Revision of the Most Primitive Taxa of the Family Gyrodactylidae (van Beneden et Hesse, 1864) (Platyhelminthes, Monopisthocotyla) Based on ITS rDNA Phylogeny
Abstract
:1. Introduction
2. Materials and Methods
3. Results
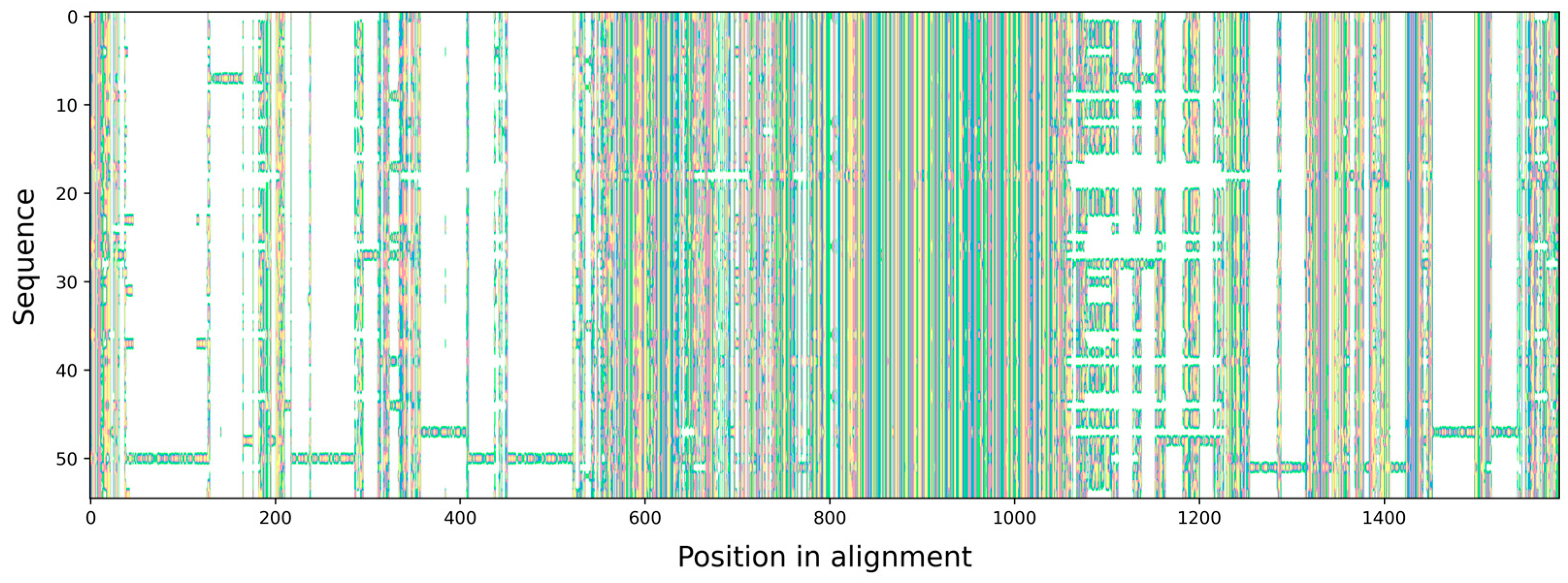
3.1. Characteristics of the ITS rDNA Marker MSA
3.2. Molecular Analysis
3.2.1. Intra- and Interspecific Genetic Distances
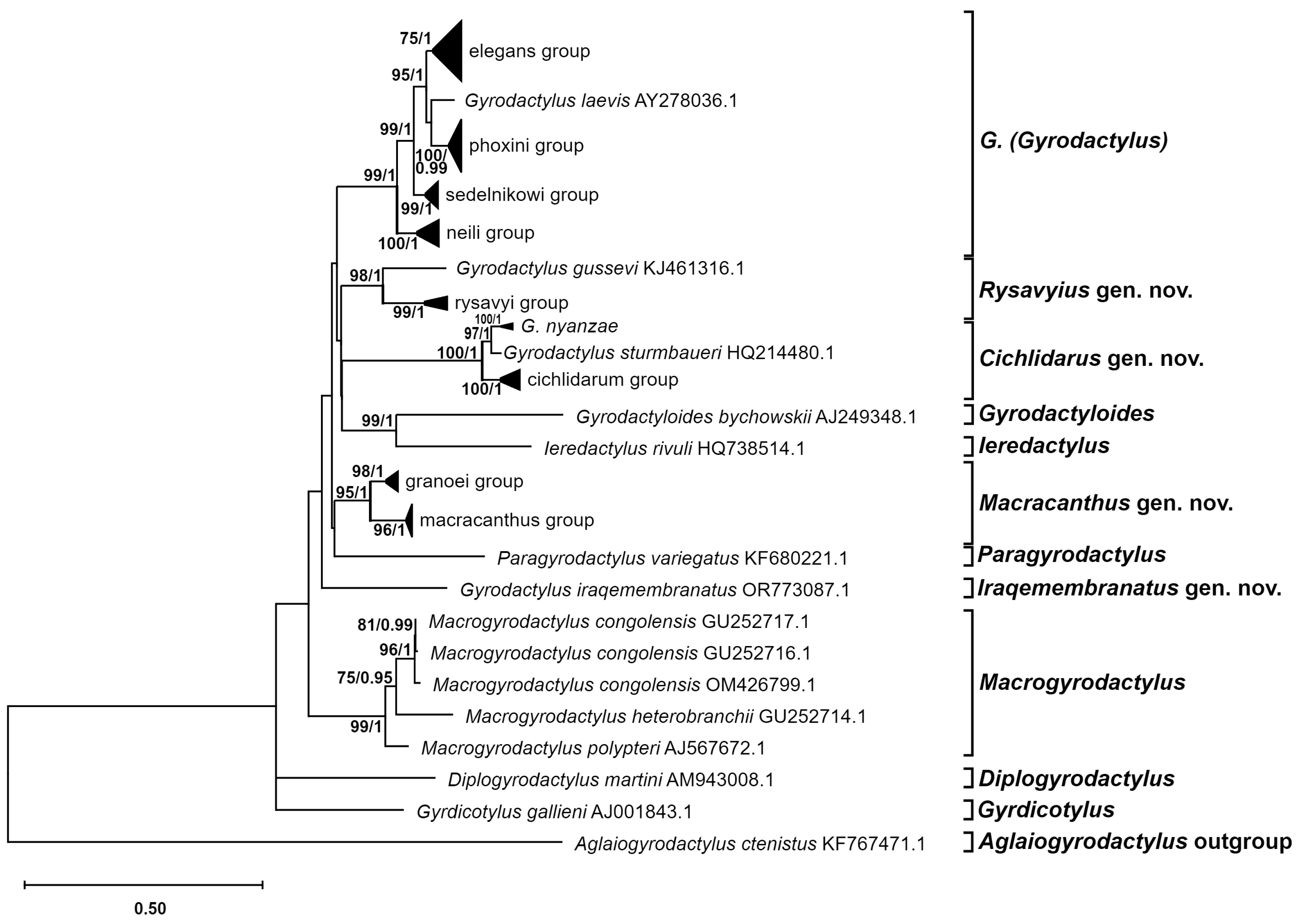
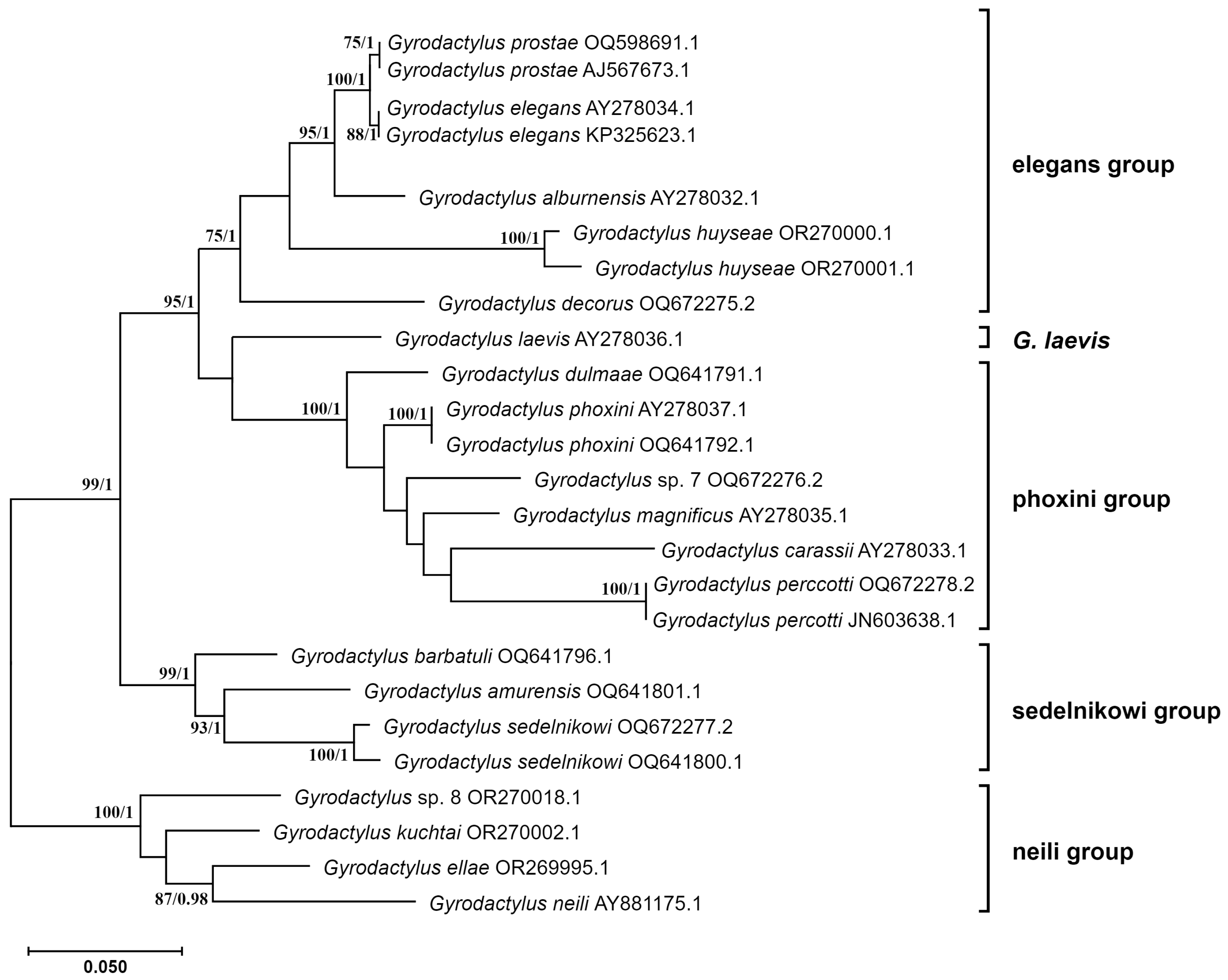
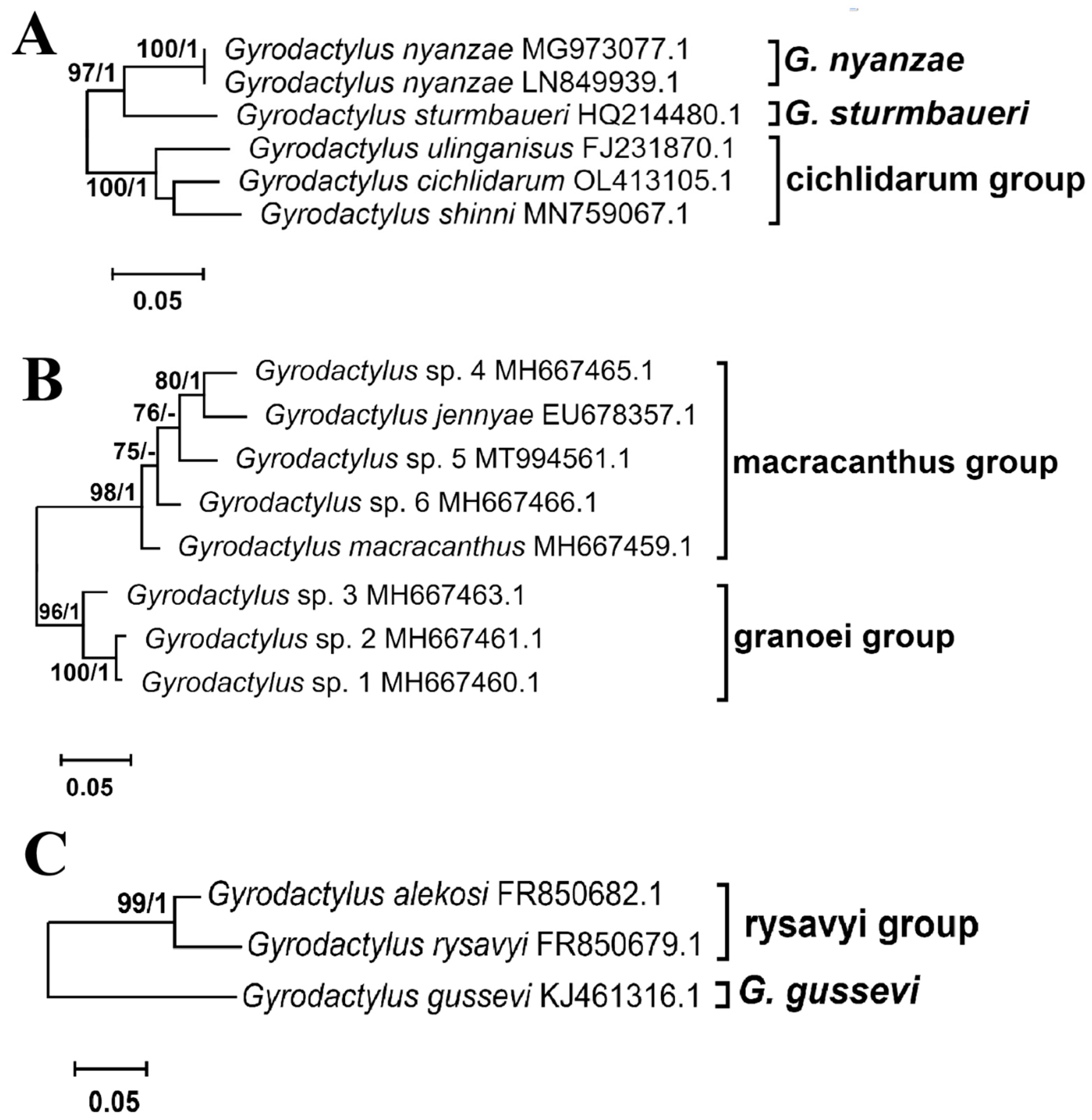
3.2.2. Phylogenic Phylogenetic Inference
3.3. Description of New Genera
- Platyhelminthes Monot, 1876;
- Monopisthocotyla (Odhner, 1912);
- Gyrodactylidea Bykhovsky, 1937;
- Gyrodactylidae (Beneden et Hesse, 1864)
3.3.1. Cichlidarus gen. nov.
- Cichlidarus gen. nov. Figures 1–3 and Table 1 in [89].
- Type species: Gyrodactylus cichlidarum Paperna, 1968.
- Current name of type species: Cichlidarus gen. nov. cichlidarum (Paperna, 1968).
- Other species: G. nyanzae Paperna, 1973;
- G. shinni Garcia-Vasques, Pinacho–Pinacho, Guzman–Valdivieso, Colixto–Rojas et Rubio–Gody, 2021;
- G. sturmbaueri Vanhove, Snoeks, Volckaert et Huyse, 2011;
- Gyrodactylus ulinganisus Garcia–Vasquez, Hansen, Christison, Bron et Shinn, 2011.
- Current names of other species: Cichlidarus gen. nov. nyaznaze (Paperna, 1973);
- Cichlidarus gen. nov. shinni (Garcia-Vasques, Pinacho–Pinacho, Guzman–Valdivieso, Colixto–Rojas et Rubio–Gody, 2021);
- Cichlidarus gen. nov. sturmbaueri (Vanhove, Snoeks, Volckaert et Huyse, 2011);
- Cichlidarus gen. nov. ulinganisus (Garcia–Vasquez, Hansen, Christison, Bron et Shinn, 2011).
- Hologenotype for type species: ITS rDNA (ITS1–5.8S–ITS2 rDNA) sequences:
- Cichlidarus gen. nov. cichlidarum OL413105.1.
- Hologenotypes for other species:
- Cichlidarus gen. nov. nyanzae MG973077.1;
- C. gen. nov. shinni MN759067.1;
- C. gen. nov. sturmbaueri HQ214480.1;
- C. gen. nov. ulinganisus FJ231870.1;Cichlidarus gen. nov. sp. MN759067.
- Type host: Sarotherodon galilaeus (L.) (Cichliformes: Cichlidae) [90].
- Additional hosts: Coptodon zillii (Gervais) (Cichliformes: Cichlidae) [90];
- Haplochromis flaviijosephi (Lortet) (Cichliformes: Cichlidae) [91];
