Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants
Abstract
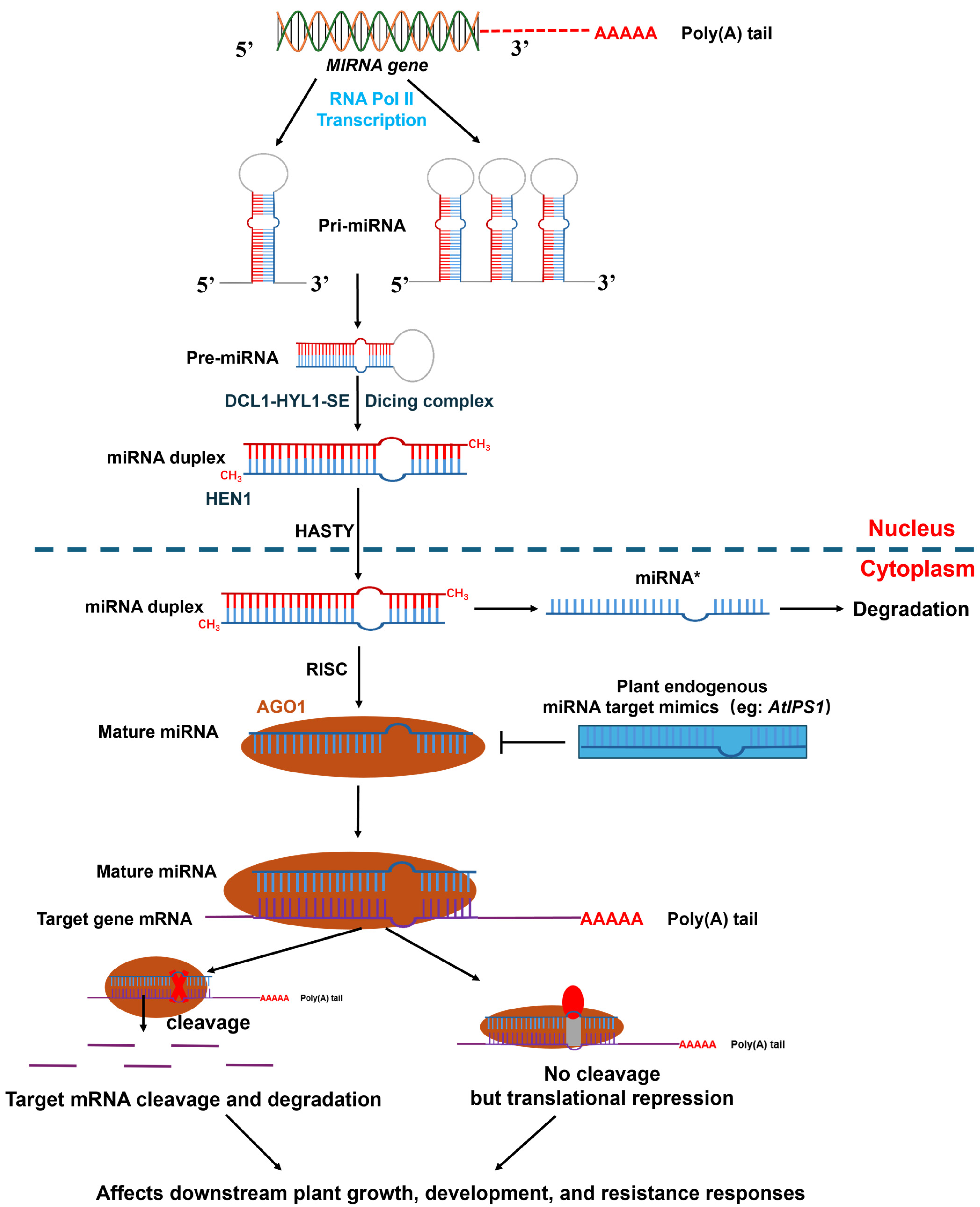
1. Introduction
2. Progress in miRNA Research on Insect Resistance and Breeding in Plants
2.1. Plant Endogenous miRNAs
2.2. Insect Endogenous miRNAs
3. Progress in miRNA Research on Disease Resistance and Breeding in Plants
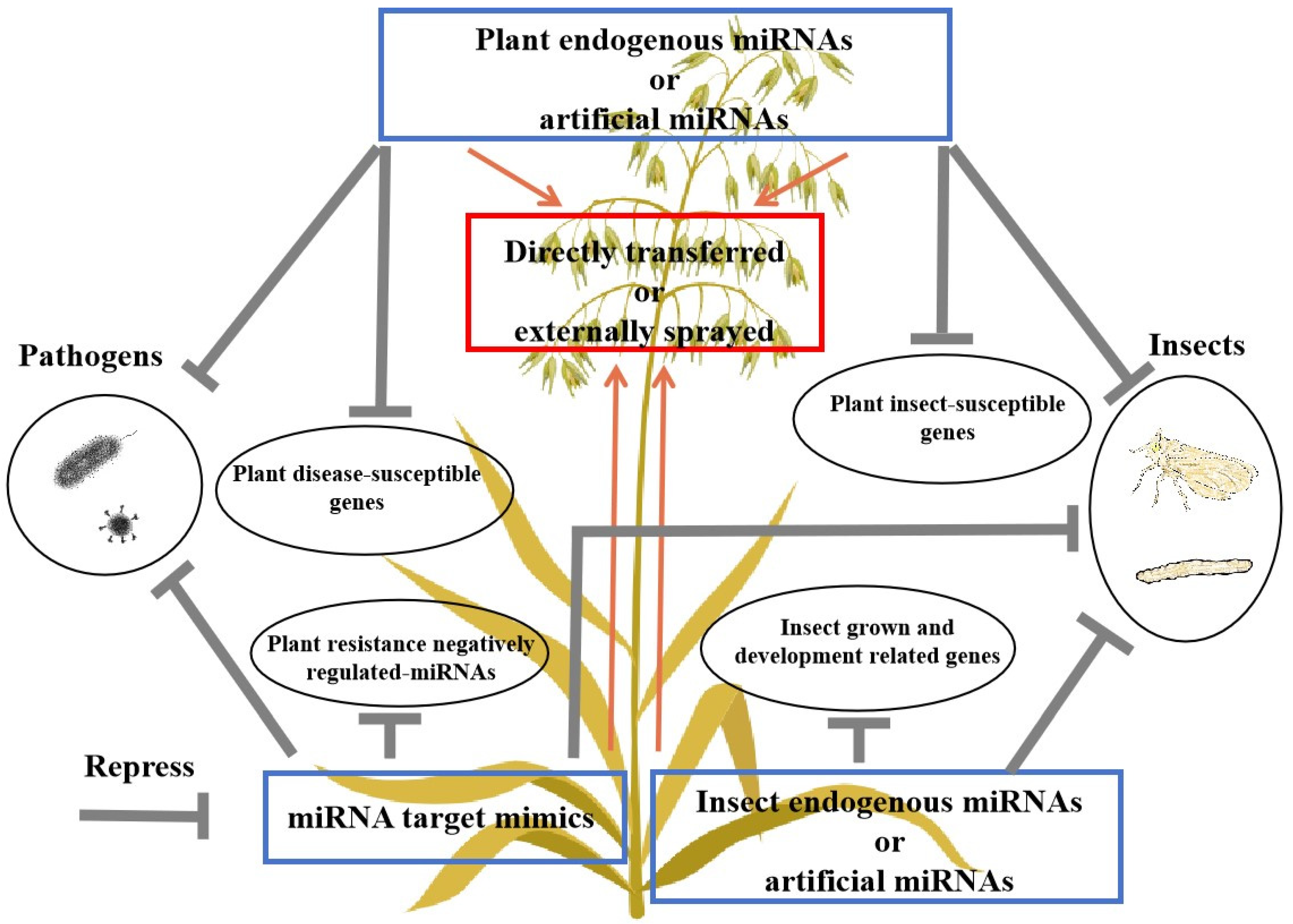
4. Application of Artificial miRNAs (amiRNAs) in Research on Plant Resistance
4.1. Application of amiRNAs for Insecticidal Effects in Plants
4.2. Application of amiRNAs for Disease Resistance in Plants
5. Prospects and Conclusions
5.1. Applications of RNAi Technology in Crop Production
5.2. Challenges in the Application of miRNA Technology in Crop Breeding
5.3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ action through miRNA editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, A.; Zielenkiewicz, P. Plant microRNAs—Novel players in natural medicine? Int. J. Mol. Sci. 2016, 18, 9. [Google Scholar] [CrossRef]
- Ameres, S.; Zamore, P. Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 2013, 14, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Gebert, L.; Macrae, I. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, J. The Crosstalk between MicroRNAs and Gibberellin Signaling in Plants. Plant Cell Physiol. 2020, 61, 1880–1890. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRbase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, 155–162. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Y.; Chen, D.; Chen, F.; Fang, X.; Hong, G.; Wang, L.; Wang, J.; Chen, X. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef]
- Wamiq, G.; Khan, J. Overexpression of ghr-miR166b generates resistance against Bemisia tabaci infestation in Gossypium hirsutum plants. Planta 2018, 247, 1175–1189. [Google Scholar] [CrossRef]
- Agrawal, A.; Rajamani, V.; Reddy, V.; Mukherjee, S.; Bhatnagar, R. Transgenic plants over-expressing insect-specific microRNA acquire insecticidal activity against Helicoverpa armigera: An alternative to Bt-toxin technology. Transgenic Res. 2015, 24, 791–801. [Google Scholar] [CrossRef]
- Ge, Y.; Han, J.; Zhou, G.; Xu, Y.; Ding, Y.; Shi, M.; Guo, C.; Wu, G. Silencing of miR156 confers enhanced resistance to brown planthopper in rice. Planta 2018, 248, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, G.; Miao, X.; Shi, Z. OsmiR159 Modulate BPH Resistance Through Regulating G-Protein γ Subunit GS3 Gene in Rice. Rice 2023, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; Yuan, L.; Shen, W.; Shi, Q.; Qi, G.; Zhang, Z. Osa-miR162a Enhances the Resistance to the Brown Planthopper via α-Linolenic Acid Metabolism in Rice (Oryza sativa). J. Agric. Food Chem. 2023, 71, 11847–11859. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Ran, L.; Hu, J.; Ye, X.; Xu, D.; Li, J.; Su, H.; Wang, X.; Ren, S.; Luo, K. miR319a/TCP module and DELLA protein regulate trichome initiation synergistically and improve insect defenses in Populus tomentosa. New Phytol. 2020, 227, 867–883. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Fan, J.; Hu, C.; Yang, R.; Qi, X.; Chen, H.; Zhao, F.; Wang, S. Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J. Exp. Bot. 2015, 66, 4653–4667. [Google Scholar] [CrossRef]
