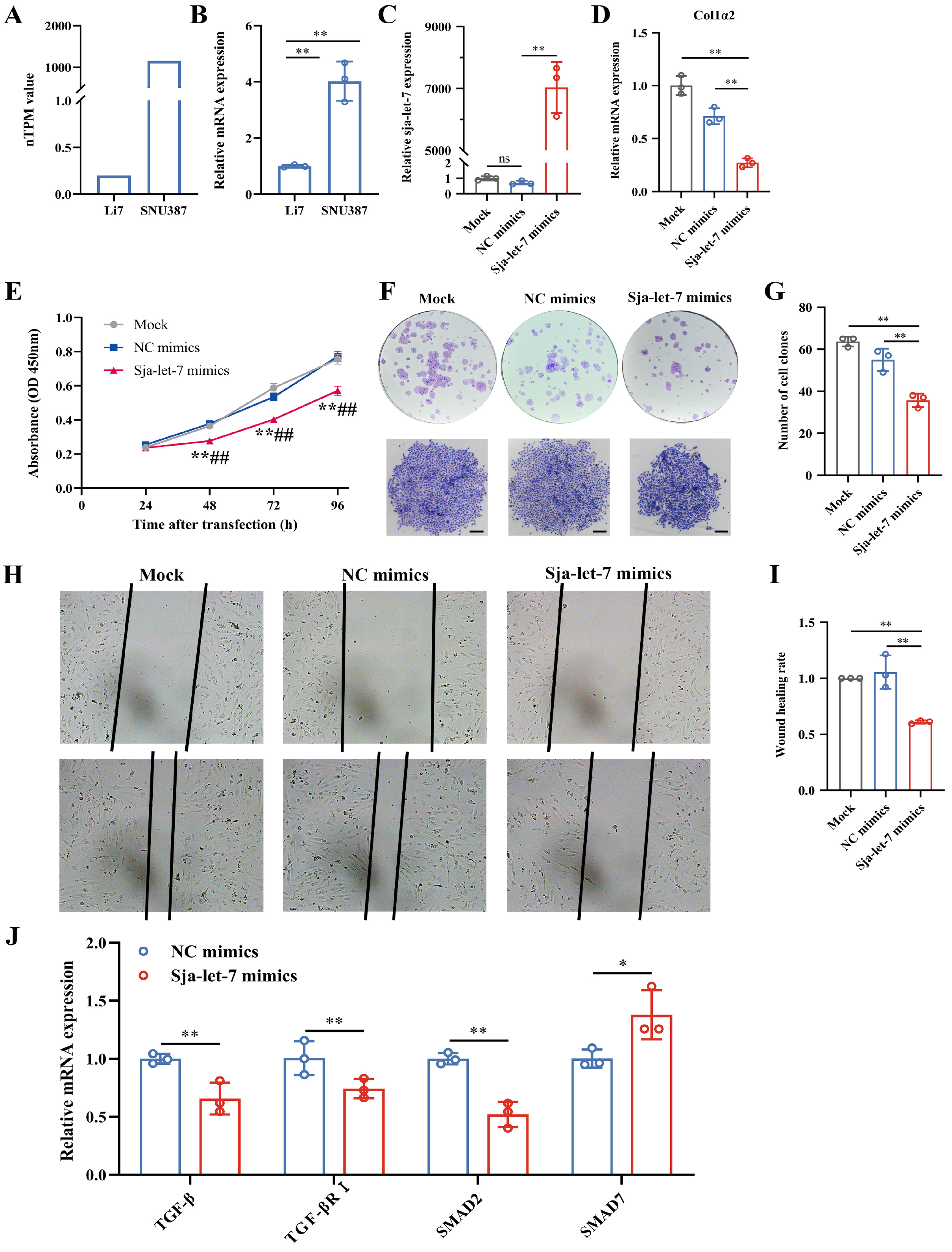
3.4. Inhibition of Hepatoma Cells Growth by sja-let-7 Targeting Col1α2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.R.; Wong, E.Y.T.; Wong, S.H.; Ng, A.W.T.; Loo, L.H.; Chow, P.K.; Ngeow, J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology 2023, 164, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Sidali, S.; Trépo, E.; Sutter, O.; Nault, J.C. New concepts in the treatment of hepatocellular carcinoma. United Eur. Gastroenterol. J. 2022, 10, 765–774. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Pan, W. Host-parasite interactions mediated by cross-species microRNAs. Trends Parasitol 2022, 38, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, Y.; Koganezawa, S. Metrical and histopathological studies on Japanese schistosomiasis of the colon associated with cancer. Nihon Shokakibyo Gakkai Zasshi—Jpn. J. Gastro-Enterol. 1976, 73, 972–985. [Google Scholar]
- Inaba, Y.; Maruchi, N.; Matsuda, M.; Yoshihara, N.; Yamamoto, S. A case-control study on liver cancer with special emphasis on the possible aetiological role of schistosomiasis. Int. J. Epidemiol. 1984, 13, 408–412. [Google Scholar] [CrossRef]
- El-Tonsy, M.M.; Hussein, H.M.; Helal Tel, S.; Tawfik, R.A.; Koriem, K.M.; Hussein, H.M. Schistosoma mansoni infection: Is it a risk factor for development of hepatocellular carcinoma? Acta Trop. 2013, 128, 542–547. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Yang, R.; Yu, Z.; Zhang, L.; Zhu, Z.; Wu, X.; Shen, J.; Liu, J.; Xu, L.; et al. Sja-miR-71a in Schistosome egg-derived extracellular vesicles suppresses liver fibrosis caused by schistosomiasis via targeting semaphorin 4D. J. Extracell. Vesicles 2020, 9, 1785738. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Fan, X.; Lei, N.; Tian, Y.; Zhang, D.; Pan, W. A schistosome miRNA promotes host hepatic fibrosis by targeting transforming growth factor β receptor III. J. Hepatol. 2020, 72, 519–527. [Google Scholar] [CrossRef]
- Zhong, H.; Dong, B.; Zhu, D.; Fu, Z.; Liu, J.; Jin, Y. Sja-let-7 suppresses the development of liver fibrosis via Schistosoma japonicum extracellular vesicles. PLoS Pathog. 2024, 20, e1012153. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, S.; Wang, J.; Lin, Y.; Ma, L.; Zhu, L.; Jiang, P.; Li, Z.; Pan, W. Schistosoma japonicum MiRNA-7-5p Inhibits the Growth and Migration of Hepatoma Cells via Cross-Species Regulation of S-Phase Kinase-Associated Protein 2. Front. Oncol. 2019, 9, 175. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Pan, D.; Wang, J.; Zhu, L.; Lin, Y.; Zhu, S.; Pan, W. A Schistosoma japonicum MicroRNA Exerts Antitumor Effects Through Inhibition of Both Cell Migration and Angiogenesis by Targeting PGAM1. Front. Oncol. 