Advances in the Structure, Function, and Regulatory Mechanism of Plant Plasma Membrane Intrinsic Proteins
Abstract
1. Introduction
2. Structure and Classification of PIPs
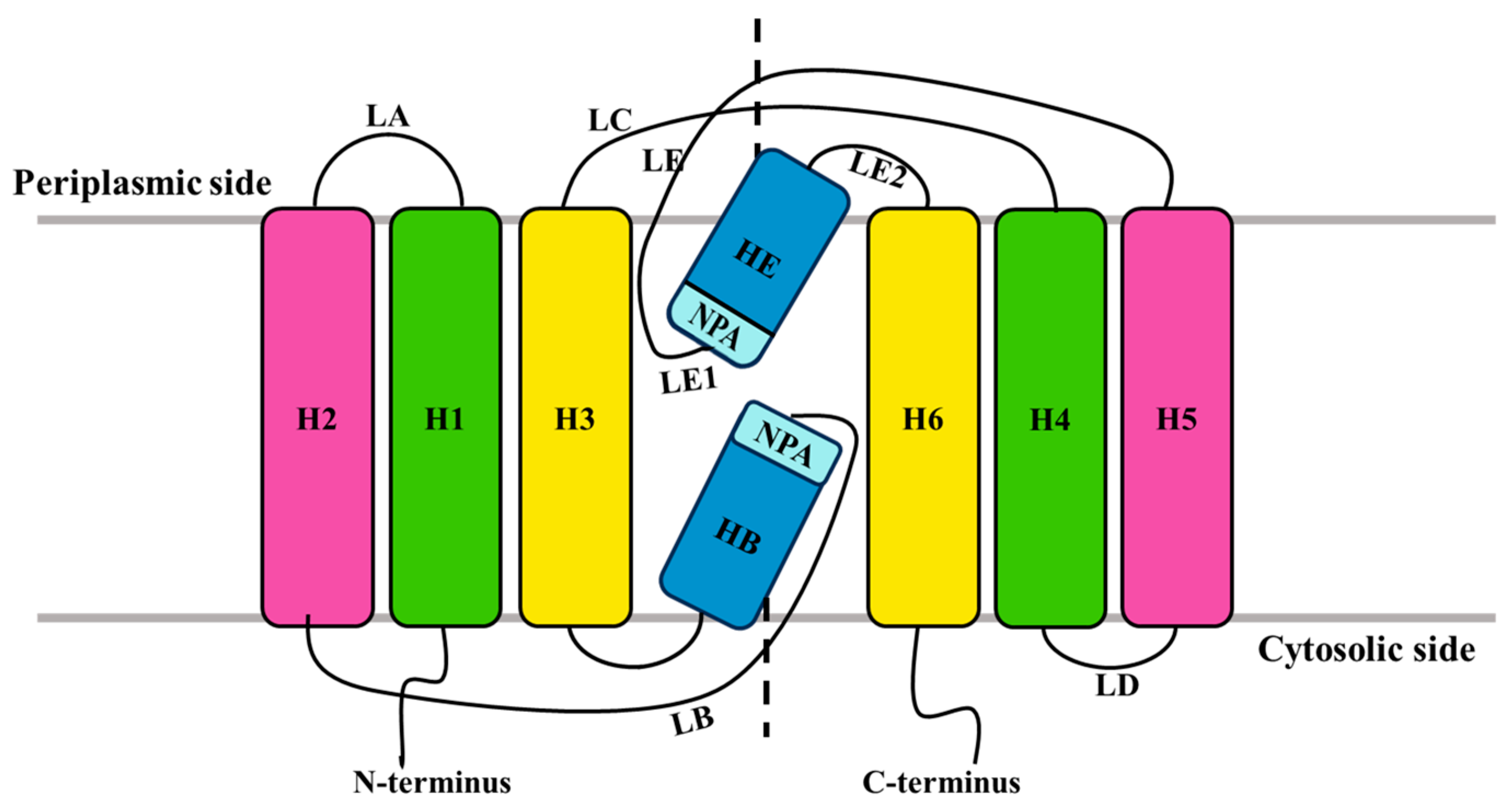
2.1. Origin, Classification, and Structure of AQPs
2.2. Conserved Regions and Classification of PIPs
3. The Function of PIPs
3.1. Physiological Function
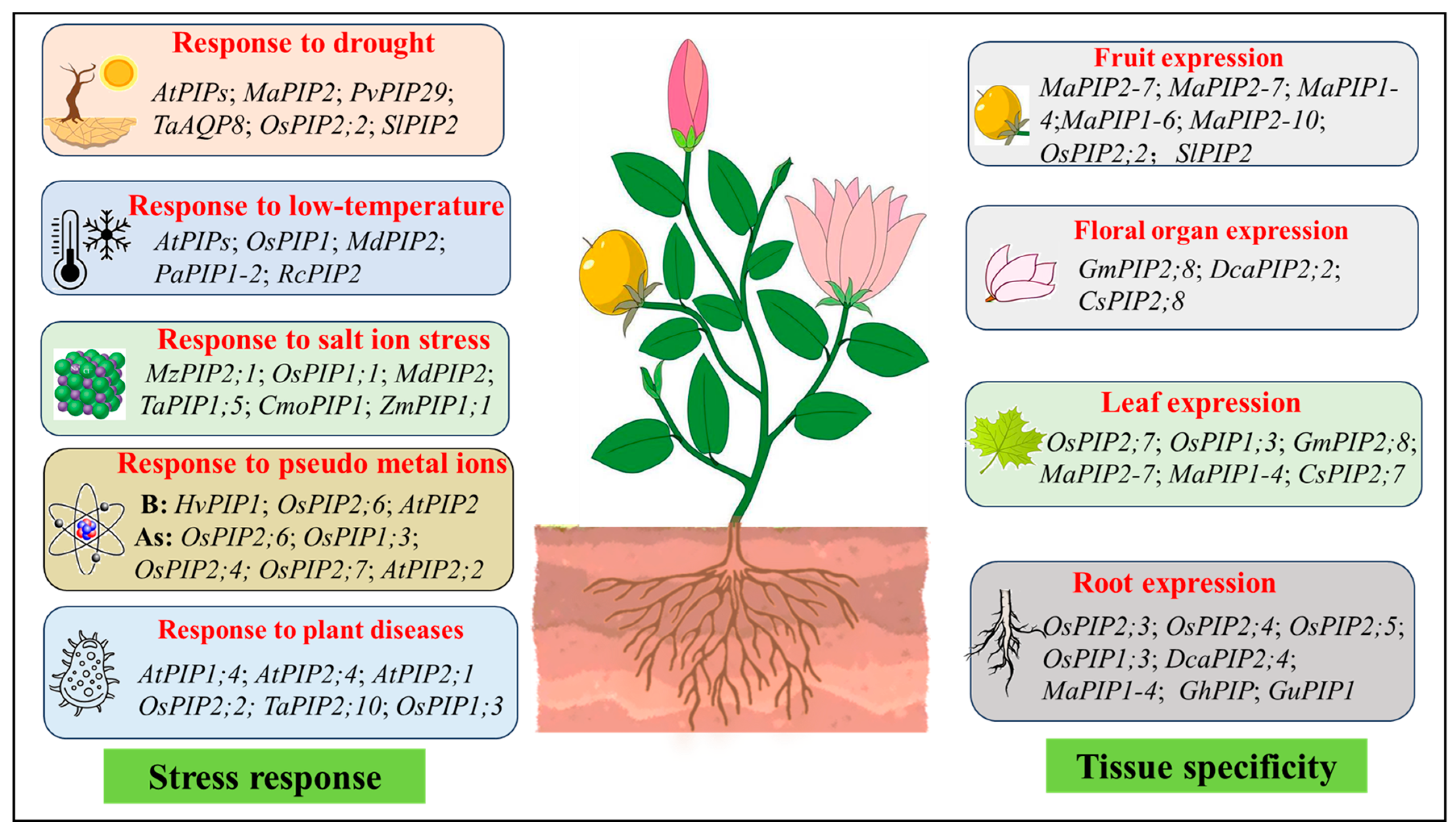
3.2. Function Under Abiotic Stress
3.2.1. Response to Drought
3.2.2. Response to Low Temperature
3.2.3. Response to Salt Ion Stress
3.2.4. Response to Pseudo Metal Ions
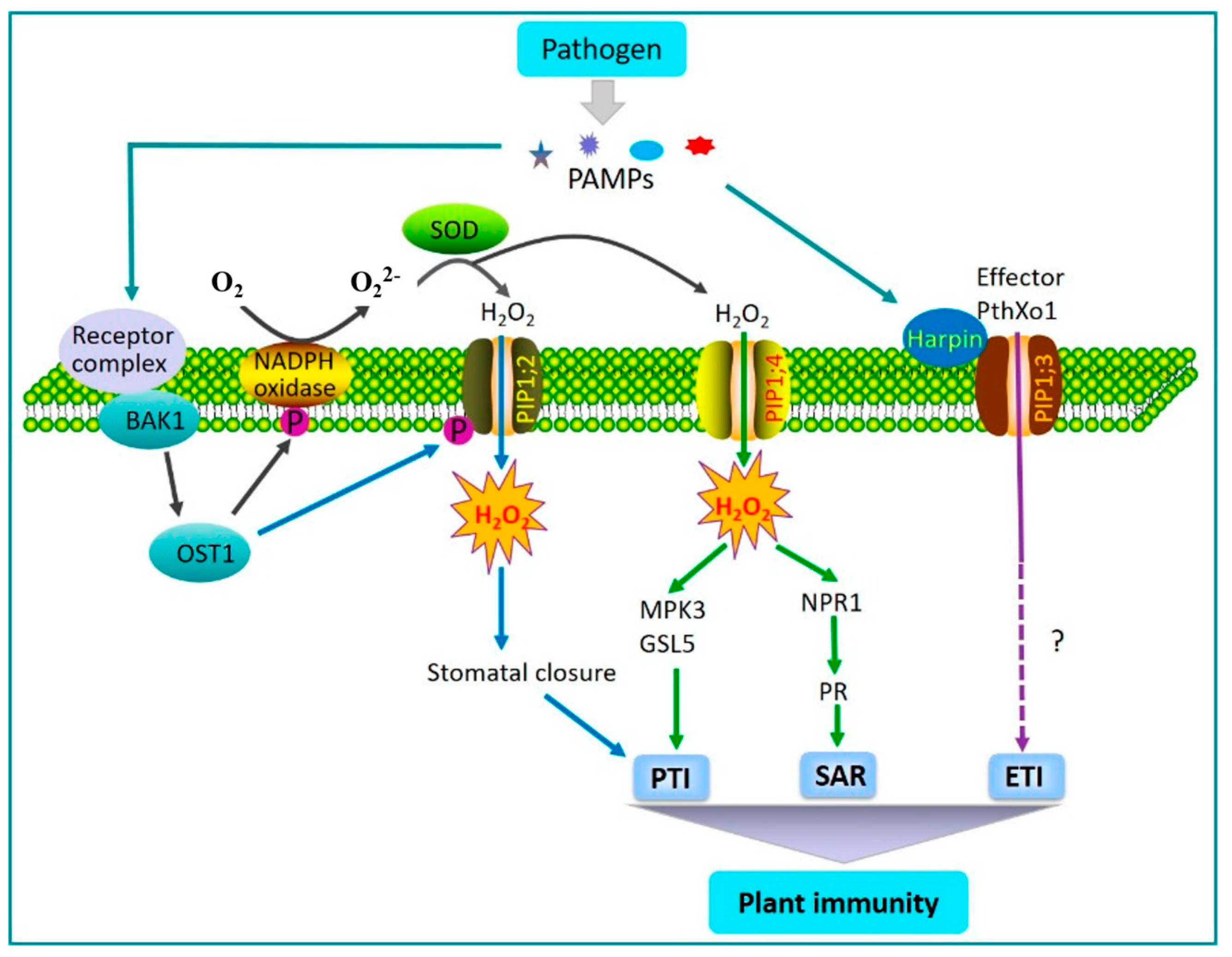
3.3. Function Under Plant Diseases
4. The Expression and Regulatory Mechanism of PIP
4.1. The Specificity of PIP Expression Mechanisms in Different Organizations
4.2. PIP Regulatory Mechanism
4.2.1. Phosphorylation Modification
4.2.2. Other Proteins Translated Post Modification
4.2.3. Other Regulatory Mechanisms
5. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaldenhoff, R.; Fischer, M. Aquaporins in plants. Acta Physiol. 2006, 187, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole Gene Family Expression and Drought Stress Regulation of Aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef]
- Lucía, Y.; Micaela, C.; Carmen, M. Detergent Resistant Membrane Domains in Broccoli Plasma Membrane Associated to the Response to Salinity Stress. Int. J. Mol. Sci. 2020, 21, 7694. [Google Scholar] [CrossRef]
- Hewawitharanage, H.; Sarvananda, L. Aquaporins: Multifunctional Players in Plant Growth, Development and Stress Responses. Silva Balc. 2024, 25, 73–86. [Google Scholar] [CrossRef]
- Yu, Q.J.; Wu, Q.; Lin, Z.P.; Li, J.F. Advance of Plant Aquaporins Research. Acta Sci. Nat. Univ. Pekin. 2002, 38, 855–866. [Google Scholar]
- Preston, G.M.; Agre, P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta 2014, 1840, 1468–1481. [Google Scholar] [CrossRef]
