Physiological and Transcriptome Analyses Reveal the Effects of Fertilization on the Yield of Winter Wheat and on the Photosynthetic Performance of Leaves during the Flowering Period
Abstract
:1. Introduction
2. Materials and Methods
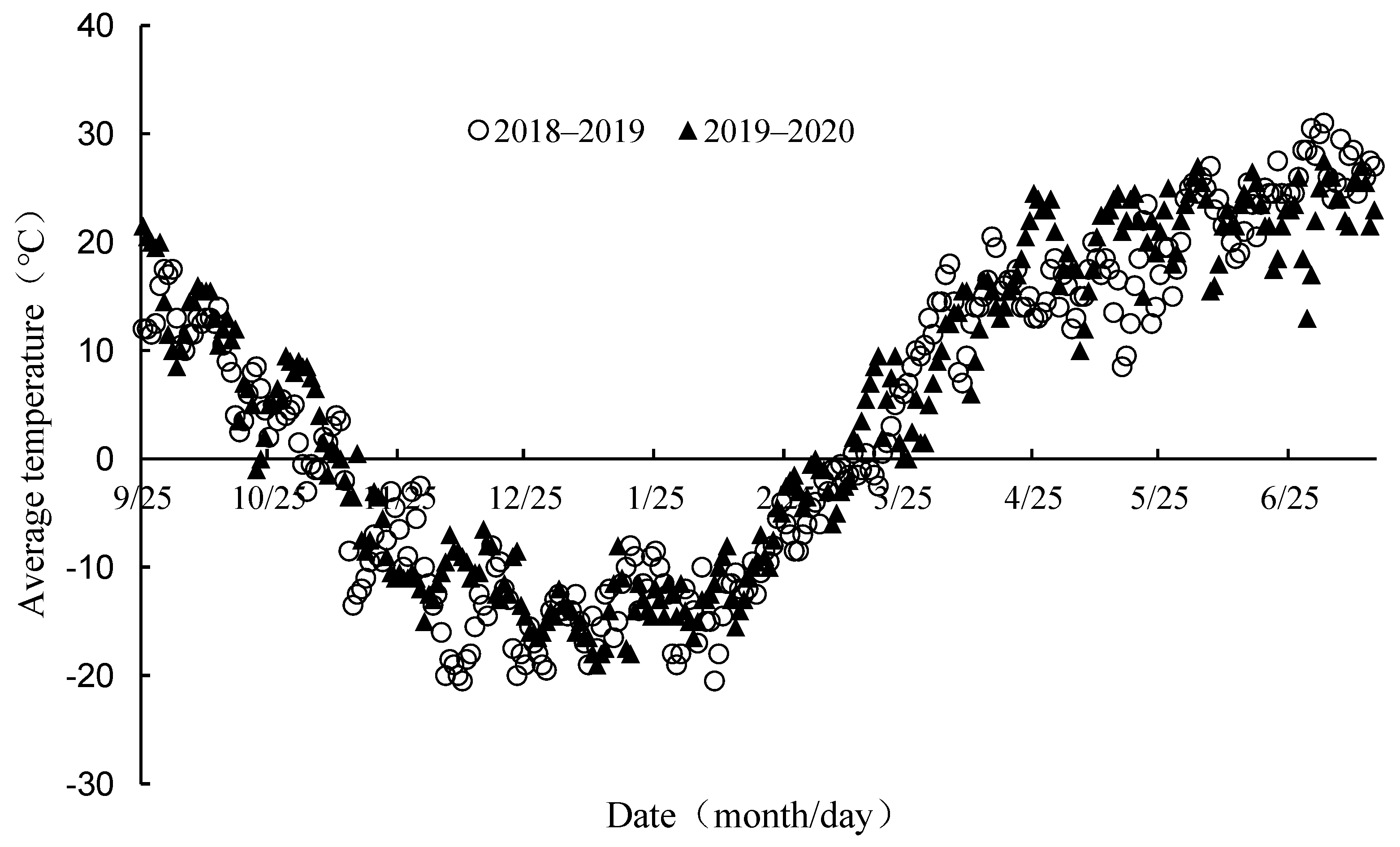
2.1. Experimental Site and Cultivar
2.2. Experiment Design
2.3. Methods
2.3.1. Yield and Its Components
2.3.2. Measurement of Blade Gas Exchange Parameters
2.3.3. Chlorophyll Content
2.3.4. Antioxidant Enzyme Activity and Endogenous Hormone Content
2.3.5. Transcriptome Sequencing
2.3.6. Validation of DEGs Using Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.4. Transcriptomic Data Analysis
2.5. Data Analysis
3. Results
3.1. Yield and Yield Components
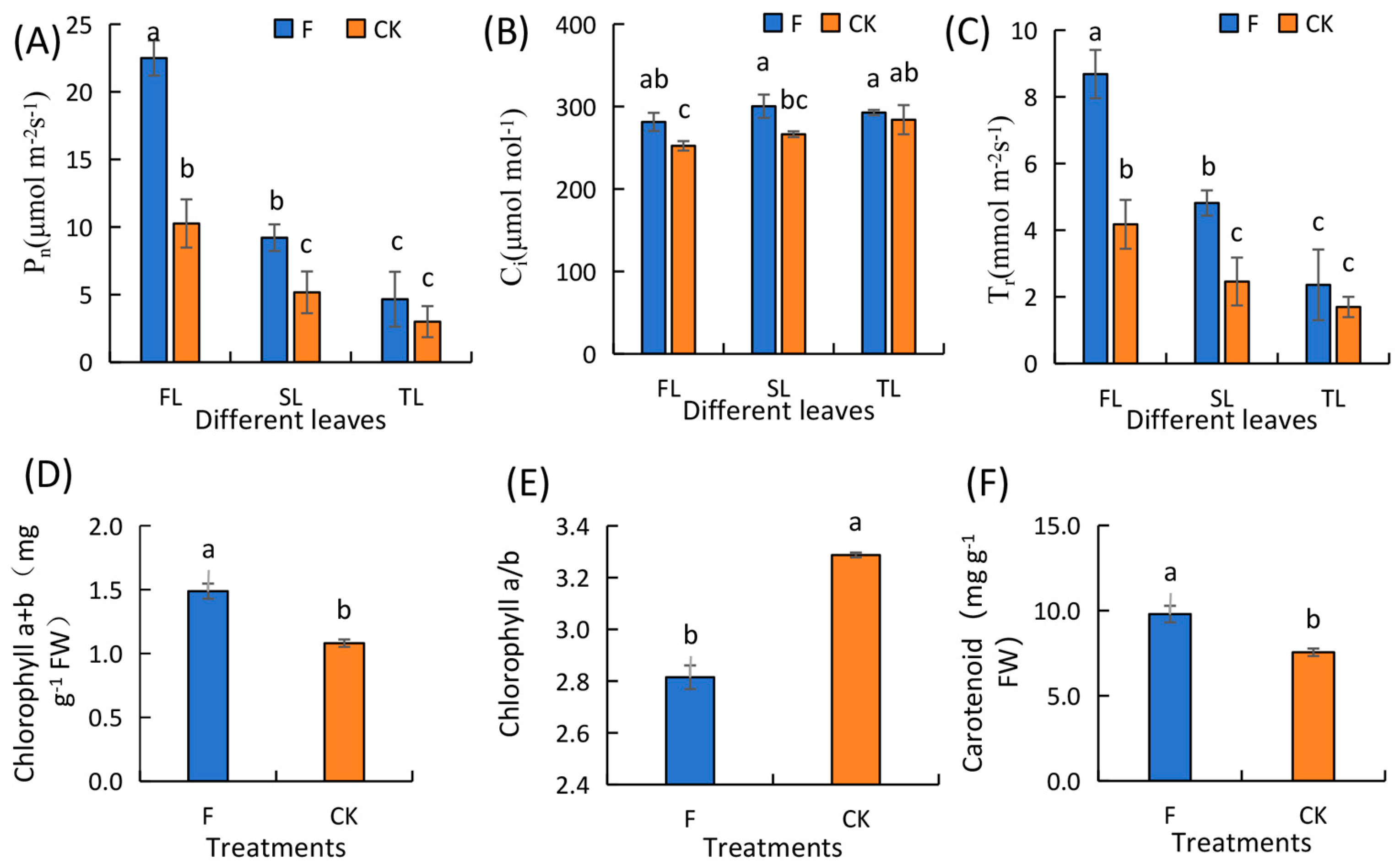
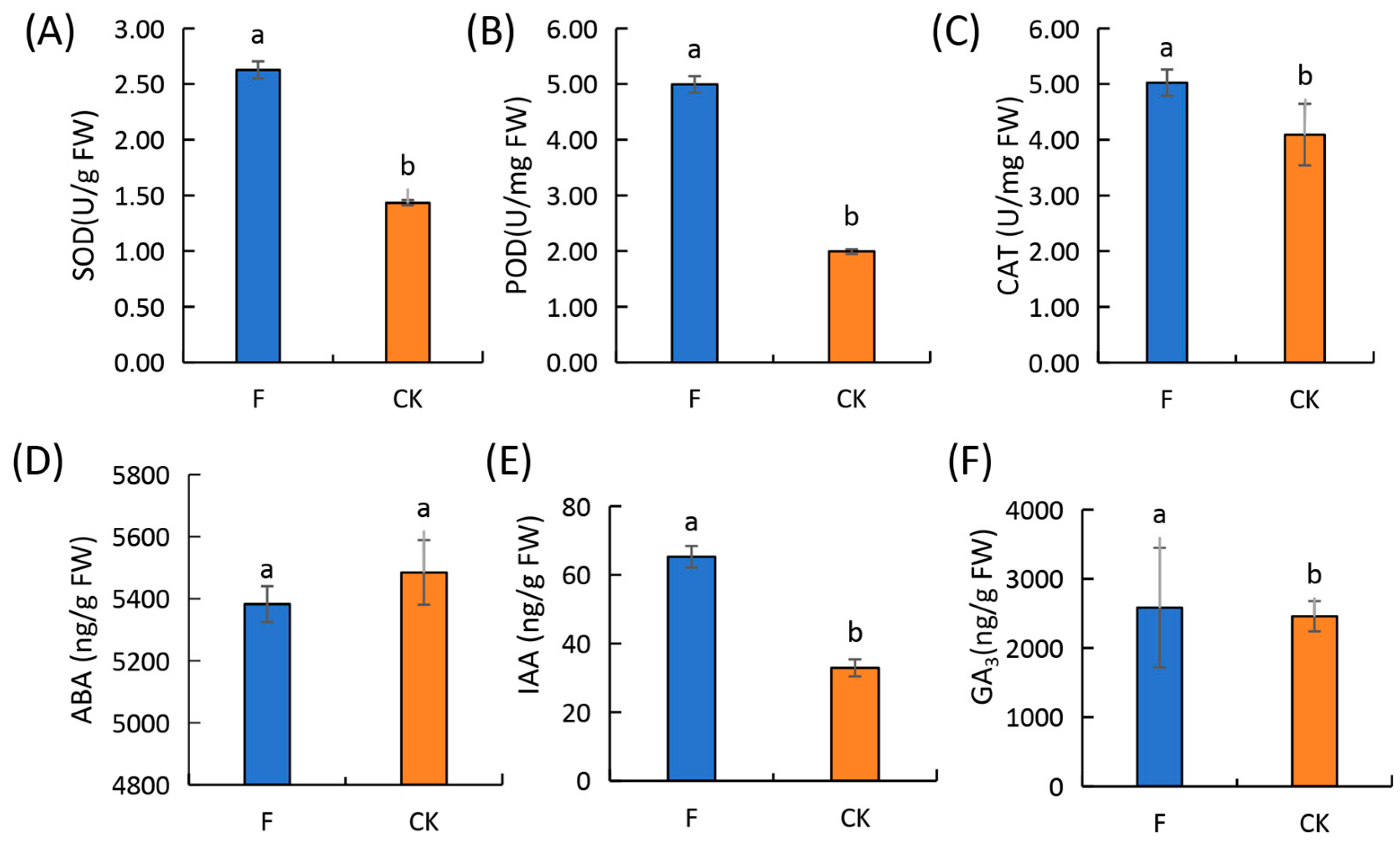
3.2. Changes in the Photosynthetic Parameters, Chlorophyll Content, Antioxidant Enzyme Activity, and Endogenous Hormone Content of Wheat Leaves during the Flowering Stage
3.3. Characterization of the Sequenced Solexa/Illumina Libraries and Identification of DEGs
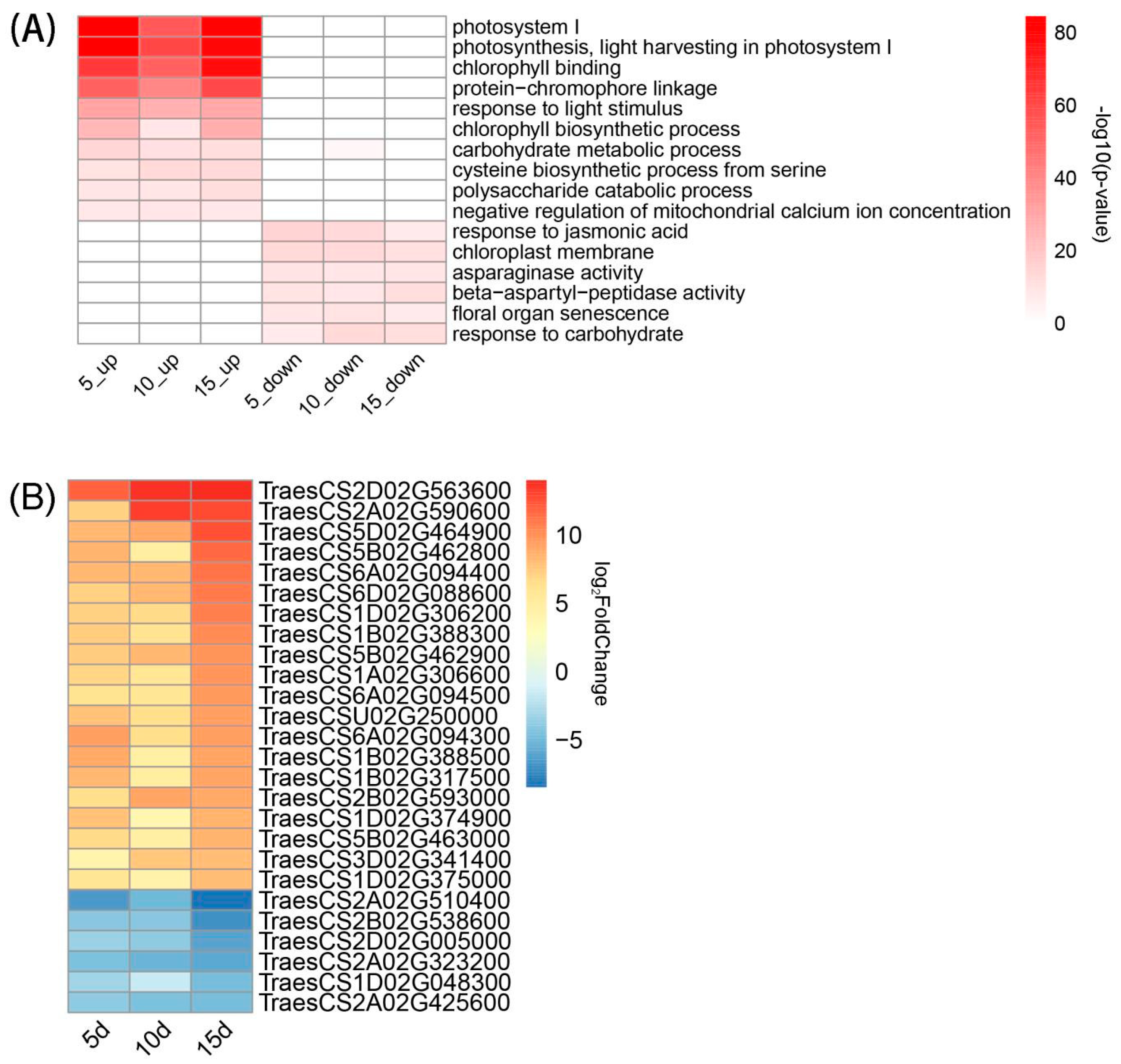
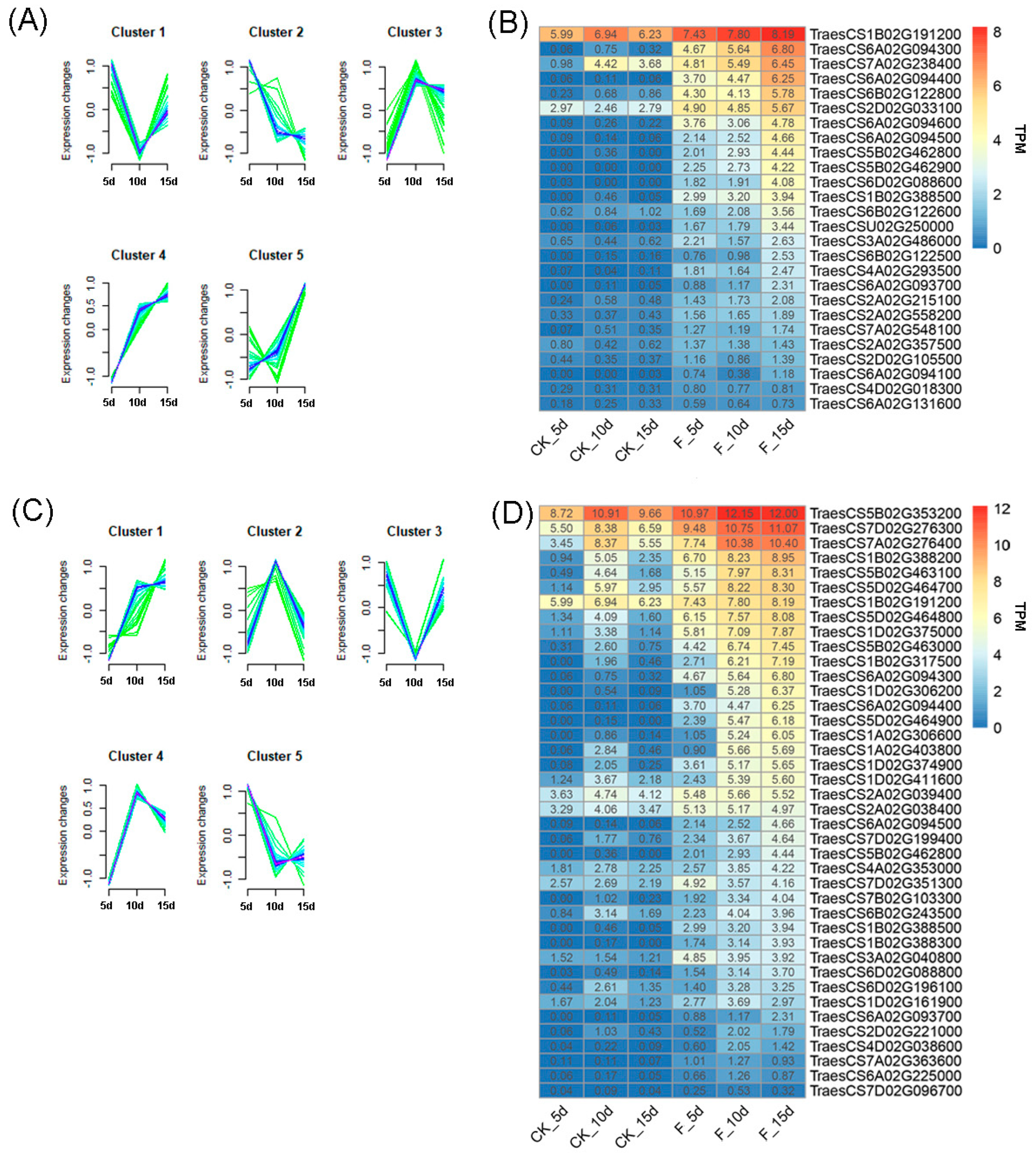
3.4. GO Enrichment Analysis of DEGs
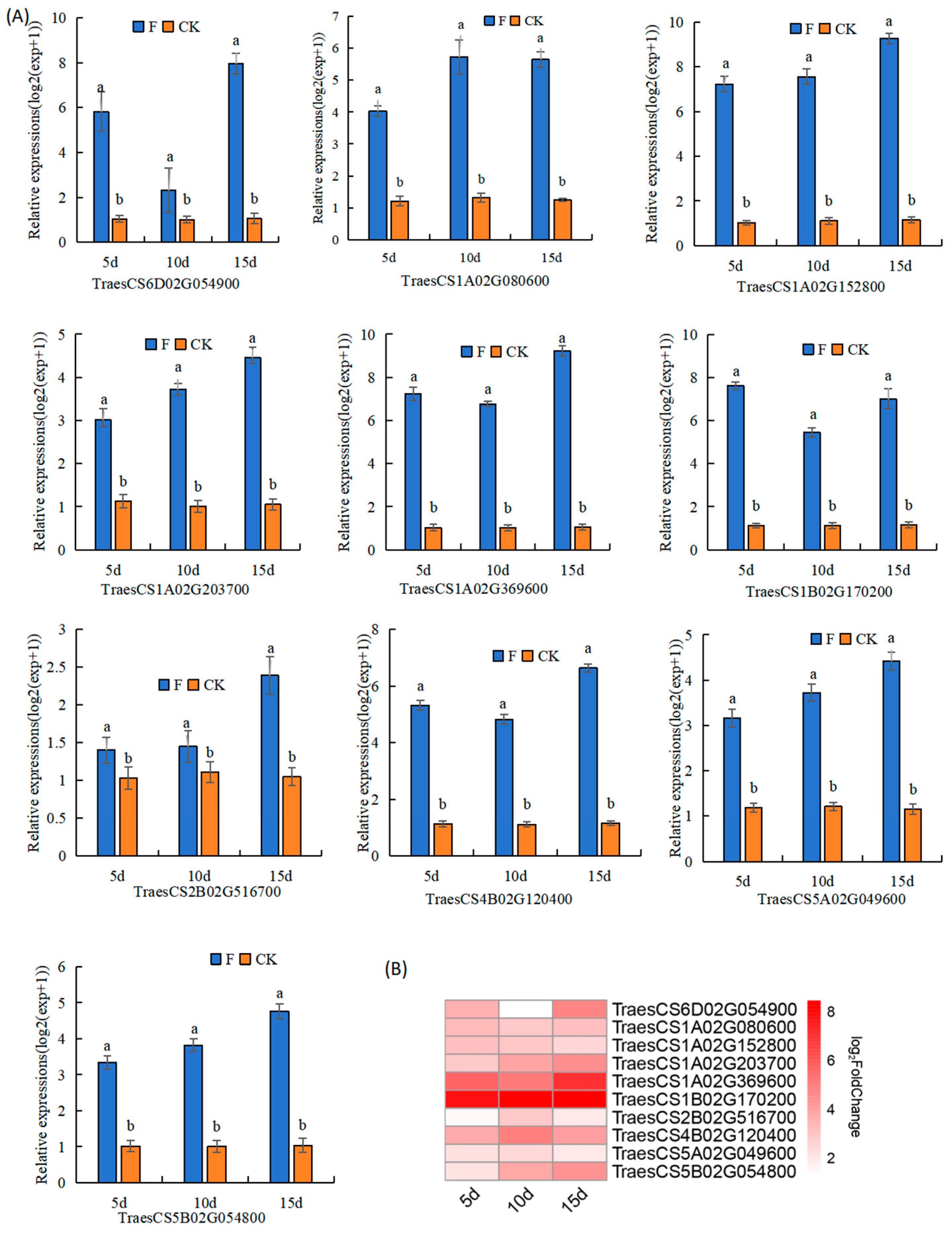
3.5. qRT-PCR-Based Verification of the Expression of Genes Involved in Fertilizer Utilisation Efficiency
4. Discussion
4.1. Effect of Fertilization on the Physiological Indicators of Winter Wheat Leaves during the Flowering Period