- Hemichromis fasciatus Peters (Cichliformes: Cichlidae) [90];
- Oreochromis niloticus (L.) (Cichliformes: Cichlidae) [92];
- Oreochromis aureus Steindachner (Cichliformes: Cichlidae) [91];
- Poeciliopsis gracilis (Heckel) (Cyprinodontiformes: Poeciliidae) [93];
- Poecilia mexicana Steindachner (Cyprinodontiformes: Poeciliidae) [93];
- Pseudoxiphophorus bimaculatus (Heckel) (Cyprinodontiformes: Poeciliidae) [93];
- Rubricatochromis bimaculatus (Gill) (Cichliformes: Cichlidae) [90];
- S. galilaeus galilaeus (L.) (Cichliformes: Cichlidae) [91];
- Sarotherodon melanotheron heudelotii (Duméril) (Cichliformes: Cichlidae) [91];
- Tilapia guineensis (Günther) (Cichliformes: Cichlidae) [91];
- Tristamella simonis simonis (Günther) (Cichliformes: Cichlidae) [91].
- Site on hosts: skin, fins, and gills.
- Type locality: Accra plains and Akuse lagoon, Lower Volta, Ghana.
- Type material: Holotype 35584, MRAC, Vouchers MRAC 37,560–37,562, Vouchers 2004.12.8.9–11 Natural History Museum, London, Vouchers M-406, Institute of Parasitology, Academy of Sciences of the Czech Republic, České Budějovice.
- ZooBank registration: The Life Science Identifier (LSID) for Gyrodactylus cichlidarum Paperna, 1968 is urn:lsid:zoobank.org:act:18730161-3470-4E74-BEC4-29D3F526E1DA; the LSID for Cichlidarus gen. nov. is urn:lsid:zoobank.org:act:A90817FF-7DAA-4F9B-ABD6-5781883DC83D.
- Etymology: The Cichlidarus gen. nov. name is derived from the species that is typical of the genus and was the first to be described.
- Diagnosis: Hologenotype ITS rDNA (OL413105.1) 788 bp: ITS1 complete TTAAATT to AATTATA 343 bp, 5.8S complete CAACTCT to GTCGGCT 157 bp, ITS2 complete TTAACCT to TACTATT 289 bp. Opisthaptor typical for Gyrodactylidae, with 16 marginal hooks and a pair of anchors with ventral and dorsal bars. The ventral bar membrane is approximately square in shape with medial, spatulate ridges and with characteristic crescent-shaped depressions. Male copulatory organ (MCO): globular, with a single prominent apical spine and a single row of spikes, one set of robust “terminal” spikes, and two pairs of increasingly gracile “sub-terminal” and “medial” spikes. Excretory bladders present.
