Smoltification of Atlantic Salmon (Salmo salar L.) Is Associated with Enhanced Traffic and Renewal of B Cell Repertoire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish
2.2. RNA Extraction, Gene Expression
2.3. IgM Repertoire Sequencing
2.4. Statistics
3. Results
3.1. Gene Expression: Differences between Tissues and Temporal Changes
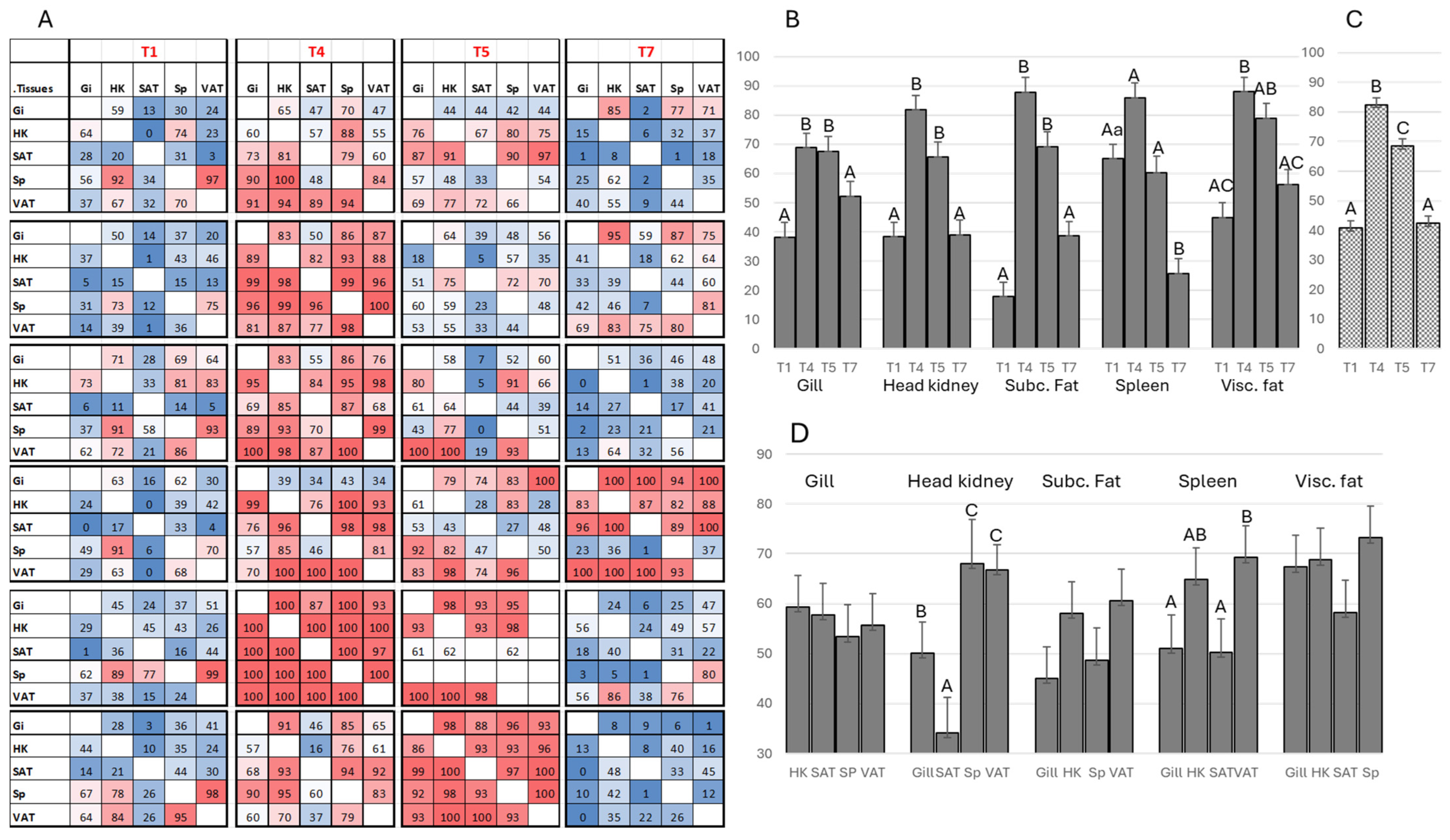
3.2. Sequencing of the Variable Region of igm Heavy Chain (Ig-seq) Reveals B Cell Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barron, M.G. Endocrine control of smoltification in anadromous salmonids. J. Endocrinol. 1986, 108, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2010, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.-H.; Timmerhaus, G.; Afanasyev, S.; Jørgensen, S.M.; Krasnov, A. Smoltification and seawater transfer of Atlantic salmon (Salmo salar L.) is associated with systemic repression of the immune transcriptome. Fish Shellfish Immunol. 2016, 58, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Timmerhaus, G.; Baranski, M.; Andersen, Ø.; Takle, H.; Krasnov, A. Training the salmon’s genes: Influence of aerobic exercise, swimming performance and selection on gene expression in Atlantic salmon. BMC Genom. 2017, 18, 971. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, C.; Ytteborg, E.; Timmerhaus, G.; Høst, V.; Handeland, S.; Jørgensen, S.M.; Krasnov, A. Atlantic salmon skin barrier functions gradually enhance after seawater transfer. Sci. Rep. 2018, 8, 9510. [Google Scholar] [CrossRef]
- Shwe, A.; Krasnov, A.; Visnovska, T.; Ramberg, S.; Østbye, T.-K.K.; Andreassen, R. Differential Expression of miRNAs and Their Predicted Target Genes Indicates That Gene Expression in Atlantic Salmon Gill Is Post-Transcriptionally Regulated by miRNAs in the Parr-Smolt Transformation and Adaptation to Sea Water. Int. J. Mol. Sci. 2022, 23, 8831. [Google Scholar] [CrossRef]
- Krasnov, A.; Burgerhout, E.; Johnsen, H.; Tveiten, H.; Bakke, A.F.; Lund, H.; Afanasyev, S.; Rebl, A.; Johansen, L.-H. Development of Atlantic Salmon (Salmo salar L.) Under Hypoxic Conditions Induced Sustained Changes in Expression of Immune Genes and Reduced Resistance to Moritella viscosa. Front. Ecol. Evol. 2021, 9, 722218. [Google Scholar] [CrossRef]
- Lund, H.; Bakke, A.; Boysen, P.; Afanasyev, S.; Rebl, A.; Manji, F.; Ritchie, G.; Krasnov, A. Evaluation of Immune Status in Two Cohorts of Atlantic Salmon Raised in Different Aquaculture Systems (Case Study). Genes 2022, 13, 736. [Google Scholar] [CrossRef]