4.2. Transcriptome Analysis of the Effect of Fertilization on the Leaves of Winter Wheat at the Flowering Stage
4.3. Verification and Regulatory Analysis of Key Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.; Zhu, S.; Schultze-Kraft, R.; Liu, G.; Chen, Z. Dissection of crop metabolome responses to nitrogen, phosphorus, potassium, and other nutrient deficiencies. Int. J. Mol. Sci. 2022, 23, 9079. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Xu, M.; Li, R.; Zheng, L.; Wang, H.; Liu, S.; Zhang, W.; Duan, Y.; Lu, C. Achieving high yield and nitrogen agronomic efficiency by coupling wheat varieties with soil fertility. Sci. Total Environ. 2023, 881, 163531. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shi, K.; Zhu, C.-W.; Jiang, G.-Y.; Luo, L.; Meng, W.-W.; Liu, F.; Shen, F.-M.; Liu, S.-L. Effects of planting density and nitrogen application rate on wheat photosynthetic characteristics, yield, and soil nitrogen content in fluvo-aquic soil in northern Henan Province. J. Agric. Sci. Technol. 2023, 25, 24–33. [Google Scholar]
- Vicente, R.; Vergara-Díaz, O.; Uberegui, E.; Martínez-Peña, R.; Morcuende, R.; Kefauver, S.C.; López-Cristoffanini, C.; Aparicio, N.; Serret, M.D.; Araus, J.L. Non-foliar photosynthesis and nitrogen assimilation influence grain yield in durum wheat regardless of water conditions. J. Exp. Bot. 2024, 75, 