- Zardoya, R. Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 2005, 97, 397–414. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Heymann, J.B.; Engel, A. Aquaporins: Phylogeny, structure, and physiology of water channels. News Physiol. Sci. 1999, 14, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, M.; Frick, A.; Wang, Y.; Ekvall, M.; Hallgren, K.; Hedfalk, K.; Neutze, R.; Tajkhorshid, E.; Törnroth-Horsefield, S. Structural and Functional Analysis of SoPIP2;1 Mutants Adds Insight into Plant Aquaporin Gating. J. Mol. Biol. 2009, 387, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Luang, S.; Hrmova, M. Structural Basis of the Permeation Function of Plant Aquaporins; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–28. [Google Scholar]
- Kammerloher, W.; Fischer, U.; Piechottka, G.P.; Schaffner, A.R. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 2010, 6, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Karlsson, M.; Johanson, U.; Larsson, C.; Kjellbom, P. The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta-Biomembr. 2000, 1465, 324–342. [Google Scholar] [CrossRef]
- Johansson, I.; Karlsson, M.; Shukla, V.K.; Chrispeels, M.J.; Larsson, C.; Kjellbom, P. Water Transport Activity of the Plasma Membrane Aquaporin PM28A Is Regulated by Phosphorylation. Plant Cell 1998, 10, 451–460. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Deokar, A.A.; Tar’an, B. Genome-Wide Analysis of the Aquaporin Gene Family in Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 1802. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, Q.; Ma, Z.; Zhou, G.F.; Feng, F.F.; Le, S.; Lei, C.Y.; Gu, Q.Q. Genome-wide identification and characterization of sweet orange (Citrus sinensis) aquaporin genes and their expression in two citrus cultivars differing in drought tolerance. Tree Genet. Genomes 2019, 15, 17. [Google Scholar] [CrossRef]
- Ariani, A.; Gepts, P. Genome-wide identification and characterization of aquaporin gene family in common bean (Phaseolus vulgaris L.). Mol. Genet. Genom. 2015, 290, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Yaguinuma, D.H.; Dos Santos, T.B.; De Souza, S.G.H.; Vieira, L.G.E.; Ribas, A.F. Genome-Wide Identification, Evolution, and Expression Profile of Aquaporin Genes in Coffea canephora in Response to Water Deficit. Plant Mol. Biol. Report. 2021, 39, 146–162. [Google Scholar] [CrossRef]
- Min, X.; Wu, H.; Zhang, Z.; Wei, X.Y.; Jin, X.Y.; Ndayambaza, B.; Wang, Y.R.; Liu, W.X. Genome-wide identification and characterization of the aquaporin gene family in Medicago truncatula. J. Plant Biochem. Biotechnol. 2019, 28, 320–335. [Google Scholar] [CrossRef]