- Annotations for other species’ ITS rDNA:
- (MG973077.1), 801 bp—ITS1 complete TTAAATT to AATTATA 342 bp, 5.8S complete CAACTCT to GTCGGCT 157 bp, ITS2 TTTACCT to ATTTATT 302 bp;
- (LN849939.1), not determined—ITS1 incomplete to TAATTATA, 5.8S complete CAACTCT to GTCGGCT 157 bp, ITS2 TTTACCT to incomplete;
- (MN759067.1), 802 bp—ITS1 complete TTAAATT to AATAATA 342 bp, 5.8S complete CAACTCT to GTCGGCT 157 bp, ITS2 complete TTAACCT to CTTTAGT 303 bp;
- (HQ214480.1), 799 bp—ITS1 complete TTAAATT to AATTATA 339 bp, 5.8S complete CAACTCT to GTCGGCT 157 bp, ITS2 complete ITS2 complete TTAATCT to AACTATT 303 bp;
- (FJ231870.1) 802 bp—ITS1 complete TTAAATT to AATTATA 342bp, 5.8S complete CAACTCT to GTCGGCT 157 bp, ITS2 complete TTAACCT to CATTATT 303.
- Remark: Detailed type species redescription is presented in [90]. Local phylogenies presented in [61,94] demonstrated that valid names for Gyrodactylus chitandiri Zahradničková, Barson, Luus-Powell et Přikrylová, 2016, Gyrodactylus ergensi Přikrylová, Matĕjusová, Musilová et Gelnar, 2009, Gyrodactylus malalai Přikrylová, Blažek et Gelnar, 2012, Gyrodactylus occupatus Zahradničková, Barson, Luus-Powell et Přikrylová, 2016, and Gyrodactylus parisellei Zahradnıčková, Barson, Luus-Powell et Přikrylová, 2016 should be changed into Cichlidarus gen. nov. chitandiri Zahradničková, Barson, Luus-Powell et Přikrylová, 2016, Cichlidarus gen. nov. ergensi Přikrylová, Matĕjusová, Musilová et Gelnar, 2009, Cichlidarus gen. nov. malalai Přikrylová, Blažek et Gelnar, 2012, Cichlidarus gen. nov. occupatus Zahradničková, Barson, Luus-Powell et Přikrylová, 2016, and Cichlidarus gen. nov. parisellei Zahradnıčková, Barson, Luus-Powell et Přikrylová, 2016, respectively. The ITS sequences of the above species were incomplete and therefore excluded from the analyses, but rDNA of C. gen. nov. chitandiri formed the sturmbaueri group with the singleton. C. gen. nov. ergensi and C. gen. nov. malalai formed the nyanzae group, and C. gen. nov. occupatus and C. gen. nov. parisellei joined the cichlidarum group.
3.3.2. Iraquemembranatus gen. nov.
- Iraquemembranatus gen. nov. Figure 10, Table 3, and Figure S5C in [68].
- Type and the only species: G. iraqemembranatus Rahmouni, 2024 [68].
- Current name: Iraqemembranatus iraqemembranatus (Rahmouni, 2024).
- Hologenotype: ITS rDNA (ITS1-5.8S-ITS2 rDNA) sequence: I. iraqemembranatus OR773087.1.
- Type host: Paracapoeta trutta (Heckel, 1843) (Cyprinoidei: Cyprinidae).
- Additional hosts: Alburnus sellal Heckel, 1843 (Cyprinoidei: Leuciscidae); Barbus lacerta Heckel, 1843 (Cyprinoidei: Cyprinidae).
- Site on the host: gill filaments for P. trutta and B. lacerta; fins for A. sellal.
- Type locality: Kani Shok, tributary of the Tabin River, Sulaymaniyah Province, Iraq.
- Type material: holotype and six paratypes (IPCAS M-784/1-3).
- ZooBank registration: the Life Science Identifier (LSID) for G. iraqemembranatus Rahmouni, 2024 is urn:lsid:zoobank.org:act: B4738C07-9748-4217-80C0-D5510AC31E4F; the LSID for Iraqemembranatus gen. nov. is urn:lsid:zoobank.org:act:35264468-9620-45E3-9B70-9237899E06F8.
- Etymology: the name derived from the original name G. iraqemembranatus.
- Diagnosis: Hologenotype ITS rDNA (OR773087.1) 834 bp: ITS1 complete TGTATTG to TAATTTT 345 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTAGCCT 332 bp. Opisthaptor typical for Gyrodactylidae, with 16 marginal hooks and a pair of anchors with ventral and dorsal bars. The ventral bar is lacking bilateral processes and membrane. Male copulatory organ (MCO) with single prominent apical spine and a single row of spikes. Excretory bladders not investigated.
- Remark: Detailed species description in [68]. G. emembranatus Malmberg, 1970 is another species with a ventral bar without bilateral processes and a membrane. Its phylogenetic position is uncertain. It is a marine species from Gadus morhua L. collected in the Norwegian Sea near Tromsø, classified by Malmberg [69] to the subgenus G. (Metanephrotus). The ITS rDNA sequence JF836148.1 [16] is from worms collected on G. morhua in the Atlantic Ocean near Nova Scotia. The sequence is incomplete and, therefore, was not used in the present analysis. Its phylogenetic position, inferred from partial 18S rDNA sequences, groups it with Gyrocerviceanseris passamaquoddyensis Cone, Abbott, Gilmore et Burt, 2010 (ITS1 rDNA not available either). When its ITS2 rDNA is blasted against the nucleotide database, it is grouped within the subgenus G. (Gyrodactylus).
3.3.3. Macracanthus gen. nov.
- Type species: Gyrodactylus macracanthus Hukuda, 1940.
- Current name of type species: Macracanthus gen. nov. macracanthus (Hukuda, 1940).
- Other species: G. jennyae Paetow, Cone, Huyse, McLaughlinand et Marcogliese, 2009;
- G. granoei You, Guo, King et Cone, 2010.
- Current names of other species: Macracanthus gen. nov. jennyae (Paetow, Cone, Huyse, McLaughlin et Marcogliese, 2009);
- Macracanthus gen. nov. granoei (You, Guo, King et Cone, 2010).
- Hologenotype: ITS rDNA (ITS1-5.8S-ITS2 rDNA) sequences: Macracanthus gen. nov. macracanthus MH667459.1.
- Hologenotypes for other species:
- M. gen. nov. jennyae EU678357.1;
- Macracanthus gen. nov. sp. 1 MH667460.1.
- Macracanthus gen. nov. sp. 2 MH667461.1;
- Macracanthus gen. nov. sp. 3 MH667463.1;
- Macracanthus gen. nov. sp. 4 MH667465.1;
- Macracanthus gen. nov. sp. 5 MT994561.1;
- Macracanthus gen. nov. sp. 6 MH667466.1;
- Type host: Misgurnus anguillicaudatus (Cantor) (Cypriniformes: Cobitidae).
- Additional hosts: not known.
- Site on hosts: skin, fins, gills.
- Type locality: Seoul, South Korea.
- Type material: N/A.
- ZooBank registration: the Life Science Identifier (LSID) for G. macracanthus Hukuda, 1940 is urn:lsid:zoobank.org:act:9B9904AC-684E-4095-8765-6FEA80C57B6A; the LSID for Macracanthus gen. nov. is urn:lsid:zoobank.org:act:FA3B583C-9FF5-42B9-BFE5-2D766A545BCC.
- Etymology: The Macracanthus gen. nov. name is derived from the species typical of the genus, which was also the first to be described.
- Diagnosis: Hologenetype ITS rDNA (MH667459.1) 838 bp): ITS1 complete TGTATTT to ATATGTA 359 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp and ITS2 complete TTTACCT to ATTACTT 322 bp. Opisthaptor typical for Gyrodactylidae, with 16 marginal hooks and a pair of anchors with ventral and dorsal bars. Male copulatory organ (MCO) is globular. A pair of large triangular excretory bladders is present.Annotations for other species’ ITS rDNA:(EU678357.1)—855 bp—ITS1 complete CGTATTT to AATTATA 379 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTACTTG 319 bp;(MH667460.1)—842 bp, ITS1 complete TGTATTG to ATTTGTA 366 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTTGCCT 319 bp;(MH667461.1)—842 bp, ITS1 complete TGTATTG to ATTTGTA 366 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTTGCCT 319bp;(MH667463.1)—845 bp, ITS1 complete TGTATTG to ATTTGTA 368 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTAGCCT 320 bp;(MH667465.1)—840 bp, ITS1 complete CGTATTT to ATTTGTA 371 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTTCTAG 312 bp;(MT994561.1)—841 bp, ITS1 complete CGTATTT to ATTTGTA 365 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTATTTG 319 bp;(MH667466.1)—830 bp, ITS1 complete CGTATTT to ATTTGTA 354 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp, ITS2 complete TTTACCT to TTACTTG 319 bp.
- Remark: Detailed species description in [95,96]. In the local phylogeny presented in [67], Gyrodactylus granoei You, Guo, King et David Cone, 2010 is included. The ITS rDNA sequence of the species HM185817.1 is incomplete; therefore, it was not included in the analysis. However, the species belongs to Macracantsus gen. nov., so its name should be changed to Macracantsus granoei (You, Guo, King et David Cone, 2010).
3.3.4. Rysavyius gen. nov.
- Rysavyius gen. nov. (Figures 2 (j,k), 3 (a-c), Table 2 in [97]).
- Type species: Gyrodactylus rysavyi Ergens, 1973.
- Current name of type species: Rysavyius gen. nov. rysavyi (Ergens, 1973).
- Other species: G. alekosi Přikrylová, Blažek et Vanhove, 2012;
- G. gussevi Dubey, Gupta et Agarwal, 1990.
- Current names of other species: Rysavyius gen. nov. alekosi (Přikrylová, Blažek et Vanhove, 2012);
- Rysavyius gen. nov. gussevi (Dubey, Gupta et Agarwal,1990).