3412–3430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, J.; Tan, Q.; Hu, H.; Sun, C.; Lei, K.; Tian, Z.; Dai, T. Improved chloroplast Pi allocation helps sustain electron transfer to enhance photosynthetic low-phosphorus tolerance of wheat. Plant Physiol. Biochem. 2023, 201, 107880. [Google Scholar] [CrossRef]
- Kubar, M.S.; Wang, C.; Noor, R.S.; Feng, M.; Yang, W.; Kubar, K.A.; Soomro, K.; Yang, C.; Sun, H.; Hasan, M.E. Nitrogen fertilizer application rates and ratios promote the biochemical and physiological attributes of winter wheat. Front. Plant Sci. 2022, 13, 1011515. [Google Scholar] [CrossRef]
- Chen, X.-X.; Zhang, W.; Liang, X.-Y.; Liu, Y.-M.; Xu, S.-J.; Zhao, Q.-Y.; Du, Y.-F.; Zhang, L.; Chen, X.-P.; Zou, C.-Q. Physiological and developmental traits associated with the grain yield of winter wheat as affected by phosphorus fertilizer management. Sci. Rep. 2019, 9, 16580. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, F.; Peng, S.; Chai, R.; Zhang, L.; Zhang, C.; Luo, L. Trade-Off Strategy for Usage of Phosphorus Fertilizer in Calcareous Soil-Grown Winter Wheat: Yield, Phosphorus Use Efficiency, and Zinc Nutrition Response. Agriculture 2024, 14, 373. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Hou, Z.; Li, J. Unlike chemical fertilizer reduction, organic fertilizer substitution increases soil organic carbon stock and soil fertility in wheat fields. J. Sci. Food Agric. Agric. Sci. Procedia 2024, 104, 2798–2808. [Google Scholar] [CrossRef]