- Shivaraj, S.M.; Deshmukh, R.K.; Rai, R.; Bélanger, R.; Agrawal, P.K.; Dash, P.K. Genome-wide identification, characterization, and expression profile of aquaporin gene family in flax (Linum usitatissimum). Sci. Rep. 2017, 7, 46137. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins Constitute a Large and Highly Divergent Protein Family in Maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef]
- Schuurmans, J.A.M.J.; van Dongen, J.T.; Rutjens, B.P.W.; Boonman, A.; Pieterse, C.M.J.; Borstlap, A.C. Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Mol. Biol. 2003, 53, 633–645. [Google Scholar] [CrossRef]
- Belugin, B.V.; Zhestkova, I.M.; Piotrovskii, M.S.; Lapshin, N.K.; Trofimova, M.S. PIP1 aquaporins, sterols, and osmotic water permeability of plasma membranes from etiolated pea seedlings. Biol. Membr. 2017, 11, 168–176. [Google Scholar] [CrossRef]
- Danielson, J.Å.H.; Johanson, U. Unexpected complexity of the Aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef]
- MacRobbie, E.A.C. Osmotic effects on vacuolar ion release in guard cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Hanba, Y.T.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K.; Terashima, I.; Katsuhara, M. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004, 45, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Rivers, R.L.; Dean, R.M.; Chandy, G.; Hall, J.E.; Roberts, D.M.; Zeidel, M.L. Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J. Biol. Chem. 1997, 272, 16256–16261. [Google Scholar] [CrossRef] [PubMed]
- Dominique, L.; Uwe, L.; Lixing, Y.; von Wirén, N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005, 137, 671–680. [Google Scholar]
- Kojima, S.; Bohner, A.; von Wirén, N. Molecular mechanisms of urea transport in plants. J. Membr. Biol. 2006, 212, 83–91. [Google Scholar] [CrossRef]
- Junpei, T.; Motoko, W.; Uwe, L.; Schaaf, G.; von Wirén, N.; Fujiwara, T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar]