- Hologenotypes: ITS rDNA (ITS1-5.8S-ITS2 rDNA) sequences: Rysavyius gen. nov. rysavyi FR850679.1;
- Hologenotypes for other species:
- R. gen. nov. alekosi FR850682.1;
- R. gen. nov. gussevi KJ461316.1.
- Type locality: River Nile and Abu Sarda, Egypt.
- Type material: N/A.
- ZooBank registration: the Life Science Identifier (LSID) for G. rysavyi Ergens, 1973 is urn:lsid:zoobank.org:act:68513FE2-E78D-40C1-9DD6-356A267C0491; the LSID for Rysavyius gen. nov. is urn:lsid:zoobank.org:act:6700E94B-7960-4848-9154-5A8E866B23B8.
- Etymology: the name Rysavyius gen. nov. is derived from the species that is typical of the genus.
- Diagnosis: Hologenetype ITS rDNA (FR850679.1) length not determined: ITS1 5′ end unreliable to ATTTGTA, 5.8S complete CAACTCC to GTCGGCT 157 bp, and ITS2 complete TTAACCT to TAAGCCT 384 bp. Opisthaptor typical for Gyrodactylidae, with 16 marginal hooks and a pair of anchors with ventral and dorsal bars. Anchors have a characteristic flattened area on the inner part of the root. Sickles have broad shafts and point downward, extending beyond the toe. Male copulatory organ (MCO) globular, with one large hook and eleven thin, small spikes in a single row. A pair of excretory bladders is present.
- Annotation for other species’ ITS rDNA:
- (FR850682.1)—not determined, ITS1 5′ end unreliable to ATTTGTA, 5.8S complete CAACTCC to GTCGGCT 157? bp and ITS2 complete TTAACCT to TAAGCCT 384 bp;
- (KJ461316.1)—908 bp, ITS1 complete CGTATTG to ATTTGTA 367 bp, 5.8S complete CAACTCC to GTCGGCT 157 bp and ITS2 complete TTTACCT to AAACCTT 384 bp.
- Remark: Detailed species description in [97]. An additional three species are present in the local phylogeny [97]—Gyrodactylus nigritae Přikrylová, Blažekand et Vanhove, 2012, Gyrodactylus synodonti Přikrylová, Blažekand et Vanhove, 2012, and Gyrodactylus sp. (FR850688). Their ITS rDNA sequences were incomplete; therefore, they were not included in the analysis. However, they belong to the new genus Rysavyius gen. nov., so their names should be changed to Rysavyius gen. nov. nigritae (Přikrylová, Blažek et Vanhove, 2012), R. gen. nov. nigritae (Přikrylová, Blažek et Vanhove, 2012) and Rysavyius gen. nov. sp., respectively.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Littlewood, D.T.J.; Rhode, K.; Clough, K.A. The Interrelationships of All Major Groups of Platyhelminthes: Phylogenetic Evidence from Morphology and Molecules. Biol. J. Linn. Soc. 1999, 66, 75–114. [Google Scholar] [CrossRef]
- Olson, P.D.; Tkach, V.V. Advances and Trends in the Molecular Systematics of the Parasitic Platyhelminthes. Adv. Parasitol. 2005, 60, 165–243. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.J. The Evolution of Parasitism in Flatworms. In Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology; Maule, A.G., Marks, N.J., Eds.; CABI Publishing: Wallingford, UK, 2006; pp. 1–36. [Google Scholar] [CrossRef]
- Littlewood, D.T.J. Platyhelminth Systematics and the Emergence of New Characters. Parasite 2008, 15, 333–341. [Google Scholar] [CrossRef]
- Hahn, C.; Fromm, B.; Bachmann, L. Comparative Genomics of Flatworms (Platyhelminthes) Reveals Shared Genomic Features of Ecto- and Endoparasitic Neodermata. Genome Biol. Evol. 2014, 6, 1105–1117. [Google Scholar] [CrossRef]
- Bakke, T.A.; Harris, P.D.; Shinn, A.P.; Cable, J. The Biology of Gyrodactylid Monogeneans: The “Russian-Doll Killers”. Adv. Parasitol. 2007, 64, 164–169. [Google Scholar] [CrossRef]
- Brabec, J.; Salomaki, E.D.; Kolisko, M.; Scholz, T.; Kuchta, R. The Evolution of Endoparasitism and Complex Life Cycles in Parasitic Platyhelminths. Curr. Biol. 2023, 33, 4269–4275. [Google Scholar] [CrossRef]
- Boeger, W.A.; Kristsky, D.C.; Patella, L.; Bueno-Silva, M. Phylogenetic Status and Historical Origins of the Oviparous and Viviparous Gyrodactylids (Monogenoidea, Gyrodactylidea). Zool. Scr. 2021, 50, 112–124. [Google Scholar] [CrossRef]
- Cable, J.; Harris, P.D.; Tinsley, R.C.; Lazarus, C.M. Phylogenetic Analysis of Gyrodactylus spp. (Platyhelminthes: Monogenea) Using rDNA Sequences. Can. J. Zool. 1999, 77, 1439–1449. [Google Scholar] [CrossRef]
- Kritsky, D.C.; Boeger, W.A. Phylogeny of the Gyrodactylidae and the Phylogenetic Status of Gyrodactylus von Nordmann, 1832 (Platyhelminthes, Monogenoidea). In Taxonomy, Ecology and Evolution of Metazoan Parasites; Combes, C., Jourdaine, J., Eds.; Presses Universitaires de Perpignan: Perpignan, France, 2003; Volume 2, pp. 37–58. [Google Scholar]
- Matejusová, I.; Gelnar, M.; Verneau, O.; Cunningham, C.O.; Littlewood, D.T.J. Molecular Phylogenetic Analysis of the Genus Gyrodactylus (Platyhelminthes: Monogenea) Inferred from rDNA ITS Region: Subgenera versus Species Groups. Parasitology 2003, 127, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ziętara, M.S.; Lumme, J. Comparison of molecular phylogeny and morphological systematics in fish parasite genus Gyrodactylus Nordmann, 1832 (Monogenea, Gyrodactylidae). Zool. Pol. 2004, 49, 5–28. [Google Scholar]
- King, S.D.; Cone, D.K. Morphological and Molecular Taxonomy of a New Species of Fundulotrema and Comments on Gyrodactylus stephani (Monogenea: Gyrodactylidae) from Fundulus heteroclitus (Actinopterygii). J. Parasitol. 2009, 95, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Barson, M.; Přikrylová, I.; Vanhove, M.P.M.; Huyse, T. Parasite Hybridization in African Macrogyrodactylus spp. (Monogenea, Platyhelminthes) Signals Historical Host Distribution. Parasitology 2010, 137, 1585–1595. [Google Scholar] [CrossRef]
- Cone, D.; Abbott, C.; Gilmore, S.; Burt, M. A New Genus and Species of Gyrodactylid (Monogenea) from Silver Hake, Merluccius bilinearis, in the Bay of Fundy, New Brunswick, Canada. J. Parasitol. 2010, 96, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.R.; Cone, D.K.; Lowe, G.; King, S.K.; Jones, S.R.M.; Abbott, C.L. Molecular Phylogeny of Gyrodactylus (Monogenea) Parasitizing Fishes in Fresh Water, Estuarine, and Marine Habitats in Canada. Can. J. Zool. 2012, 90, 776–786. [Google Scholar] [CrossRef]
- Kritsky, D.C.; Boeger, W.A.; Mendoza-Franco, E.F.; Vianna, R.T. Neotropical Monogenoidea. 57. Revision and Phylogenetic Position of Scleroductus Jara & Cone, 1989 (Gyrodactylidae), with Descriptions of New Species from the Guatemalan Chulin Rhamdia guatemalensis (Gunther) (Siluriformes: Heptapteridae) in Mexico and the Barred Sorubim Pseudoplatystoma fasciatum (Linnaeus) (Siluriformes: Pimelodidae) in Brazil. Syst. Parasitol. 2013, 84, 1–15. [Google Scholar] [CrossRef]
- Přikrylová, I.; Vanhove, M.P.; Janssens, S.B.; Billeter, P.A.; Huyse, T. Tiny Worms from a Mighty Continent: High Diversity and New Phylogenetic Lineages of African Monogeneans. Mol. Phylogenet. Evol. 2013, 67, 43–52. [Google Scholar] [CrossRef]
- Ye, F.; King, S.D.; Cone, D.K.; You, P. The Mitochondrial Genome of Paragyrodactylus variegatus (Platyhelminthes: Monogenea): Differences in Major Non-Coding Region and Gene Order Compared to Gyrodactylus. Parasites Vectors 2014, 7, 377. [Google Scholar] [CrossRef]
- You, P.; King, S.D.; Ye, F.; Cone, D.K. Paragyrodactylus variegatus n. sp. (Gyrodactylidae) from Homatula variegata (Dabry De Thiersant, 1874) (Nemacheilidae) in Central China. J. Parasitol. 2014, 100, 350–355. [Google Scholar] [CrossRef]