- Xia, X.; Shao, Y.; Jiang, J.; Du, X.; Sheng, L.; Chen, F.; Fang, W.; Guan, Z.; Chen, S. MicroRNA expression pro file during aphid feeding in chrysanthemum (Chrysanthemum morifolium). PLoS ONE 2015, 10, e0143720. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, W.; Taning, C.; Smagghe, G.; Wang, J. Regulatory roles of microRNAs in insect pests: Prospective targets for insect pest control. Curr. Opin. Biotechnol. 2021, 70, 158–166. [Google Scholar] [CrossRef]
- Muthu Lakshmi Bavithra, C.; Murugan, M.; Pavithran, S.; Naveena, K. Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: Role of transcriptional and post-transcriptional events. Front. Mol. Biosci. 2023, 10, 1257859. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, K.; Agarwala, N. MicroRNAs in plant insect interaction and insect pest control. Plant Gene 2021, 26, 100271. [Google Scholar] [CrossRef]
- Sempere, L.; Dubrovsky, E.; Dubrovskaya, V.; Berger, E. The expression of the let-7 small regulatory RNA is controlled by ecdysone duriniz metamorphosis in Drosophila melanogaster. Dev. Biol. 2002, 244, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.; Sokol, N.; Dubrovsky, E.; Berger, E.; Ambrosa, V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and Broad-Complex gene activity. Dev. Biol. 2003, 259, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Cohen, S. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev. 2007, 21, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xiao, H.; Sun, Y.; Ding, S.; Situ, G.; Li, F. Transgenic microRNA-14 rice shows high resistance to rice stemborer. Plant Biotechnol. J. 2019, 17, 461–471. [Google Scholar] [CrossRef]
- He, K.; Xiao, H.; Sun, Y.; Situ, G.; Xi, Y.; Li, F. microRNA-14 as an efficient suppressor to switch off ecdysone production after ecdysis in insects. RNA Biol. 2019, 16, 1313–1325. [Google Scholar] [CrossRef]
- Epstein, Y.; Perry, N.; Volin, M.; Zohar-Fux, M.; Braun, R.; Porat-Kuperstein, L.; Toledano, H. miR-9a modulates maintenance and ageing of Drosophila germline stem cells by limiting N-cadherin expression. Nat. Commun. 2017, 8, 600. [Google Scholar] [CrossRef]
- Li, X.; Zhao, M.; Tian, M.; Zhao, J.; Cai, W.; Hua, H. An InR/mir-9a/NlUbx regulatory cascade regulates wing diphenism in brown planthoppers. Insect Sci. 2021, 28, 1300–1313. [Google Scholar] [CrossRef]
- Guo, H.; Song, X.; Wang, G.; Yang, K.; Wang, Y.; Niu, L.; Chen, X.; Fang, R. Plant-generated artificial small RNAs mediated aphid resistance. PLoS ONE 2014, 9, e97410. [Google Scholar] [CrossRef]
- Pruss, G.J.; Nester, E.W.; Vance, V. Infiltration with Agrobacterium tumefaciens induces host defense and development-dependent responses in the infiltrated zone. Mol. Plant Microbe Interact. 2008, 21, 1528–1538. [Google Scholar] [CrossRef]
- Djami-Tchatchou, A.; Dubery, I. miR393 regulation of lectin receptor-like kinases associated with LPS perception in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2019, 513, 88–92. [Google Scholar] [CrossRef]