- Harmut, A. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–383. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of antioxidant compounds from blueberry fruit waste and evaluation of their in vitro biological activity in human keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Liao, S.; Cui, K. Appropriate ultra-low seed moisture content stabilizes the seed longevity of Calocedrus macrolepis, associated with changes in endogenous hormones, antioxidant enzymes, soluble sugars and unsaturated fatty acids. New For. 2019, 50, 455–468. [Google Scholar] [CrossRef]
- Soneson, C.; Love, M.; Robinson, M. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Kumar, L.; Mfuzz, M.F. A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef]
- Bojović, B.M.; Stojanović, J.; Marković, A. Effects of fertilization on chloroplasts pigments content, leaf surface and dry matter weight in some wheat cultivars. Acta Agric. Serbica 2005, 10, 29–37. [Google Scholar]
- Tranavičienė, T.; Šikšnianienė, J.B.; Urbonavičiūtė, A.; Vagusevičienė, I.; Samuolienė, G.; Duchovskis, P.; Sliesaravičius, A. Effects of nitrogen fertilizers on wheat photosynthetic pigment and carbohydrate contents. Biologija 2007, 53, 80–84. [Google Scholar]
- Jiang, D.; Dai, T.; Jing, Q.; Cao, W.; Zhou, Q.; Zhao, H.; Fan, X. Effects of long-term fertilization on leaf photosynthetic characteristics and grain yield in winter wheat. Photosynthetica 2004, 42, 439–446. [Google Scholar] [CrossRef]