- Přikrylová, I.; Shinn, A.P.; Paladini, G. Description of Citharodactylus gagei n. gen. et n. sp. (Monogenea: Gyrodactylidae) from the Moon Fish, Citharinus citharus (Geoffroy Saint-Hilaire), from Lake Turkana. Parasitol. Res. 2017, 116, 281–292. [Google Scholar] [CrossRef]
- Vanhove, M.P.M.; Briscoe, A.G.; Jorissen, M.W.P.; Littlewood, D.T.J.; Huyse, T. The First Next-Generation Sequencing Approach to the Mitochondrial Phylogeny of African Monogenean Parasites (Platyhelminthes: Gyrodactylidae and Dactylogyridae). Genomics 2018, 19, 520. [Google Scholar] [CrossRef]
- Přikrylová, I.; Barson, M.; Shinn, A.P. Description of Tresuncinidactylus wilmienae Gen. et Sp. N. (Monogenea: Gyrodactylidae), from the Gills of the Bulldog, Marcusenius macrolepidotus (Peters) from Lake Kariba, Zimbabwe. Folia Parasitol. 2021, 68, 25. [Google Scholar] [CrossRef] [PubMed]
- Truter, M.; Acosta, A.A.; Wey, O.L.F.; Smit, N.J. Novel Distribution Records and Molecular Data for Species of Macrogyrodactylus Malmberg, 1957 (Monogenea: Gyrodactylidae) from Clarias gariepinus (Burchell) (Siluriformes: Clariidae) in Southern Africa. Acta Parasitol. 2022, 67, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Maduenyane, M.; Dos Santos, Q.M.; Avenant-Oldewage, A. First Isolation and Scanning Electron Microscopy of Haptoral Sclerites of Macrogyrodactylus (Monogenea). J. Helminthol. 2022, 96, e17. [Google Scholar] [CrossRef]
- Ciccheto, J.R.M.; Razzolini, E.L.; de Buron, I.; Boeger, W.A. Position of Polyclithrum within Gyrodactylidae (Monogenoidea): Incongruences between Morphological and Molecular Phylogenies. Syst. Parasitol. 2023, 100, 633–645. [Google Scholar] [CrossRef]
- Přikrylová, I.; Matejusová, I.; Musilová, N.; Gelnar, M.; Harris, P.D. A New Gyrodactylid (Monogenea) Genus on Gray Bichir, Polypterus senegalus (Polypteridae) from Senegal (West Africa). J. Parasitol. 2009, 95, 555–560. [Google Scholar] [CrossRef]
- Schelkle, B.; Paladini, G.; Shinn, A.P.; King, S.; Johnson, M.; van Oosterhout, C.; Mohammed, R.S.; Cable, J. Ieredactylus rivuli gen. et sp. nov. (Monogenea, Gyrodactylidae) from Rivulus hartii (Cyprinodontiformes, Rivulidae) in Trinidad. Acta Parasitol. 2011, 56, 360–370. [Google Scholar] [CrossRef]
- Malmberg, G. Taxonomical and Ecological Problems in Gyrodactylus (Trematoda, Monogenea). In Parasitic Worms and Aquatic Conditions; Ergens, R., Rysavy, B., Eds.; Publ. House, Czechoslovak Academy of Sciences: Prague, Czech Republic, 1964; pp. 203–230. [Google Scholar]
- Rokicka, M.; Lumme, J.; Ziętara, M.S. Identification of Gyrodactylus Ectoparasites in Polish Salmonid Farms by PCR-RFLP of the Nuclear ITS Segment of Ribosomal DNA (Monogenea, Gyrodactylidae). Acta Parasitol. 2007, 52, 185–195. [Google Scholar] [CrossRef]
- Harris, P.; Shinn, A.; Cable, J.; Bakke, T.A. Nominal Species of the Genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a List of Principal Host Species. Syst. Parasitol. 2004, 59, 1–27. [Google Scholar] [CrossRef]
- Harris, P.D.; Cable, J. Gyrodactylus poeciliae n. sp. and G. milleri n. sp. (Monogenea: Gyrodactylidae) from Poecilia caucana (Steindachner) in Venezuela. Syst. Parasitol. 2000, 47, 79–85. [Google Scholar] [CrossRef]
- Huyse, T.; Volckaert, F.A.M. Identification of a Host-Associated Species Complex Using Molecular and Morphometric Analyses, with the Description of Gyrodactylus rugiensoides n. sp. (Gyrodactylidae, Monogenea). Int. J. Parasitol. 2002, 32, 907–919. [Google Scholar] [CrossRef]
- Huyse, T.; Audenaert, V.; Volckaert, F.A.M. Speciation and Host–Parasite Relationships in the Parasite Genus Gyrodactylus (Monogenea, Platyhelminthes) Infecting Gobies of the Genus Pomatoschistus (Gobiidae, Teleostei). Int. J. Parasitol. 2003, 33, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Huyse, T.; Malmberg, G. Molecular and Morphological Comparisons between Gyrodactylus ostendicus n. sp. (Monogenea: Gyrodactylidae) on Pomatoschistus microps (Krøyer) and G. harengi Malmberg, 1957 on Clupea harengus membras L. Syst. Parasitol. 2004, 58, 105–113. [Google Scholar] [CrossRef]
- Huyse, T.; Malmberg, G.; Volckaert, F.A.M. Four New Species of Gyrodactylus von Nordmann, 1832 (Monogenea, Gyrodactylidae) on Gobiid Fishes: Combined DNA and Morphological Analyses. Syst. Parasitol. 2004, 59, 103–120. [Google Scholar] [CrossRef]
- LeBlanc, J.; Hansen, H.; Burt, M.; Cone, D. Gyrodactylus neili n. sp. (Monogenea: Gyrodactylidae), a parasite of chain pickerel Esox niger Lesueur (Esocidae) from freshwaters of New Brunswick, Canada. Syst. Parasitol. 2006, 65, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, J.; Ziętara, M.S.; Lumme, J. Description of Three New European Cryptic Species of Gyrodactylus Nordmann, 1832 Supported by Nuclear and Mitochondrial Phylogenetic Characterization. Acta Parasit. 2008, 53, 120–126. [Google Scholar] [CrossRef]
- Přikrylová, I.; Matejusová, I.; Jarkovský, J.; Gelnar, M. Morphometric Comparison of Three Members of the Gyrodactylus nemachili-like Species Group (Monogenea: Gyrodactylidae) on Barbatula barbatula L. in the Czech Republic, with a Reinstatement of G. papernai Ergens & Bychowsky, 1967. Syst. Parasitol. 2008, 69, 33–44. [Google Scholar]
- You, P.; Easy, R.H.; Cone, D.K. Gyrodactylus parvae n. sp. (Monogenea) from the fins and body surface of Pseudorasbora parva (Cyprinidae) in Central China. Comp. Parasitol. 2008, 75, 28–32. [Google Scholar] [CrossRef]
- You, P.; Guo, Z.; King, S.D.; Cone, D.K. A new Gyrodactylid species from Cobitis granoei (Rendahl) (Cobitidae) in Central China. J. Parasitol. 2010, 96, 897–899. [Google Scholar] [CrossRef]
- You, P.; Li, X.; King, S.D.; Cone, D.K. Gyrodactylus rivularae n. sp. (Monogenoidea: Gyrodactylidae) from Abbottina rivularis (Basilewsky, 1855) (Pisces: Cyprinidae) in Central China. Comp. Parasitol. 2011, 78, 257–260. [Google Scholar] [CrossRef]
- Bueno-Silva, M.; Boeger, W.A. Neotropical Monogenoidea. 53. Gyrodactylus corydori sp. n. and Redescription of Gyrodactylus anisopharynx (Gyrodactylidea: Gyrodactylidae), Parasites of Corydoras spp. (Siluriformes: Callichthyidae) from Southern Brazil. Folia Parasitol. 2009, 56, 13–20. [Google Scholar] [CrossRef]
- King, S.D.; Forest, J.J.H.; Cone, D.K. Gyrodactylus notatae n. sp. (Monogenea: Gyrodactylidae) from Menidia menidia (L.) (Actinopterygii: Atherinidae) in Nova Scotia, Canada. Syst. Parasitol. 2009, 74, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Paetow, L.; Cone, D.K.; Huyse, T.; McLaughlin, J.D.; Marcogliese, D.J. Gyrodactylus jennyae n. sp. (Monogenea) from Tadpoles of Captive Rana catesbeiana Shaw (Anura), with a Review of the Species of Gyrodactylus Nordmann, 1832 Parasitizing Amphibians. Syst. Parasitol. 2009, 73, 219–227. [Google Scholar] [CrossRef]
- Paladini, G.; Cable, J.; Fioravanti, M.L.; Faria, P.J.; Di Cave, D.; Shinn, A.P. Gyrodactylus orecchiae sp. n. (Monogenea: Gyrodactylidae) from Farmed Populations of Gilthead Seabream (Sparus aurata) in the Adriatic Sea. Folia Parasitol. 2009, 56, 21–28. [Google Scholar] [CrossRef]
- Rokicka, M.; Lumme, J.; Ziętara, M.S. Two New Antarctic Gyrodactylus Species (Monogenea): Description and Phylogenetic Characterization. J. Parasitol. 2009, 95, 1112–1119. [Google Scholar] [CrossRef]