- Krasnov, A.; Jørgensen, S.M.; Afanasyev, S. Ig-seq: Deep sequencing of the variable region of Atlantic salmon IgM heavy chain transcripts. Mol. Immunol. 2017, 88, 99–105. [Google Scholar] [CrossRef]
- Krasnov, A.; Afanasyev, S.; Nylund, S.; Rebl, A. Multigene Expression Assay for Assessment of the Immune Status of Atlantic Salmon. Genes 2020, 11, 1236. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, R.; Karlsen, C.; Boudinot, P.; Afanasyev, S.; Mørkøre, T.; Krasnov, A. Differentiation and traffic of IgM+ B cells between focal dark spots in skeletal muscle of Atlantic salmon, lymphoid and adipose tissues. Fish Shellfish Immunol. 2023, 139, 108858. [Google Scholar] [CrossRef] [PubMed]
- Mota, V.C.; Verstege, G.C.; Striberny, A.; Lutfi, E.; Dessen, J.-E.; Sveen, L.; Burgerhout, E.; Bou, M. Smoltification, seawater performance, and maturation in Atlantic salmon (Salmo salar) fed different fat levels. Front. Aquac. 2024, 3, 1323818. [Google Scholar] [CrossRef]
- Clarke, W. Evaluation of the seawater challenge test as an index of marine survival. Aquaculture 1982, 28, 177–183. [Google Scholar] [CrossRef]
- McCormick, S.D.; Shrimpton, J.M.; Moriyama, S.; Björnsson, B.T. Differential hormonal responses of Atlantic salmon parr and smolt to increased daylength: A possible developmental basis for smolting. Aquaculture 2007, 273, 337–344. [Google Scholar] [CrossRef]
- Holmes, M.L.; Pridans, C.; Nutt, S.L. The regulation of the B-cell gene expression programme by Pax5. Immunol. Cell Biol. 2008, 86, 47–53. [Google Scholar] [CrossRef]
- Chu, P.G.; Arber, D.A. CD79: A review. Appl. Immunohistochem. Mol. Morphol. 2001, 9, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Braden, L.M.; Rasmussen, K.J.; Purcell, S.L.; Ellis, L.; Mahony, A.; Cho, S.; Whyte, S.K.; Jones, S.R.M.; Fast, M.D. Acquired Protective Immunity in Atlantic Salmon Salmo salar against the Myxozoan Kudoa thyrsites Involves Induction of MHIIβ(+) CD83(+) Antigen-Presenting Cells. Infect. Immun. 2018, 86, e00556-17. [Google Scholar] [CrossRef]
- Lagos, L.X.; Iliev, D.B.; Helland, R.; Rosemblatt, M.; Jørgensen, J.B. CD40L—A costimulatory molecule involved in the maturation of antigen presenting cells in Atlantic salmon (Salmo salar). Dev. Comp. Immunol. 2012, 38, 416–430. [Google Scholar] [CrossRef]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–30. [Google Scholar]