- Kasschau, K.; Xie, Z.; Allen, E.; Llave, C.; Chapman, E.; Krizan, K.; Carrington, J. P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell 2003, 4, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.; Prokhnevsky, A.; Gopinath, K.; Dolja, V.; Carrington, J. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004, 18, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Windels, D.; Vazquez, F. miR393: Integrator of environmental cues in auxin signaling? Plant Signal. Behav. 2011, 6, 1672–1675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.; Zhu, Y.; Yang, X.; Zhang, K.; Xiao, Z.; Wang, H.; Zhao, J.; Zhang, L.; Li, G.; et al. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Zhang, Q.; Fu, Y.; Feng, C.; Wang, B.; Huang, L.; Kang, Z. Monodehydroascorbate reductase gene, regulated by the wheat PN-2013 miRNA, contributes to adult wheat plant resistance to stripe rust through ROS metabolism. Biochim. Biophys. Acta 2014, 1839, 1–12. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants 2017, 3, 485–510. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Han, X.; Shen, F. Genome-wide profiling of miRNAs and other small non-coding RNAs in the Verticillium dahliae-inoculated cotton roots. PLoS ONE 2012, 7, e35765. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Zhao, J.; Wang, S.; Jin, Y.; Chen, Z.; Fang, Y.; Hua, C.; Ding, S.; Guo, H. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Hu, G.; Lei, Y.; Liu, J.; Hao, M.; Zhang, Z.; Tang, Y.; Chen, A.; Wu, J. The ghr-miR164 and GhNAC100 modulate cotton plant resistance against Verticillium dahlia. Plant Sci. 2020, 293, 110438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cui, J.; Shi, Y.; Yang, G.; Zhou, X.; Hou, X.; Meng, J.; Luan, Y. Tomato lncRNA23468 functions as a competing endogenous RNA to modulate NBSLRR genes by decoying miR482b in the tomato-Phytophthora infestans interaction. Hortic. Res. 2019, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Chern, M.; Zhu, Y.; Zhang, L.; Lu, J.; Li, X.; Dang, W.; Ma, X.; Yang, Z.; et al. Suppression of rice miR168 improves yield, flowering time and immunity. Nat. Plants 2021, 7, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hui, S.; Lv, Y.; Zhang, M.; Chen, D.; Tian, J.; Zhang, H.; Liu, H.; Cao, J.; Xie, W.; et al. miR395-regulated sulfate metabolism exploits pathogen sensitivity to sulfate to boost immunity in rice. Mol. Plant 2022, 15, 671–688. [Google Scholar] [CrossRef]
- Li, F.; Pignatta, D.; Bendix, C.; Brunkard, J.O.; Cohn, M.M.; Tung, J.; Sun, H.; Kumar, P.; Baker, B. MicroRNA regulation of plant innate immune receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1790–1795. [Google Scholar] [CrossRef]
- Luo, M.; Peng, H.; Gao, J.; Pan, G.; Zhang, Z. Identification and functional analysis of miRNAs in response to banded leaf and sheath blight in Zea mays. Chin. J. Biochem. Mol. Biol. 2012, 28, 1122–1132. [Google Scholar]
- Loreti, E.; Perata, P. Mobile plant microRNAs allow communication within and between organisms. New Phytol. 2022, 235, 2176–2182. [Google Scholar] [CrossRef]