- Gilmore, S.R.; Abbott, C.L.; Cone, D.K. The Placement of Gyrodactylus salmonis (Yin & Sproston) in the Molecular Phylogeny of Studied Members of the Gyrodactylus wageneri-Group Parasitizing Salmonids. J. Fish Dis. 2010, 33, 461–467. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Hansen, H.; Christison, K.W.; Bron, J.E.; Shinn, A.P. Description of Three New Species of Gyrodactylus von Nordmann, 1832 (Monogenea) Parasitizing Oreochromis niloticus niloticus (L.) and O. mossambicus (Peters) (Cichlidae). Acta Parasitol. 2011, 56, 20–33. [Google Scholar] [CrossRef]
- Paladini, G.; Hansen, H.; Fioravanti, M.L.; Shinn, A.P. Gyrodactylus longipes n. sp. (Monogenea: Gyrodactylidae) from Farmed Gilthead Seabream (Sparus aurata L.) from the Mediterranean. Parasitol. Int. 2011, 60, 410–418. [Google Scholar] [CrossRef]
- Paladini, G.; Huyse, T.; Shinn, A.P. Gyrodactylus salinae n. sp. (Platyhelminthes: Monogenea) Infecting the South European Toothcarp Aphanius fasciatus (Valenciennes) (Teleostei: Cyprinodontidae) from a Hypersaline Environment in Italy. Parasites Vectors 2011, 4, 100. [Google Scholar] [CrossRef]
- Vanhove, M.P.M.; Snoeks, J.; Volckaert, F.A.M.; Huyse, T. First Description of Monogenean Parasites in Lake Tanganyika: The Cichlid Simochromis diagramma (Teleostei, Cichlidae) Harbours a High Diversity of Gyrodactylus Species (Platyhelminthes, Monogenea). Parasitology 2011, 138, 364–380. [Google Scholar] [CrossRef]
- Cone, D.K.; Appy, R.; Baggett, L.; King, S.; Gilmore, S.; Abbott, C. A new gyrodactylid (Monogenea) parasitizing bay pipefish (Syngnathus leptorhynchus) from the Pacific coast of North America. J. Parasitol. 2013, 99, 183–188. [Google Scholar] [CrossRef]
- King, S.D.; Cone, D.K.; Mackley, M.P.; Bentzen, P. Gyrodactylus laevisoides n. sp. (Monogenea: Gyrodactylidae) infecting northern redbelly dace Phoxinus eos Cope (Cyprinidae) from Nova Scotia, Canada. Syst. Parasitol. 2013, 86, 285–291. [Google Scholar] [CrossRef] [PubMed]
- King, S.D.; Marcogliese, D.J.; Forest, J.J.H.; McLaughlin, J.D.; Bentzen, P. Gyrodactylus mediotorus n. sp. (Monogenea: Gyrodactylidae) infecting spottail shiner (Notropis hudsonius) from the St. Lawrence River, Canada. J. Parasitol. 2013, 99, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, M.; Boeger, W.A. Neotropical Monogenoidea. 58. Three New Species of Gyrodactylus (Gyrodactylidae) from Scleromystax spp. (Callichthyidae) and the Proposal of COII Gene as an Additional Fragment for Barcoding Gyrodactylids. Folia Parasitol. 2014, 61, 213–222. [Google Scholar] [CrossRef] [PubMed]
- King, S.D.; Bentzen, P.; Cone, D.K. Gyrodactylus patersoni n. sp. (Monogenea: Gyrodactylidae) infecting Atlantic silverside Menidia menidia from Nova Scotia, Canada. Comp. Parasitol. 2014, 81, 27–32. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Razo-Mendivil, U.; Rubio-Godoy, M. Morphological and Molecular Description of Eight New Species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from Poeciliid Fishes, Collected in Their Natural Distribution Range in the Gulf of Mexico Slope, Mexico. Parasitol. Res. 2015, 114, 3337–3355. [Google Scholar] [CrossRef]
- Leis, E.; King, S.D.; Leis, S.; Cone, D.K. Infections of Gyrodactylus crysoleucas and Gyrodactylus sp. (Monogenea) at a Golden Shiner (Notemigonus crysoleucas) Farm in Minnesota. Comp. Parasitol. 2016, 83, 105–110. [Google Scholar] [CrossRef]
- Razo-Mendivil, U.; García-Vásquez, A.; Rubio-Godoy, M. Spot the difference: Two cryptic species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) infecting Astyanax aeneus (Actinopterygii, Characidae) in Mexico. Parasitol. Int. 2016, 65, 389–400. [Google Scholar] [CrossRef]
- Zahradníčková, P.; Barson, M.; Luus-Powell, W.J.; Přikrylová, I. Species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from cichlids from Zambezi and Limpopo river basins in Zimbabwe and South Africa: Evidence for unexplored species richness. Syst. Parasitol. 2016, 93, 679–700. [Google Scholar] [CrossRef]
- Lumme, J.; Ziętara, M.S.; Lebedeva, D. Ancient and Modern Genome Shuffling: Reticulate Mitonuclear Phylogeny of Four Related Allopatric Species of Gyrodactylus von Nordmann, 1832 (Monogenea: Gyrodactylidae), Ectoparasites on the Eurasian Minnow Phoxinus phoxinus (L.) (Cyprinidae). Syst. Parasitol. 2017, 94, 183–200. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Pinacho-Pinacho, C.D.; Guzmán-Valdivieso, I.; Salgado-Maldonado, G.; Rubio-Godoy, M. New Species of Gyrodactylus von Nordmann, 1832 from Native Fish from Chiapas, Mexico, Studied by Morphology and Molecular Analyses. Acta Parasitol. 2019, 64, 551–565. [Google Scholar] [CrossRef]
- Kvach, Y.; Ondračková, M.; Seifertová, M.; Hulak, B. Gyrodactylus ginestrae n. sp. (Monogenea: Gyrodactylidae), a Parasite of the Big-Scale Sand Smelt, Atherina boyeri Risso, 1810 (Actinopterygii: Atherinidae) from the Black Sea. Parasitol. Res. 2019, 118, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Razzolini, E.; Murari, A.L.; Baldisserotto, B.; Boeger, W.A. Gyrodactylus lilianae n. sp. (Polyonchoinea: Gyrodactylidae) from Rhamdia quelen (Quoy & Gaimard) (Siluriformes: Heptapteridae) from southern Brazil: A potential nuisance for aquaculture. Syst. Parasitol. 2019, 96, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Heglasová, I.; Nezhybová, V.; Přikrylová, I. An Amended Description of Two Gyrodactylus Species (Platyhelminthes: Monogenea) Parasitizing Antarctic Notothenioid Fish. J. Helminthol. 2020, 94, e20. [Google Scholar] [CrossRef] [PubMed]
- Reyda, F.B.; Wells, S.M.; Ermolenko, A.V.; Ziętara, M.S.; Lumme, J.I. Global parasite trafficking: Asian Gyrodactylus (Monogenea) arrived to the U.S.A. via invasive fish Misgurnus anguillicaudatus as a threat to amphibians. Biol. Invasions 2020, 22, 391–402. [Google Scholar] [CrossRef]
- Benovics, M.; Rahmouni, C.; Řehulková, E.; Nejat, F.; Šimková, A. Uncovering the Monogenean Species Diversity of Cyprinoid Fish in Iraq Using an Integrative Approach. Parasitology 2024, 151, 220–246. [Google Scholar] [CrossRef]
- Malmberg, G. The Excretory Systems and the Marginal Hooks as a Basis for the Systematics of Gyrodactylus (Trematoda, Monogenea). Ark. Zool. 1970, 23, 27–29. [Google Scholar]
- Gläser, H.J. Monogenea. In Limnofauna Europea; Illies, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1978; pp. 21–27. [Google Scholar]
- Cunningham, C.O. Species Variation within the Internal Transcribed Spacer (ITS) Region of Gyrodactylus (Monogenea: Gyrodactylidae) Ribosomal RNA Genes. J. Parasitol. 1997, 83, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Ziętara, M.S.; Arndt, A.; Geets, A.; Hellemans, B.; Volckaert, F.A.M. The Nuclear rDNA Region of Gyrodactylus arcuatus and G. branchicus (Monogenea: Gyrodactylidae). J. Parasitol. 2000, 86, 1368–1373. [Google Scholar] [CrossRef]
- Ziętara, M.S.; Lumme, J. The crossroads of molecular, typological, and biological species concepts: Two new species of Gyrodactylus Nordmann, 1832 (Monogenea: Gyrodactylidae). Syst. Parasitol. 2003, 55, 39–52. [Google Scholar] [CrossRef]
- Ziętara, M.S.; Huyse, T.; Lumme, J.; Volckaert, F.A. Deep Divergence Among Subgenera of Gyrodactylus Inferred from rDNA ITS Region. Parasitology 2002, 124, 39–52. [Google Scholar] [CrossRef]
- Ziętara, M.S.; Lumme, J. Speciation by Host Switch and Adaptive Radiation in a Fish Parasite Genus Gyrodactylus (Monogenea, Gyrodactylidae). Evolution 2002, 56, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, G. Gyrodactylidae and Gyrodactylosis of Salmonidae. Bull. Fr. Pêche Piscic. 1993, 328, 5–46. [Google Scholar] [CrossRef]