2021, 11, 652395. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, S.; Hu, C.; Wang, J.; Jiang, P.; Zhu, L.; Li, Z.; Wang, S.; Zhang, Y.; Xu, X.; et al. Cross-Species Suppression of Hepatoma Cell Growth and Migration by a Schistosoma japonicum MicroRNA. Mol. Ther. Nucleic Acids 2019, 18, 400–412. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, J.; Zhu, S.; Hu, C.; Lin, Y.; Pan, W. Identification of a Schistosoma japonicum MicroRNA That Suppresses Hepatoma Cell Growth and Migration by Targeting Host FZD4 Gene. Front. Cell. Infect. Microbiol. 2022, 12, 786543. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Dong, B.; Zhu, D.; Li, H.; Lu, K.; Fu, Z.; Liu, J.; Jin, Y. Sja-Let-7 Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in a Mouse Model via Col1α2. Biology 2023, 12, 1465. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Y.; Wang, N. Systematic identification of key extracellular proteins as the potential biomarkers in lupus nephritis. Front. Immunol. 2022, 13, 915784. [Google Scholar] [CrossRef]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas--a tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sawamura, S.; Makino, K.; Ide, M.; Shimada, S.; Kajihara, I.; Makino, T.; Jinnin, M.; Fukushima, S. Elevated α 1(I) to α 2(I) Collagen Ratio in Dermal Fibroblasts Possibly Contributes to Fibrosis in Systemic Sclerosis. Int. J. Mol. Sci. 2022, 23, 6811. [Google Scholar] [CrossRef]
- Le Saux, O.; Teeters, K.; Miyasato, S.; Choi, J.; Nakamatsu, G.; Richardson, J.A.; Starcher, B.; Davis, E.C.; Tam, E.K.; Jourdan-Le Saux, C. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L1007–L1017. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Ghosh, A.K. Factors involved in the regulation of type I collagen gene expression: Implication in fibrosis. Exp. Biol. Med. 2002, 227, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Doh, J. The extracellular matrix in solid tumor immunotherapy. Trends Immunol. 2024, S1471-4906(24)00184-4. [Google Scholar] [CrossRef]
- Li, X.; Shepard, H.M.; Cowell, J.A.; Zhao, C.; Osgood, R.J.; Rosengren, S.; Blouw, B.; Garrovillo, S.A.; Pagel, M.D.; Whatcott, C.J.; et al. Parallel Accumulation of Tumor Hyaluronan, Collagen, and Other Drivers of Tumor Progression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Romualdo, G.R.; Grassi, T.F.; Goto, R.L.; Tablas, M.B.; Bidinotto, L.T.; Fernandes, A.A.H.; Cogliati, B.; Barbisan, L.F. An integrative analysis of chemically-induced cirrhosis-associated hepatocarcinogenesis: Histological, biochemical and molecular features. Toxicol. Lett. 2017, 281, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhao, L.; Budhu, A.; Forgues, M.; Jia, H.