- Niu, D.; Lii, Y.; Chellappan, P.; Lei, L.; Peralta, K.; Jiang, C.; Guo, J.; Coaker, G.; Jin, H. miRNA863-3p sequentially targets negative immune regulator ARLPKs and positive regulator SERRATE upon bacterial infection. Nat. Commun. 2016, 7, 11324. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Y.R.; Pei, Y.; Lin, S.; Tuschl, T.; Patel, D.; Chua, N. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006, 20, 3255–3268. [Google Scholar] [CrossRef]
- Schwab, R.; Ossowski, S.; Riester, M.; Warthmann, N.; Weigel, D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 2006, 18, 1121–1133. [Google Scholar] [CrossRef]
- Kryovrysanaki, N.; James, A.; Tselika, M.; Bardani, E.; Kalantidis, K. RNA silencing pathways in plant development and defense. Int. J. Dev. Biol. 2022, 66, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ding, S. RNA silencing. Curr. Opin. Biotechnol. 2000, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Baldrich, P.; Kakar, K.; Sire, C.; Moreno, A.; Berger, A.; GarcíaChapa, M.; López-Moya, J.; Riechmann, J.; San Segundo, B. Small RNA profiling reveals regulation of Arabidopsis miR168 and heterochromatic siRNA415 in response to fungal elicitors. BMC Genom. 2014, 15, 1083. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Srivastava, A.; Pan, Y.; Bai, J.; Fang, J.; Shi, H.; Zhu, J. Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018, 176, 2082–2094. [Google Scholar] [CrossRef]
- Mengistu, A.; Tenkegna, T. The role of miRNA in plant-virus interaction: A review. Mol. Biol. Rep. 2021, 48, 2853–2861. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Saini, A.; Sunkar, R. Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis. Planta 2009, 229, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Kiran, S.; Du, D.; Gmitter, F.; Deng, Z. Comprehensive meta-analysis, co-expression, and miRNA nested network analysis identifies gene candidates in citrus against Huanglongbing disease. BMC Plant Biol. 2015, 15, 184. [Google Scholar] [CrossRef]
- Chisholm, S.; Coaker, G.; Day, B.; Staskawicz, B. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 810–814. [Google Scholar] [CrossRef]
- Ngou, B.; Ding, P.; Jones, J. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Shivaprasad, P.; Chen, H.; Patel, K.; Bond, D.; Santos, B.; Baulcombe, D. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 2012, 24, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sun, Y.; Chiang, V. Stress-responsive microRNAs in populus. Plant J. 2008, 55, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Peris-Peris, C.; Siré, C.; Moreno, A.; Donaire, L.; Zytnicki, M.; Notredame, C.; Llave, C.; San Segundo, B. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytol. 2013, 199, 212–227. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Shi, Y.; Wu, L.; Xu, Y.; Huang, F. Multiple rice micrornas are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol. 2014, 164, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Wang, Y.; Yao, Y.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Diverse set of microRNAs are responsive tompowdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biol. 2010, 10, 123. [Google Scholar] [CrossRef]