- Jin, X.; Li, W.; Cheng, Y.; Li, M.; Wu, S.; Zou, H.; Wang, G. Description of Gyrodactylus banmae n. sp. (Monogenea, Gyrodactylidae) Parasitic on Zebrafish, Danio rerio. Parasitol. Int. 2022, 87, 102531. [Google Scholar] [CrossRef] [PubMed]
- Gläser, H.J. Eine Neue Artengruppe des Subgenus Gyrodactylus (Paranephrotus) (Monogenea, Gyrodactylidae). Zool. Ann. 1974, 192, 271–278. [Google Scholar]
- Lebedeva, D.; Muñoz, G.; Lumme, J. New Salinity Tolerant Species of Gyrodactylus (Platyhelminthes, Monogenea) on Intertidal and Supratidal Fish Species from the Chilean Coast. Acta Parasitol. 2021, 66, 174–185. [Google Scholar] [CrossRef]
- Pinacho-Pinacho, C.D.; Calixto-Rojas, M.; García-Vásquez, A.; Guzmán-Valdivieso, I.; Barrios-Gutiérrez, J.J.; Rubio-Godoy, M. Species Delimitation of Gyrodactylus (Monogenea: Gyrodactylidae) Infecting the Southernmost Cyprinids (Actinopterygii: Cyprinidae) in the New World. Parasitol. Res. 2021, 120, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 2022, 13, 6968. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bui Quang Minh; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Sugiura, N. Further Analysis of the Data by Akaike’s Information Criterion and the Finite Corrections: Further Analysis of the Data by Akaike’s. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An Advanced Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Bouckaert, R.R. An Efficient Coalescent Epoch Model for Bayesian Phylogenetic Inference. Syst. Biol. 2022, 71, 1549–1560. [Google Scholar] [CrossRef]
- Bianchini, G.; Sánchez-Baracaldo, P. TreeViewer: Flexible, Modular Software to Visualise and Manipulate Phylogenetic Trees. Ecol. Evol. 2024, 14, e10873. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vasquez, A.; Hansen, H.; Shinn, A. A Revised Description of Gyrodactylus cichlidarum Paperna, 1968 (Gyrodactylidae) from the Nile Tilapia, Oreochromis niloticus niloticus (Cichlidae), and Its Synonymy with G. niloticus Cone, Arthur et Bondad-Reantaso, 1995. Folia Parasitol. 2007, 54, 129–140. [Google Scholar] [CrossRef]
- Paperna, I. Monogenetic Trematodes Collected from Fresh Water Fish in Ghana. Second Report. Bamidgeh 1968, 20, 88–99. [Google Scholar]
- Paperna, I. Monogenea of Inland Water Fishes in Africa. Musée R. De L’afrique Cent. Tervuren 1979, 226, viii+131. [Google Scholar]
- Abdel-Latif, H.M.R.; Khafaga, A.F. Natural Co-Infection of Cultured Nile Tilapia Oreochromis niloticus with Aeromonas hydrophila and Gyrodactylus cichlidarum Experiencing High Mortality During Summer. Aquac. Res. 2020, 51, 1880–1892. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Razo-Mendivil, U.; Rubio-Godoy, M. Triple Trouble? Invasive Poeciliid Fishes Carry the Introduced Tilapia Pathogen Gyrodactylus cichlidarum in the Mexican Highlands. Vet. Parasitol. 2017, 235, 37–40. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Pinacho-Pinacho, C.D.; Guzmán-Valdivieso, I.; Calixto-Rojas, M.; Rubio-Godoy, M. Morpho-Molecular Characterization of Gyrodactylus Parasites of Farmed Tilapia and Their Spillover to Native Fishes in Mexico. Sci. Rep. 2021, 11, 13957. [Google Scholar] [CrossRef]
- Hukuda, M. Three New Species of Japanese Gyrodactylus (Trematoda). Sci. Rep. Tohoku Imp. Univ. 1940, 7, 37–44. [Google Scholar]
- Ergens, R. Redescription of Two Species of the Genus Gyrodactylus Nordmann, 1832 (Monogenoidea) from Misgurnus anguillicaudatus. Folia Parasitol. 1975, 22, 363–368. [Google Scholar]
- Přikrylová, I.; Blažek, R.; Vanhove, M.P.M. An overview of the Gyrodactylus (Monogenea: Gyrodactylidae) species parasitizing African catfishes, and their morphological and molecular diversity. Parasitol. Res. 2012, 110, 1185–1200. [Google Scholar] [CrossRef]
- Ergens, R. Two New Species of Gyrodactylus from Clarias lazera (Vermes, Trematoda, Monogenoidea). Rev. Zool. Bot. Afr. 1973, 87, 77–80. [Google Scholar]
- Matejusová, I.; Cunningham, C.O. The First Complete Monogenean Ribosomal RNA Gene Operon: Sequence and Secondary Structure of the Gyrodactylus salaris Malmberg, 1957, Large Subunit Ribosomal RNA Gene. J. Parasitol. 2004, 90, 146–151. [Google Scholar] [CrossRef]
- Sterud, E.; Mo, T.A.; Collins, C.M.; Cunningham, C.O. The Use of Host Specificity, Pathogenicity, and Molecular Markers to Differentiate Between Gyrodactylus salaris Malmberg, 1957 and G. thymalli Zitnan, 1960 (Monogenea: Gyrodactylidae). Parasitology 2002, 124, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.O.; Collins, C.M.; Malmberg, G.; Mo, T.A. Analysis of Ribosomal RNA Intergenic Spacer (IGS) Sequences in Species and Populations of Gyrodactylus (Platyhelminthes: Monogenea) from Salmonid Fish in Northern Europe. Dis. Aquat. Org. 2003, 57, 237–246. [Google Scholar] [CrossRef]
- Rahmouni, C.; Seifertová, M.; Šimková, A. Revealing the hidden diversity of Gyrodactylus communities (Monogenea, Gyrodactylidae) from Nearctic Catostomidae and Leuciscidae fish hosts (Teleostei, Cypriniformes), with descriptions of ten new species. Parasite 2023, 30, 40. [Google Scholar] [CrossRef]
- Rahmouni, C.; Seifertová, M.; Benovics, M.; Šimková, A. Diversity and Phylogeny of Gyrodactylus spp. (Monogenea: Gyrodactylidae) across the Strait of Gibraltar: Parasite Speciation and Historical Biogeography of West Mediterranean Cyprinid Hosts. Diversity 2023, 15, 1152. [Google Scholar] [CrossRef]
- Jin, X.; Cheng, H.; Li, M.; Zou, H.; Cai, J.; Amoah, K.; Li, W.; Wang, G. Description of three new species of Gyrodactylus von Nordmann, 1832 (Monogenea: Gyrodactylidae) on bitterling fishes (Acheilognathinae) from China. Parasitol. Int. 2024, 101, 102893. [Google Scholar] [CrossRef]
- Perkins, E.M.; Donnellan, S.C.; Bertozzi, T.; Chisholm, L.A.; Whittington, I.D. Looks Can Deceive: Molecular Phylogeny of a Family of Flatworm Ectoparasites (Monogenea: Capsalidae) Does Not Reflect Current Morphological Classification. Mol. Phylogenet. Evol. 2009, 52, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Pietrak, M.; Rosser, T.G. Morphologic and Molecular Characterization of Gyrodactylus cyclopteri Scyborskaja, 1948, from Cyclopterus lumpus L., 1758. Parasitol. Res. 2019, 119, 879–884. [Google Scholar] [CrossRef]
- Rochat, E.C.; Blasco-Costa, I.; Scholz, T.; Unmack, P.J. High Diversity of Metazoan Parasites in Carp Gudgeons (Eleotridae: Hypseleotris spp.) from Eastern Australia. J. Helminthol. 2020, 94, e146. [Google Scholar] [CrossRef] [PubMed]
- Anshary, H.; Sriwulan, S.; Amriana, A. High Prevalence and Mean Intensity of Trichodinids and Monogeneans on Nile Tilapia (Oreochromis niloticus) in Indonesian Hatcheries. Vet. Parasitol. Reg. Stud. Rep. 2023, 43, 100898. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Pinacho-Pinacho, C.D.; Guzmán-Valdivieso, I.; Calixto-Rojas, M.; Rubio-Godoy, M. Neotropical sisterhood: New species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) infecting Rhamdia guatemalensis and Rhamdia laticauda (Siluriformes: Heptapteridae) in Mexico. J. Helminthol. 2023, 97, e20. [Google Scholar] [CrossRef]