- Guo, L.; Ma, M.; Wu, L.; Zhou, M.; Li, M.; Wu, B.; Li, L.; Liu, X.; Jing, R.; Chen, W. Modified expression of TaCYP78A5 enhances grain weight with yield potential by accumulating auxin in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2022, 20, 168–182. [Google Scholar] [CrossRef]
- Liu, H.; Si, X.; Wang, Z.; Cao, L.; Gao, L.; Zhou, X.; Wang, W.; Wang, K.; Jiao, C.; Zhuang, L. TaTPP-7A positively feedback regulates grain filling and wheat grain yield through T6P-SnRK1 signalling pathway and sugar–ABA interaction. Plant Biotechnol. J. 2023, 21, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, Q.; Pei, X.; Xing, G.; Ou, X.; Li, H. Comparative analysis of the photosynthetic physiology and transcriptome of a high-yielding wheat variety and its parents. Crop J. 2020, 8, 1037–1048. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Q.; Shan, F.; Tian, L.; Gong, J.; Quan, W.; Yang, W.; Hou, Q.; Zhang, F.; Zhang, S. Transcriptional regulation mechanism of wheat varieties with different nitrogen use efficiencies in response to nitrogen deficiency stress. BMC Genom. 2022, 23, 727. [Google Scholar] [CrossRef]
- Fu, Y.-L.; Cui, Z.-H.; Yao, J.-Q.; Ji, F.-Y.; Lu, W.-Y.; He, Z.-J.; Gao, Z.-K.; Wang, S. Effects of water and nitrogen coupling on photosynthetic characteristics and yield of winter wheat in film hole irrigation fields. Desalin. Water Treat. 2022, 268, 273–284. [Google Scholar] [CrossRef]