- David, I.; Hee, L.S.; Matthew, R.T.; Zwiazek, J.J. Plasma membrane aquaporins of the PIP1 and PIP2 subfamilies facilitate hydrogen peroxide diffusion into plant roots. BMC Plant Biol. 2022, 22, 566. [Google Scholar]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Fetter, K.; Van Wilder, V.; Moshelion, M.; Chaumont, F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 2004, 16, 215–228. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Fischer, M. Functional aquaporin diversity in plants. Biochim. Et Biophys. Acta 2006, 1758, 1134–1141. [Google Scholar] [CrossRef]
- Hedfalk, K.; Tornroth-Horsefield, S.; Nyblom, M.; Johanson, U.; Kjellbom, P.; Neutze, R. Aquaporin gating. Curr. Opin. Struct. Biol. 2006, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Morillon, R.; Catterou, M.; Sangwan, R.S.; Sangwan, B.S.; Lassalles, J.P. Brassinolide may control aquaporin activities in Arabidopsis thaliana. Planta 2001, 212, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Siefritz, F.; Biela, A.; Eckert, M.; Otto, B.; Uehlein, N.; Kaldenhoff, R. The tobacco plasma membrane aquaporin NtAQP1. J. Exp. Bot. 2001, 52, 1953–1957. [Google Scholar] [CrossRef]
- Maurel, C.; Chrispeels, M.J. Aquaporins. A molecular entry into plant water relations. Plant Physiol. 2001, 125, 135–138. [Google Scholar] [CrossRef]
- Clare, V.W.; Olivier, P.; Colette, T.-R.; Boursiac, Y.; Maurel, C. Expression and inhibition of aquaporins in germinating Arabidopsis seeds. Plant Cell Physiol. 2006, 47, 1241–1250. [Google Scholar]
- Hélène, J.; Virginie, L.; Véronique, S.; Martin-Laurent, F.; Güçlü, J.; Vinh, J.; Heyes, J.; Franck, K.I.; Schäffner, A.R.; Bouchez, D.; et al. Role of a single aquaporin isoform in root water uptake. Plant Cell 2003, 15, 509–522. [Google Scholar]
- Soto, G.; Alleva, K.; Mazzella, M.A.; Amodeo, G.; Muschietti, J.P. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett. 2008, 582, 4077–4082. [Google Scholar] [CrossRef]
- Gabriela, S.; Romina, F.; Nicolas, A.; Alleva, K.; Guaimas, F.; Erijman, E.J.; Mazzella, A.; Amodeo, G.; Muschietti, J. TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2010, 64, 1038–1047. [Google Scholar]
- Andrea, P.D.G.J.; Laura, B.M.; Gabriela, A.; Muschietti, J.P. Pollen aquaporins: What are they there for? Plant Signal. Behav. 2016, 11, e1217375. [Google Scholar]