- Hua, C.; Zhao, J.; Guo, H. Trans-Kingdom RNA Silencing in Plant-Fungal Pathogen Interactions. Mol. Plant 2018, 11, 235–244. [Google Scholar] [CrossRef]
- Tang, J.; Gu, X.; Liu, J.; He, Z. Roles of small RNAs in crop disease resistance. Stress Biol. 2021, 1, 6. [Google Scholar] [CrossRef]
- Ding, T.; Li, W.; Li, F.; Ren, M.; Wang, W. microRNAs: Key Regulators in Plant Responses to Abiotic and Biotic Stresses via Endogenous and Cross-Kingdom Mechanisms. Int. J. Mol. Sci. 2024, 25, 1154. [Google Scholar] [CrossRef]
- Sablok, G.; Pérez-Quintero, Á.; Hassan, M.; Tatarinova, T.; López, C. Artificial microRNAs (amiRNAs) engineering—On how microRNAbased silencing methods have affected current plant silencing research. Biochem. Biophys. Res. Commun. 2011, 406, 315–319. [Google Scholar] [CrossRef]
- Ai, T.; Zhang, L.; Gao, Z.; Zhu, C.; Guo, X. Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants. Plant Biol. 2011, 13, 304–316. [Google Scholar] [CrossRef]
- Park, W.; Zhai, J.; Lee, J. Highly efficient gene silencing using perfect complementary artificial miRNA targeting AP1 or heteromeric artificial miRNA targeting AP1 and CAL genes. Plant Cell Rep. 2009, 28, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.; Palatnik, J.; Riester, M.; Schommer, C.; Schmidet, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Lin, S.; Reyes, J.; Chen, K.; Wu, H.; Yeh, S.; Chua, N. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat. Biotechnol. 2006, 24, 1420–1428. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Zhang, J.; Zhang, C.; Gong, P.; Ziaf, K.; Xiao, F.; Ye, Z. Expression of artificial microRNAs in tomato confers efficient and stable virus resistance in a cellautonomous manner. Transgenic Res. 2011, 20, 569–581. [Google Scholar] [CrossRef]
- Saini, R.; Raman, V.; Dhandapani, G.; Malhotra, E.; Sreevathsa, R.; Kumar, P.; Sharma, T.; Pattanayak, D. Silencing of HaAce1 gene by host-delivered artificial microRNA disrupts growth and development of Helicoverpa armigera. PLoS ONE 2018, 13, e0194150. [Google Scholar] [CrossRef]
- Qu, J.; Ye, J.; Fang, R. Artificial microRNA-mediated virus resistance in plants. J. Virol. 2007, 81, 6690–6699. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Han, Q.; Jiang, F.; Sun, R.; Fan, Z.; Zhu, C.; Wen, F. Effects of the sequence characteristics of miRNAs on multi-viral resistance mediated by single amiRNAs in transgenic tobacco. Plant Physiol. Biochem. 2014, 77, 90–98. [Google Scholar] [CrossRef]
- Zheng, X.; Weng, Z.; Li, H.; Kong, Z.; Zhou, Z.; Li, F.; Ma, W.; Lin, Y.; Chen, H. Transgenic rice overexpressing insect endogenous microRNA csu-novel-260 is resistant to striped stem borer under field conditions. Plant Biotechnol. J. 2021, 19, 421–423. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, H.; Liu, H.; Zheng, J.; Lin, Y.; Chen, H. The overexpression of insect endogenous small RNAs in transgenic rice inhibits growth and delays pupation of striped stem borer (Chilo suppressalis). Pest Manag. Sci. 2017, 73, 1453–1461. [Google Scholar] [CrossRef]