- Rodrigues, V.A.; Crusciol, C.A.C.; Bossolani, J.W.; Portugal, J.R.; Moretti, L.G.; Bernart, L.; Vilela, R.G.; Galeriani, T.; Lollato, R.P. Foliar nitrogen as stimulant fertilization alters carbon metabolism, reactive oxygen species scavenging, and enhances grain yield in a soybean–maize rotation. Crop Sci. 2021, 61, 3687–3701. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Lopez-Bellido, R.; Hawkesford, M.J. Genotypic variation in the uptake, partitioning and remobilisation of nitrogen during grain-filling in wheat. Field Crops Res. 2014, 156, 242–248. [Google Scholar] [CrossRef]
- Kong, L.; Xie, Y.; Hu, L.; Feng, B.; Li, S. Remobilization of vegetative nitrogen to developing grain in wheat (Triticum aestivum L.). Field Crops Res. 2016, 196, 134–144. [Google Scholar] [CrossRef]
- Oaks, A. Primary nitrogen assimilation in higher plants and its regulation. Can. J. Bot. 1994, 72, 739–750. [Google Scholar] [CrossRef]
- Han, L.; Li, G.J.; Yang, K.Y.; Mao, G.; Wang, R.; Liu, Y.; Zhang, S. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010, 64, 114–127. [Google Scholar] [CrossRef]
- Chen, L.; Hamada, S.; Fujiwara, M.; Zhu, T.; Thao, N.P.; Wong, H.L.; Krishna, P.; Ueda, T.; Kaku, H.; Shibuya, N. The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbes 2010, 7, 185–196. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y. The research progress on the functions of plant aspartic proteases. Chin. Bull. Life Sci 2016, 28, 384–390. [Google Scholar]