- Ryosuke, S.; Masayoshi, M. The ER-localized aquaporin SIP2;1 is involved in pollen germination and pollen tube elongation in Arabidopsis thaliana. Plant Mol. Biol. 2019, 100, 335–349. [Google Scholar]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2020, 171, 66–76. [Google Scholar] [CrossRef]
- Yanru, H.; Yanjuan, J.; Xiao, H.; Wang, H.P.; Pan, J.J.; Yu, D.Q. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar]
- Christian, Z.; Christoph-Martin, G.; Karl-Josef, D. Salinity and Crop Yield. Plant Biol. 2018, 21, 31–38. [Google Scholar]
- Ahmed, M. Effects of Abiotic Stress in Crop Production. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Cham, Switzerland, 2017; pp. 165–180. [Google Scholar]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Maurel, C. Plant aquaporins: Novel functions and regulation properties. FEBS Lett. 2007, 581, 2227–2236. [Google Scholar] [CrossRef]
- Li, D.D.; Wu, Y.J.; Ruan, X.M.; Li, B.; Zhu, L.; Wang, H.; Li, X.B. Expressions of three cotton genes encoding the PIP proteins are regulated in root development and in response to stresses. Plant Cell Rep. 2009, 28, 291–300. [Google Scholar] [CrossRef]
- Yu, Q.J.; Hu, Y.L.; Li, J.F.; Qi, W.; Lin, Z. Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Sci. 2005, 169, 647–656. [Google Scholar] [CrossRef]
- Sajad, A.; Shaista, K.; Mohd, A.; Gandhi, S.G. Plant Aquaporins: A frontward to make crop plants drought resistant. Physiol. Plant. 2021, 172, 1089–1105. [Google Scholar]
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Jin, S.K.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Hou, X.W.; Huang, C.; Yan, Y.; Tie, W.W.; Ding, Z.H.; Wei, Y.X.; Liu, J.H.; Miao, H.X.; Lu, Z.W.; et al. Genome-Wide Identification and Expression Analyses of Aquaporin Gene Family during Development and Abiotic Stress in Banana. Int. J. Mol. Sci. 2015, 16, 19728–19751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, W.W.; Li, H.; Lu, Q.; Xu, B.; Huang, B.G. Overexpression of an aquaporin gene PvPIP2;9 improved biomass yield, protein content, drought tolerance and water use efficiency in switchgrass (Panicum virgatum L.). GCB Bioenergy 2020, 12, 979–991. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.; Song, S.; Hou, X.; Jia, C.; Li, J.; Miao, H.; Wang, Z.; Tie, W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. 2020, 147, 66–76. [Google Scholar] [CrossRef]