- Liu, H.; Shen, E.; Wu, H.; Ma, W.; Chen, H.; Lin, Y. Trans-kingdom expression of an insect endogenous microRNA in rice enhances resistance to striped stem borer Chilo suppressalis. Pest Manag. Sci. 2022, 78, 770–777. [Google Scholar] [CrossRef]
- Li, C.; Wei, J.; Lin, Y.; Chen, H. Gene silencing using the recessive rice bacterial blight resistance gene xa13 as a new paradigm in plant breeding. Plant Cell Rep. 2012, 31, 851–862. [Google Scholar] [CrossRef]
- Shen, W.; Cao, S.; Liu, J.; Zhang, W.; Chen, J.; Li, J. Overexpression of an Osa-miR162a Derivative in Rice Confers Cross-Kingdom RNA Interference-Mediated Brown Planthopper Resistance without Perturbing Host Development. Int. J. Mol. Sci. 2021, 22, 12652. [Google Scholar] [CrossRef]
- Daborn, P.; Lumb, C.; Harrop, T.; Blasetti, A.; Pasricha, S.; Morin, S.; Mitchell, S.; Donnelly, M.; Müller, P.; Batterham, P. Using Drosophila melanogaster to validate metabolism-based insecticide resistance from insect pests. Insect Biochem. Mol. Biol. 2012, 42, 918–924. [Google Scholar] [CrossRef]
- Kung, Y.; Lin, S.; Huang, Y.; Chen, T.; Harish, S.; Chua, N.; Yeh, S. Multiple artificial microRNAs targeting conserved motifs of the replicase gene confer robust transgenic resistance to negative-sense single-stranded RNA plant virus. Mol. Plant Pathol. 2012, 13, 303–317. [Google Scholar] [CrossRef]
- Zeng, Y.; Wagner, E.; Cullen, B. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 2002, 9, 1327–1333. [Google Scholar] [CrossRef]
- Parizotto, E.; Dunoyer, P.; Rahm, N.; Himber, C.; Voinnet, O. In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 2004, 18, 2237–2242. [Google Scholar] [CrossRef]
- Toppino, L.; Kooiker, M.; Lindner, M.; Dreni, L.; Rotino, G.; Kater, M. Reversible male sterility in eggplant (Solanum melongena L.) by artificial microRNA-mediated silencing of general transcription factor genes. Plant Biotechnol. J. 2011, 9, 684–692. [Google Scholar] [CrossRef]
- Warthmann, N.; Chen, H.; Ossowski, S.; Weigel, D.; Hervé, P. Highly specific gene silencing by artificial miRNAs in rice. PLoS ONE 2008, 3, e1829. [Google Scholar] [CrossRef]
- Wagaba, H.; Patil, B.; Mukasa, S.; Alicai, T.; Fauquet, C.; Taylor, N. Artificial microRNA-derived resistance to Cassava brown streak disease. J. Virol Methods 2016, 231, 231–243. [Google Scholar] [CrossRef]
- Peng, J.; Chen, T.; Raja, J.; Yang, C.; Chien, W.; Lin, C.; Liu, F.; Wu, H.; Yeh, S. Broad-spectrum transgenic resistance against distinct tospovirus species at the genus level. PLoS ONE 2014, 9, e96073. [Google Scholar] [CrossRef]
- Chandra, T.; Jaiswal, S.; Iquebal, M.; Singh, R.; Gautam, R.; Rai, A.; Kumar, D. Revitalizing miRNAs mediated agronomical advantageous traits improvement in rice. Plant Physiol. Biochem. 2023, 202, 107933. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wu, H.; Elena, S.; Chen, K.; Niu, Q.; Yeh, S.; Chen, C.; Chua, N. Molecular evolution of a viral noncoding sequence under the selective pressure of amiRNA-mediated silencing. PLoS Pathog. 2009, 5, e1000312. [Google Scholar] [CrossRef] [PubMed]