- Shibasaki, Y.; Afanasyev, S.; Fernández-Montero, A.; Ding, Y.; Watanabe, S.; Takizawa, F.; Lamas, J.; Fontenla-Iglesias, F.; Leiro, J.M.; Krasnov, A.; et al. Cold-blooded vertebrates evolved organized germinal center–like structures. Sci. Immunol. 2023, 8, eadf1627. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Lillehaug, A. Vaccination strategies in seawater cage culture of salmonids. Dev. Biol. Stand. 1997, 90, 401–408. [Google Scholar] [PubMed]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Matsiota, P.; Torchy, C.; De Kinkelin, P.; Avrameas, S. Natural anti-TNP antibodies from rainbow trout interfere with viral infection in vitro. Res. Immunol. 1989, 140, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Gonzalez, R.; Bonjour, E.; Avrameas, S. A concurrent increasing of natural antibodies and enhancement of resistance to furunculosis in rainbow-trout. Ann. Rech. Vet. 1990, 21, 211–218. [Google Scholar]
- Coll, J.M. Herpesvirus Infection Induces both Specific and Heterologous Antiviral Antibodies in Carp. Front. Immunol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Bakke, A.F.; Rebl, A.; Frost, P.; Afanasyev, S.; Røyset, K.A.; Søfteland, T.; Lund, H.; Boysen, P.; Krasnov, A. Effect of two constant light regimens on antibody profiles and immune gene expression in Atlantic salmon following vaccination and experimental challenge with salmonid alphavirus. Fish Shellfish Immunol. 2021, 118, 188–196. [Google Scholar] [CrossRef]
- Aslam, M.L.; Aslam, M.L.; Robledo, D.; Krasnov, A.; Moghadam, H.K.; Hillestad, B.; Houston, R.D.; Baranski, M.; Boison, S.; Robinson, N.A. Quantitative trait loci and genes associated with salmonid alphavirus load in Atlantic salmon: Implications for pancreas disease resistance and tolerance. Sci. Rep. 2020, 10, 10393. [Google Scholar] [CrossRef]
- Johansen, L.-H.; Thim, H.L.; Jørgensen, S.M.; Afanasyev, S.; Strandskog, G.; Taksdal, T.; Fremmerlid, K.; McLoughlin, M.; Jørgensen, J.B.; Krasnov, A. Comparison of transcriptomic responses to pancreas disease (PD) and heart and skeletal muscle in-flammation (HSMI) in heart of Atlantic salmon (Salmo salar L). Fish Shellfish Immunol. 2015, 46, 612–623. [Google Scholar] [CrossRef]
- Hillestad, B.; Makvandi-Nejad, S.; Krasnov, A.; Moghadam, H.K. Identification of genetic loci associated with higher resistance to pancreas disease (PD) in Atlantic salmon (Salmo salar L.). BMC Genom. 2020, 21, 388. [Google Scholar] [CrossRef]