- Sreedharan, S.; Shekhawat, U.K.S.; Ganapathi, T.R. Transgenic banana plants overexpressing a native plasma membrane aquaporin MusaPIP1;2 display high tolerance levels to different abiotic stresses. Plant Biotechnol. J. 2013, 11, 942–952. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.H.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14, 59. [Google Scholar] [CrossRef]
- Hu, W.; Yuan, Q.Q.; Wang, Y.; Cai, R.; Deng, X.M.; Wang, J.; Zhou, S.Y.; Chen, M.J.; Chen, L.H.; Huang, C.; et al. Overexpression of a Wheat Aquaporin Gene, TaAQP8, Enhances Salt Stress Tolerance in Transgenic Tobacco. Plant Cell Physiol. 2012, 53, 2127–2141. [Google Scholar] [CrossRef]
- Bai, J.Q.; Wang, X.; Yao, X.H.; Chen, X.C.; Lu, K.; Hu, Y.Q.; Wang, Z.D.; Mu, Y.J.; Zhang, L.Y.; Dong, H.S. Rice aquaporin OsPIP2;2 is a water-transporting facilitator in relevance to drought-tolerant responses. Plant Direct. 2021, 5, e338. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.F.; Li, S.T.; Zhang, L.; Qi, C.D.; Weeda, S.; Zhao, B.; Ren, S.X.; Guo, Y.D. Plasma Membrane Intrinsic Proteins SlPIP2;1, SlPIP2;7 and SlPIP2;5 Conferring Enhanced Drought Stress Tolerance in Tomato. Sci. Rep. 2016, 6, 31814. [Google Scholar] [CrossRef]
- Matsumoto, T.; Lian, H.L.; Su, W.A.; Tanaka, D.; Liu, C.W.; Iwasaki, I.; Kitagawa, Y. Role of the Aquaporin PIP1 Subfamily in the Chilling Tolerance of Rice. Plant Cell Physiol. 2009, 50, 216–229. [Google Scholar] [CrossRef]
- Xin, M.M. Cloning and Functional Analysis of Apple Plasma Membrane Intrinsic Protein Gene MdPIP2; Northwest A&F University: Xianyang, China, 2021. [Google Scholar]
- Li, S.F.; Zheng, G.S.; Wang, F.; Yu, H.; Wang, S.; Guan, H.H.; Lv, F.N.; Xia, Y.X. Expression and Functional Analysis of the PaPIP1-2 Gene during Dormancy and Germination Periods of Kernel-Using Apricot (Prunus armeniaca L.). Forests 2023, 14, 2306. [Google Scholar] [CrossRef]