-L.; Qin, L.-X.; Ye, Q.-H.; Yu, J.; Shi, X.; Tang, Z.-Y.; et al. Let-7g targets collagen type I α2 and inhibits cell migration in hepatocellular carcinoma. J. Hepatol. 2010, 52, 690–697. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK cells are never alone: Crosstalk and communication in tumour microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef]
- Andrade, Z.A. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009, 31, 656–663. [Google Scholar] [CrossRef]
- De Santis, C.; Gotte, M. The Role of microRNA Let-7d in Female Malignancies and Diseases of the Female Reproductive Tract. Int. J. Mol. Sci. 2021, 22, 7359. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Kwon, C.S.; Lee, D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 2016, 7, 100–113. [Google Scholar] [CrossRef]
- Pfefferle, P.I.; Keber, C.U.; Cohen, R.M.; Garn, H. The Hygiene Hypothesis—Learning from but Not Living in the Past. Front. Immunol. 2021, 12, 635935. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Da’dara, A.A.; Skelly, P.J. Schistosome immunomodulators. PLoS Pathog. 2021, 17, e1010064. [Google Scholar] [CrossRef]
- Mu, Y.; McManus, D.P.; Hou, N.; Cai, P. Schistosome Infection and Schistosome-Derived Products as Modulators for the Prevention and Alleviation of Immunological Disorders. Front. Immunol. 2021, 12, 619776. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.; Chambers, J.P.; Yu, J.-J.; Cheng, X.; Christenson, L.K.; Guentzel, M.N.; Gupta, R.; Arulanandam, B.P. Modulation of Immune Response to Chlamydia muridarum by Host miR-135a. Front. Cell. Infect. Microbiol. 2021, 11, 638058. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Dong, B.; Zhu, D.; Fu, Z.; Liu, J.; Guan, G.; Jin, Y. Schistosoma japonicum sja-let-7 Inhibits the Growth of Hepatocellular Carcinoma Cells via Cross-Species Regulation of Col1α2. Genes 2024, 15, 1165. https://doi.org/10.3390/genes15091165
Zhong H, Dong B, Zhu D, Fu Z, Liu J, Guan G, Jin Y. Schistosoma japonicum sja-let-7 Inhibits the Growth of Hepatocellular Carcinoma Cells via Cross-Species Regulation of Col1α2. Genes. 2024; 15(9):1165. https://doi.org/10.3390/genes15091165
Chicago/Turabian StyleZhong, Haoran, Bowen Dong, Danlin Zhu, Zhiqiang Fu, Jinming Liu, Guiquan Guan, and Yamei Jin. 2024. "Schistosoma japonicum sja-let-7 Inhibits the Growth of Hepatocellular Carcinoma Cells via Cross-Species Regulation of Col1α2" Genes 15, no. 9: 1165. https://doi.org/10.3390/genes15091165