- Bakke, A.F.; Bjørgen, H.; Koppang, E.O.; Frost, P.; Afanasyev, S.; Boysen, P.; Krasnov, A.; Lund, H. IgM+ and IgT+ B Cell Traffic to the Heart during SAV Infection in Atlantic Salmon. Vaccines 2020, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Kortner, T.M.; Afanasyev, S.; Koppang, E.O.; Bjørgen, H.; Krogdahl, Å.; Krasnov, A. A comprehensive transcriptional body map of Atlantic salmon unveils the vital role of the intestine in the immune system and highlights functional specialization within its compartments. Fish Shellfish Immunol. 2024, 146, 109422. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Lee, B.-C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 446–462. [Google Scholar] [CrossRef]
- Winer, D.A.; Winer, S.; Chng, M.H.Y.; Shen, L.; Engleman, E.G. B Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell. Mol. Life Sci. 2014, 71, 1033–1043. [Google Scholar] [CrossRef]
- Pignatelli, J.; Castro, R.; Granja, A.G.; Abós, B.; González, L.; Jensen, L.B.; Tafalla, C. Immunological Characterization of the Teleost Adipose Tissue and Its Modulation in Response to Viral Infection and Fat-Content in the Diet. PLoS ONE 2014, 9, e110920. [Google Scholar] [CrossRef]
- Simón, R.; Martín-Martín, A.; Morel, E.; Díaz-Rosales, P.; Tafalla, C. Functional and Phenotypic Characterization of B Cells in the Teleost Adipose Tissue. Front. Immunol. 2022, 13, 868551. [Google Scholar] [CrossRef]
- Veenstra, K.A.; Alnabulsi, A.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. Immunohistochemical examination of immune cells in adipose tissue of rainbow trout (Oncorhynchus mykiss) following intraperitoneal vaccination. Fish Shellfish Immunol. 2019, 87, 559–564. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnov, A.; Afanasyev, S.; Hansen, M.H.S.; Bou, M.; Sveen, L.; Dessen, J.-E. Smoltification of Atlantic Salmon (Salmo salar L.) Is Associated with Enhanced Traffic and Renewal of B Cell Repertoire. Genes 2024, 15, 1220. https://doi.org/10.3390/genes15091220
Krasnov A, Afanasyev S, Hansen MHS, Bou M, Sveen L, Dessen J-E. Smoltification of Atlantic Salmon (Salmo salar L.) Is Associated with Enhanced Traffic and Renewal of B Cell Repertoire. Genes. 2024; 15(9):1220. https://doi.org/10.3390/genes15091220
Chicago/Turabian StyleKrasnov, Aleksei, Sergey Afanasyev, Marianne H. S. Hansen, Marta Bou, Lene Sveen, and Jens-Erik Dessen. 2024. "Smoltification of Atlantic Salmon (Salmo salar L.) Is Associated with Enhanced Traffic and Renewal of B Cell Repertoire" Genes 15, no. 9: 1220. https://doi.org/10.3390/genes15091220