- Pinacho-Pinacho, C.D.; Guzmán-Valdivieso, I.; Calixto-Rojas, M.; García-Vásquez, A.; Rubio-Godoy, M. Morphological and Molecular Characterization of Three New Species of Gyrodactylus (Monogenea: Gyrodactylidae) Infecting Sicydium salvini (Teleostei: Gobiidae) in Mexican Rivers Draining into the Pacific Ocean. Parasitol. Int. 2023, 93, 102712. [Google Scholar] [CrossRef]
- Christison, K.W.; Vaughan, D.B.; Shinn, A.P.; Hansen, H. Gyrodactylus molweni sp. n. (Monogenea: Gyrodactylidae) from Chelon richardsonii (Smith, 1846) (Mugilidae) from Table Bay, South Africa. Int. J. Parasitol. Parasites Wildl. 2021, 15, 87–94. [Google Scholar] [CrossRef]
- Lebedeva, D.; Ziętara, M.S.; Mendsaikhan, B.; Ermolenko, A.; Lumme, J. Survivors from a Pliocene Climatic Catastrophe: Gyrodactylus (Platyhelminthes, Monogenea) Parasites of the Relict Fishes in the Central Asian Internal Drainage Basin of Mongolia. Diversity 2023, 15, 860. [Google Scholar] [CrossRef]
- Leis, E.; Kim Chi, T.; Lumme, J. Global Phylogeography of Salmonid Ectoparasites of the Genus Gyrodactylus, with an Emphasis on the Origin of the Circumpolar Gyrodactylus salmonis (Platyhelminthes: Monogenea). Comp. Parasitol. 2021, 88, 130–143. [Google Scholar] [CrossRef]
- Naka, M.; Nitta, M. New Host and Locality Records of Gyrodactylus rarus (Monogenea: Gyrodactylidae) from Pungitius tymensis (Gasterosteidae) in Hokkaido, Japan. Biogeography 2021, 23, 80–87. [Google Scholar] [CrossRef]
- Nitta, M. A New Monogenean Species, Gyrodactylus ajime n. sp. (Gyrodactylidae), Parasitic on Niwaella delicata (Niwa), an Endemic Loach of Japan. Syst. Parasitol. 2021, 98, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M. New Records of Monogeneans, Gyrodactylus cyprini (Gyrodactylidae) and Dactylogyrus extensus (Dactylogyridae), Parasitic on Reared Common Carp Cyprinus carpio (Cypriniformes: Cyprinidae) in Mie Prefecture, Japan. Species Divers. 2023, 28, 273–284. [Google Scholar] [CrossRef]
- Paladini, G.; Shinn, A.P.; Taylor, N.G.H.; Bron, J.E.; Hansen, H. Geographical Distribution of Gyrodactylus salaris Malmberg, 1957 (Monogenea, Gyrodactylidae). Parasites Vectors 2021, 14, 34. [Google Scholar] [CrossRef]
- Truter, M.; Smit, N.J.; Malherbe, W.; Přikrylová, I. Description of Gyrodactylus paludinosus sp. nov. (Monogenea: Gyrodactylidae) from the Straightfin Barb, Enteromius paludinosus (Peters, 1852), in South Africa. Acta Parasitol. 2022, 67, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, E.; Sanna, D.; Vodiasova, E.; Prokhorova, D.; Casu, M.; Burreddu, C.; Piras, M.C.; Garippa, G.; Merella, P. Morphological and Genetic Variability of the Cryptic Gyrodactylus sphinx and Gyrodactylus gerasevi n. sp. (Platyhelminthes: Monogenea) from the Mediterranean Sea and Black Sea: Two New Members of the Cross-Ocean Distributed Gyrodactylus orecchiae Species Group. J. Helminthol. 2022, 96, e9. [Google Scholar] [CrossRef]
- Maduenyane, M.; Dos Santos, Q.M.; Avenant-Oldewage, A. Gyrodactylus sprostonae Ling, 1962 Infects an Indigenous Cyprinid in Southern Africa: An Expanded Description. J. Helminthol. 2023, 97, e40. [Google Scholar] [CrossRef]
- Mo, T.A.; Hansen, H.; Hytterød, S. Occurrence and seasonality of Gyrodactylus salaris and G. salmonis (Monogenea) on Arctic char (Salvelinus alpinus (L.)) in the Fustvatnet lake, Northern Norway. J. Fish Dis. 2023, 46, 395–403. [Google Scholar] [CrossRef]
- Ondračková, M.; Seifertová, M.; Tkachenko, M.Y.; Vetešník, L.; Liu, H.; Demchenko, V.; Kvach, Y. The parasites of a successful invader: Monogeneans of the Asian topmouth gudgeon Pseudorasbora parva, with description of a new species of Gyrodactylus. Parasite 2023, 30, 22. [Google Scholar] [CrossRef]
- Shigoley, M.I.; Rahmouni, I.; Louizi, H.; Pariselle, A.; Vanhove, M.P.M. First Study on Gyrodactylus (Monogenea: Gyrodactylidae) in Morocco, with Description of a New Species from Luciobarbus pallaryi and Luciobarbus ksibi (Actinopterygii: Cyprinidae). Animals 2023, 13, 1624. [Google Scholar] [CrossRef]
- Zhang, W.-R.; Hao, C.-L.; Arken, K.; Rong, M.-J.; Tian, S.-L.; Kadir, M.; Yue, C. New Species of Gyrodactylus von Nordmann, 1832 (Monogenoidea: Gyrodactylidae) from Gymnodiptychus dybowskii (Kessler, 1874) (Schizothoracinae) in the Kunes River (Yili River Basin), China. Int. J. Parasitol. Parasites Wildl. 2023, 22, 136–145. [Google Scholar] [CrossRef]
- Chen, T.; Huang, J.; Zhou, L.; Kang, M.; Wang, X. Supplemental Description of Gyrodactylus pseudorasborae (Gyrodactylidae) Parasitic on Topmouth Gudgeon Pseudorasbora parva (Cyprinidae) in South China. Parasitol. Int. 2024, 98, 102817. [Google Scholar] [CrossRef]
- Malmberg, G. On a new genus of viviparous monogenetic trematodes. Ark. Zool. 1957, 10, 317–330. [Google Scholar]
- Bouer, O.N. Key to the Parasites of the Freshwater Fish Fauna of the USSR (In Russion); Nauka: Leningrad, Russia, 1985. [Google Scholar]
- Prost, M. Fish Monogenoidea of Poland. I. Parasites of Alburnus alburnus. Acta Parasitol. Pol. 1972, 20, 233–247. [Google Scholar]
- Abdullah, Y. The First Record of Gyrodactylus decorus Malmberg, 1957 (Monogenea: Gyrodactylidae) on Alburnus mossulensis in Iraq. J. Zankoy Sulaimani–Part A 2021, 23, 77–82. [Google Scholar] [CrossRef]
- Kvach, Y.; Ondračková, M.; Kutsokon, Y.; Dzyziuk, N. New Record of Monogenean Parasites on Non-Indigenous Fishes in the Ukrainian Danube Delta. BioInvasions Rec. 2018, 7, 65–72. [Google Scholar] [CrossRef]
- Ergens, R.; Yukhimenko, S.S. Two New Species of the Genus Gyrodactylus Nordmann (Monogenoidea) from Perccottus glehnii. Parazitologiya 1973, 7, 186–188, (In Russian with English summary). [Google Scholar]
- Dove, A.D.M.; Ernst, I. Concurrent invaders—Four exotic species of Monogenea now established on exotic freshwater fishes in Australia. Int. J. Parasitol. 1998, 28, 1755–1764. [Google Scholar] [CrossRef]
- Ling, M.-E. Notes on Seven New Parasitic Species of Monogenetic Rematodes—Gyrodactylus from Freshwater Fishes of China. Acta Hydrobiol. Sin. 1962, 2, 67–78, (In Chinese, but abstract in English). [Google Scholar] [CrossRef]
- Dubey, A.; Gupta, A.K.; Agarwal, S.M. Studies on Monogenean Parasites in Freshwater Fishes at Raipur III. Three New Species of Genus Gyrodactylus Nordmann (1832). Indian J. Exp. Biol. 1990, 42, 1–8. [Google Scholar]
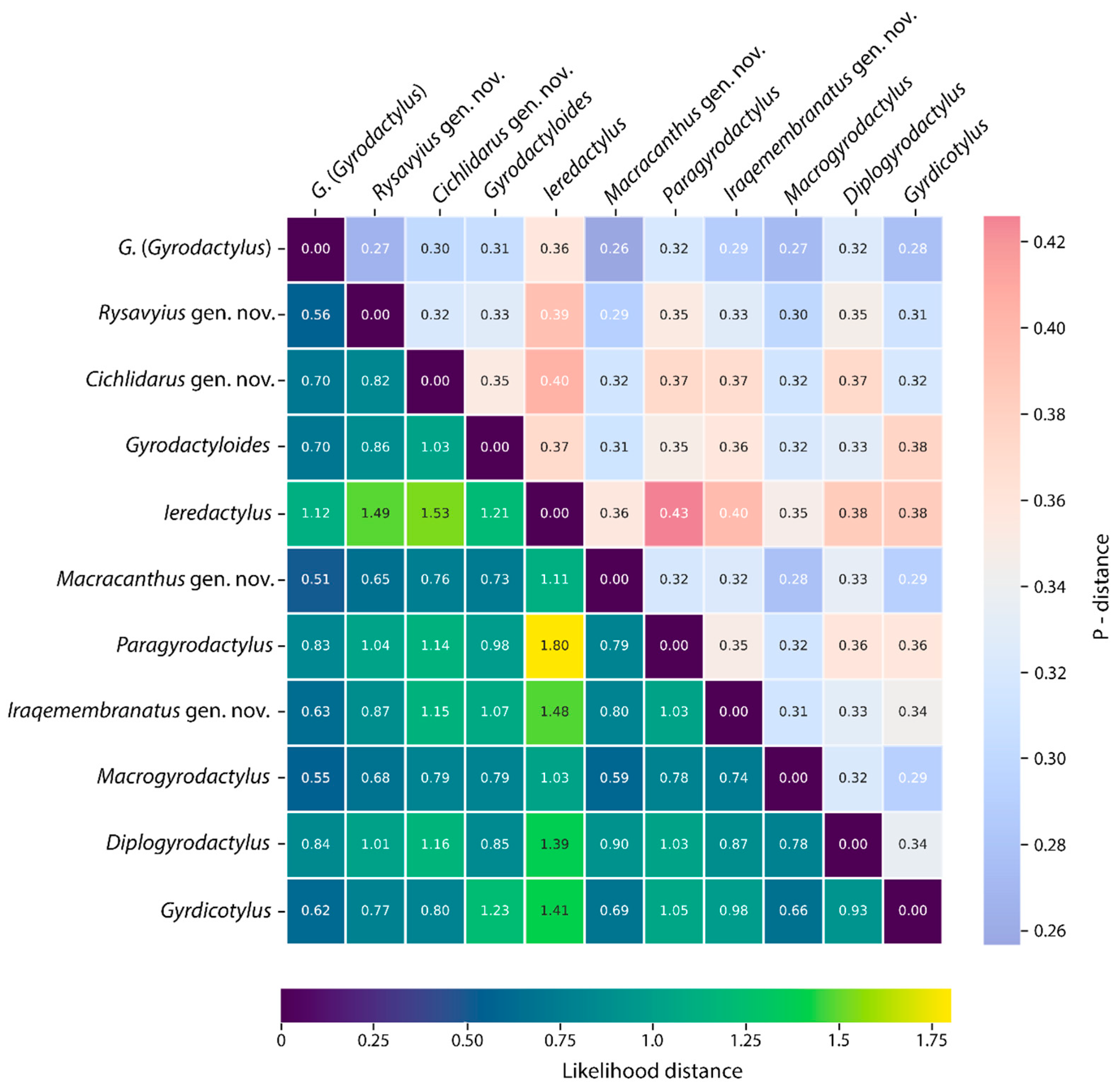
| Genus | Mean Likelihood Distance | Mean p-Distance |
|---|---|---|
| G. (Gyrodactylus) | 0.14 | 0.11 |
| Rysavyius gen. nov. | 0.39 | 0.20 |
| Cichlidarus gen. nov. | 0.09 | 0.08 |
| Macracanthus gen. nov. | 0.18 | 0.12 |
| Macrogyrodactylus | 0.10 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janulewicz, J.; Pietkiewicz, M.; Ziętara, M.S. Revision of the Most Primitive Taxa of the Family Gyrodactylidae (van Beneden et Hesse, 1864) (Platyhelminthes, Monopisthocotyla) Based on ITS rDNA Phylogeny. Genes 2024, 15, 1236. https://doi.org/10.3390/genes15091236
Janulewicz J, Pietkiewicz M, Ziętara MS. Revision of the Most Primitive Taxa of the Family Gyrodactylidae (van Beneden et Hesse, 1864) (Platyhelminthes, Monopisthocotyla) Based on ITS rDNA Phylogeny. Genes. 2024; 15(9):1236. https://doi.org/10.3390/genes15091236
Chicago/Turabian StyleJanulewicz, Jakub, Maciej Pietkiewicz, and Marek S. Ziętara. 2024. "Revision of the Most Primitive Taxa of the Family Gyrodactylidae (van Beneden et Hesse, 1864) (Platyhelminthes, Monopisthocotyla) Based on ITS rDNA Phylogeny" Genes 15, no. 9: 1236. https://doi.org/10.3390/genes15091236