- Yegin, S.; Dekker, P. Progress in the field of aspartic proteinases in cheese manufacturing: Structures, functions, catalytic mechanism, inhibition, and engineering. Dairy Sci. Technol. 2013, 93, 565–594. [Google Scholar] [CrossRef]


| Layer (cm) | Organic Substance (g kg−1) | Total Nitrogen (g kg−1) | Total Phosphorus (g kg−1) | Total Potassium (g kg−1) | Available Nitrogen (mg kg−1) | Available Potassium (mg kg−1) | Available Phosphorus (mg kg−1) | Total Salt (g kg−1) | pH |
|---|---|---|---|---|---|---|---|---|---|
| 0–20 | 11.35 | 0.807 | 1.004 | 22.078 | 43.3 | 14.3 | 136 | 0.80 | 8.76 |
| Period | F (kg ha−1) | CK | ||
|---|---|---|---|---|
| N | P2O5 | K2O | ||
| Before sowing | 97.5 | 75 | 150 | / |
| Reviving | 41.4 | / | / | / |
| Erecting | 41.4 | / | / | / |
| Jointing | 110.4 | / | 31.2 | / |
| Heading | 41.4 | / | / | / |
| Flowering | 41.4 | / | / | / |
| Filling | 34.5 | 31.2 | 20.4 | / |
| Years | Treatments | Spike Number (104 spikes·ha−1) | Grain Number per Spike | Thousand Kernel Weight (g) | Grain Yield (kg·ha−1) |
|---|---|---|---|---|---|
| 2018–2019 | F | 535.67 a | 42.18 a | 52.39 a | 9342.80 a |
| CK | 474.00 b | 19.07 b | 44.48 b | 2719.40 b | |
| 2019–2020 | F | 585.60 a | 44.55 a | 50.30 b | 9854.50 a |