- Simón-Mateo, C.; García, J.A. MicroRNA-guided processing impairs Plum pox virus replication, but the virus readily evolves to escape this silencing mechanism. J. Virol. 2006, 80, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.G.; Wang, C.H.; Fang, R.X.; Guo, H.S. Artificial MicroRNAs highly accessible to targets confer efficient virus resistance in plants. J. Virol. 2008, 82, 11084–11095. [Google Scholar] [CrossRef]
- Dhandapani, R.; Gurusamy, D.; Howell, J.; Palli, S. Development of CS-TPP-dsRNA nanoparticles to enhance RNAi efficiency in the yellow fever mosquito. Aedes Aegypti. Sci. Rep. 2019, 9, 8775. [Google Scholar] [CrossRef]
- Niehl, A.; Heinlein, M. Perception of double-stranded RNA in plant antiviral immunity. Mol. Plant Pathol. 2019, 20, 1203–1210. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H. Spray-induced gene silencing:A powerful innovative strategy for crop protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef]
- Zotti, M.; Dos Santos, E.; Cagliari, D.; Christiaens, O.; Taning, C.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Camacho, A.; Van Deynze, A.; Chi-Ham, C.; Bennett, A.B. Genetically engineered crops that fly under the US regulatory radar. Nat. Biotechnol. 2014, 32, 1087–1091. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon nanotube-mediated DNA delivery without transgene integration in intact plants. Nat. Protoc. 2019, 14, 2954–2971. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sarkar, S. Carbon dots in agricultural system. In Carbon Dots in Agricultural Systems; Khan, R., Murali, S., Gogoi, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 175–197. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Target(s) | Plant Species | Insect Species | Function | Reference |
|---|---|---|---|---|---|
| AtmiR156 | SPL9 | A. thaliana | Helicoverpa armigera Plutella xylostella | Decrease weight and prolong larval stage | [8] |
| Ghr-miR166b | ATP synthase gene | Gossypium hirsutum | Bemisia tabaci | Death | [9] |
| miR-24 | Chitinase | N. tabacum | H. armigera | Death | [10] |
| OsmiR156 | SPL14 | O. sativa | Nilaparvata lugens | Decrease fertility | [11] |
| OsmiR159 | OsGAMYBL2 | O. sativa | N. lugens | Decrease honeydew excretion | [12] |
| OsmiR396 | OsGRF8 | O. sativa | N. lugens | Decrease honeydew excretion | [13] |
| OsmiR162a | OsDCL1 | O. sativa | N. lugens | Decrease fertility | [14] |
| PtmiR319a | TCP | Populus tomentosa | Spodoptera frugiperda | Decrease feeding | [15] |
| SlmiR319 | TCP4 | Solanum lycopersicum | Meloidogyne incognita | Decrease fertility | [16] |
| miRNA | Target(s) | Plant Species | Pathogen Species | Function | Reference |
|---|---|---|---|---|---|
| AtmiR393 | TIR1 | A. thaliana | Pseudomonas syringae | Increase resistance | [38] |
| GhmiR2118 | TIR-NBS-LRR genes | G. hirsutum | Verticillium dahliae | Promote target protein accumulation, enhance root defense | [39] |
| GhmiR166, GhmiR159 | Clp-1, HiC-15 | G. hirsutum | V. dahliae | Identification and degradation | [40] |
| GhmiR164 | GhNAC100 | G. hirsutum | V. dahliae | Increase resistance | [41] |
| NtmiR482b | Solyc02g036270.2 | N. tabacum | Phytophthora parasitica | Increase NBS-LRR expression and resistance | [42] |
| OsmiR168 | Ago1 | O. sativa | Magnaporthe oryzae | Enhance resistance | [43] |
| OsmiR395 | ATP sulfurylase gene OsAPS1 | O. sativa | Xanthomonas oryzae pv. oryzae (Xoo); X. oryzae pv. oryzicola (Xoc) | Enhance broad-spectrum resistance. | [44] |
| OsmiR398b | Superoxide dismutase | O. sativa | M. oryzae | Upregulate of H2O2 level, increase resistance | [34] |
| OsmiR528 | L-Ascorbate oxidase | O. sativa | Rice stripe tenuivirus | Reduce cell death and damage | [37] |
| StmiR482, SlmiR482 | Ry, NL25, N, RB, R2, and R3a | Solanum tuberosum, S. lycopersicum | Phytophthora infestans | Genes cluster to confer resistance | [45] |
| PN-2013 | Monodehydroascorbate reductase gene | Triticum aestivum | Puccinia striiformis | Upregulate PR gene expression, increase resistance | [36] |
| ZmmiR168 | Ago1 | Z. mays | Rhizoctonia solani | Promote resistance gene accumulations, increase resistance | [46] |
| Target(s) | Plant Species | Insect/Pathogen Species | Function | Reference |
|---|---|---|---|---|
| HC-Pro, p69 gene | A. thaliana | Turnip mosaic virus, Turnip yellow mosaic virus | Mediate viral RNA cleavage, Increase resistance | [73] |
| ATP synthase gene | G. hirsutum | Bemisia Tabaci | Death | [9] |
| 2b gene | Lycopersicum esculentummill | Cucumber mosaic virus | Target viral RNA, Increase resistance | [74] |
| HaAce1 gene | N. tabacum | H. armigera | Induce larval death and deformity in adult | [75] |
| 2b gene | N. tabacum | Cucumber mosaic virus | Target viral RNA, Increase resistance | [76] |
| Nuclear inclusion b protein, coat protein | N. tabacum | Potato virus Y | Target viral RNA, Increase resistance | [77] |
| MpAChE2 | N. tabacum | M. persicae | Decrease fecundity | [28] |
| Ecdysone receptor, Spook gene | O. sativa | Chilo suppressalis | Death | [24] |
| Disembodied protein | O. sativa | C. suppressalis | Death | [78] |
| DN70206_c1_g10 | O. sativa | C. suppressalis | Decrease weight, prolong larval stage | [79] |
| DN90065_c0_g12 | O. sativa | C. suppressalis | Decrease weight, decrease eclosion rate, prolong larval stage | [80] |
| Xa13 | O. sativa | X. oryzae | Silence genes, increase resistance | [81] |
| HC-Pro, p25 gene | S. tuberosum | Potato virus Y | Reduce pathogenicity | [70] |
| HC-Pro, p25 gene | S. tuberosum | Potato virus X | Reduce pathogenicity | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wang, J.; Li, C. Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants. Genes 2024, 15, 1200. https://doi.org/10.3390/genes15091200
Ma Z, Wang J, Li C. Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants. Genes. 2024; 15(9):1200. https://doi.org/10.3390/genes15091200
Chicago/Turabian StyleMa, Zengfeng, Jianyu Wang, and Changyan Li. 2024. "Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants" Genes 15, no. 9: 1200. https://doi.org/10.3390/genes15091200
APA StyleMa, Z., Wang, J., & Li, C. (2024). Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants. Genes, 15(9), 1200. https://doi.org/10.3390/genes15091200