| CK | 475.80 b | 18.82 b | 40.97 b | 2751.36 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Shi, J.; Zhang, H.; Chen, X.; Li, J.; Wang, Z.; Li, X.; Gao, X.; Wang, C.; Xia, J.; et al. Physiological and Transcriptome Analyses Reveal the Effects of Fertilization on the Yield of Winter Wheat and on the Photosynthetic Performance of Leaves during the Flowering Period. Genes 2024, 15, 1179. https://doi.org/10.3390/genes15091179
Wang L, Shi J, Zhang H, Chen X, Li J, Wang Z, Li X, Gao X, Wang C, Xia J, et al. Physiological and Transcriptome Analyses Reveal the Effects of Fertilization on the Yield of Winter Wheat and on the Photosynthetic Performance of Leaves during the Flowering Period. Genes. 2024; 15(9):1179. https://doi.org/10.3390/genes15091179
Chicago/Turabian StyleWang, Lihong, Jia Shi, Hongzhi Zhang, Xunji Chen, Jianfeng Li, Zhong Wang, Xiaorong Li, Xin Gao, Chunsheng Wang, Jianqiang Xia, and et al. 2024. "Physiological and Transcriptome Analyses Reveal the Effects of Fertilization on the Yield of Winter Wheat and on the Photosynthetic Performance of Leaves during the Flowering Period" Genes 15, no. 9: 1179. https://doi.org/10.3390/genes15091179
APA StyleWang, L., Shi, J., Zhang, H., Chen, X., Li, J., Wang, Z., Li, X., Gao, X., Wang, C., Xia, J., Zhao, Z., Zhang, Y., Fan, Z., & Zhao, Q. (2024). Physiological and Transcriptome Analyses Reveal the Effects of Fertilization on the Yield of Winter Wheat and on the Photosynthetic Performance of Leaves during the Flowering Period. Genes, 15(9), 1179. https://doi.org/10.3390/genes15091179