- Peng, Y.H.; Arora, R.; Li, G.W.; Wang, X.; Fessehaie, A. Rhododendron catawbiense plasma membrane intrinsic proteins are aquaporins, and their over-expression compromises constitutive freezing tolerance and cold acclimation ability of transgenic Arabidopsis plants. Plant Cell Environ. 2008, 31, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Peng, Y.H.; Zhang, M.H.; Shao, Y.J.; Su, W.A.; Tang, Z.C. Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res. 2006, 16, 599–608. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, S.Y.; Hu, W.; Deng, X.M.; Wei, S.Y.; Yang, G.X.; He, G.Y. The Wheat Aquaporin Gene TaAQP7 Confers Tolerance to Cold Stress in Transgenic Tobacco. Z. Fur Naturforschung Sect. C-A J. Biosci. 2014, 69, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Hu, W.; Deng, X.M.; Ma, Z.B.; Chen, L.H.; Huang, C.; Wang, C.; Wang, J.; He, Y.Z.; Yang, G.X.; et al. Overexpression of the Wheat Aquaporin Gene, TaAQP7, Enhances Drought Tolerance in Transgenic Tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Lei, Q.; Feng, C.; Gao, Y.N.; Zheng, X.D.; Zhao, Y.; Wang, Z.; Kong, J. MzPIP2;1: An Aquaporin Involved in Radial Water Movement in Both Water Uptake and Transportation, Altered the Drought and Salt Tolerance of Transgenic Arabidopsis. PLoS ONE 2015, 10, e0142446. [Google Scholar] [CrossRef]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.H.; Sun, X.L.; Zhu, Y.M.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2012, 63, 151–158. [Google Scholar] [CrossRef]
- Sreedharan, S.; Shekhawat, U.K.S.; Ganapathi, T.R. Constitutive and stress-inducible overexpression of a native aquaporin gene (MusaPIP2;6) in transgenic banana plants signals its pivotal role in salt tolerance. Plant Mol. Biol. 2015, 88, 41–52. [Google Scholar] [CrossRef]
- Shamloo-Dashtpagerdi, R.; Sisakht, J.N.; Tahmasebi, A. MicroRNA miR1118 contributes to wheat (Triticum aestivum L.) salinity tolerance by regulating the Plasma Membrane Intrinsic Proteins1;5 (PIP1;5) gene. J. Plant Physiol. 2022, 278, 153827. [Google Scholar] [CrossRef]
- Sohail, H.; Noor, I.; Nawaz, M.A.; Ma, M.R.; Shireen, F.; Huang, Y.; Yang, L.; Bie, Z.L. Genome-wide identification of plasma-membrane intrinsic proteins in pumpkin and functional characterization of CmoPIP1-4 under salinity stress. Environ. Exp. Bot. 2022, 202, 104995. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Xiong, Y.H.; Liu, C.X.; Wang, J.G.; Wang, G.Q.; Cai, Y.L. Overexpression of a maize plasma membrane intrinsic protein ZmPIP1;1 confers drought and salt tolerance in Arabidopsis. PLoS ONE 2018, 13, e0198639. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.S.; Pedrosa, A.M.; Du, D.L.; Gonçalves, L.P.; Yu, Q.B.; Jr, F.G.; Costa, M.G. Genome-Wide Characterization and Expression Analysis of Major Intrinsic Proteins during Abiotic and Biotic Stresses in Sweet Orange (Citrus sinensis L. Osb.). PLoS ONE 2017, 10, e0138786. [Google Scholar]
- Cramer, G.R.; Ergul, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.; Bohlman, M.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef]
- Venkatesh, J.; Yu, J.W.; Park, S.W. Genome-wide analysis and expression profiling of the Solanum tuberosum aquaporins. Plant Physiol. Biochem. 2013, 73, 392–404. [Google Scholar] [CrossRef]
- Qian, W.G. Genome-Wide Characterization of AQP Gene Family and Functional Analysis on Stress Resistance of EsPIP1;2 and EsPIP2;1 in Eutrema salsugineum; Northeast Forestry University: Harbin, China, 2020. [Google Scholar]
- Jang, J.Y.; Rhee, J.Y.; Kim, D.G.; Chuang, G.C.; Lee, J.H.; Kang, H.S. Ectopic expression of a foreign aquaporin disrupts the natural expression patterns of endogenous aquaporin genes and alters plant responses to different stress conditions. Plant Cell Physiol. 2007, 48, 1331–1339. [Google Scholar] [CrossRef]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef]
- Fitzpatrick, K.L.; Reid, R.J. The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ. 2009, 32, 1357–1365. [Google Scholar] [CrossRef]
- Macho-Rivero, M.A.; Herrera-Rodríguez, M.B.; Brejcha, R.; Schäffner, A.S.; Tanaka, N.; Fujiwara, T.; Agustín, G.F.; Juan, J.C. Boron Toxicity Reduces Water Transport from Root to Shoot in Arabidopsis Plants. Evidence for a Reduced Transpiration Rate and Expression of Major PIP Aquaporin Genes. Plant Cell Physiol. 2018, 59, 836–844. [Google Scholar] [CrossRef]
- Abou, S.M.A.; Abou, E.A.A.; Yassen, A.A.; Hammad, S.A. Boron, structure, functions and its interaction with nutrients in plant physiology. Middle East J. Agric. Res. 2021, 10, 117–179. [Google Scholar]
- Meselhy, A.G.; Mosa, K.; Chhikara, S.; Kumar, K.; Musante, C.; White, J.C.; Dhankher, O.P. Plasma membrane intrinsic protein OsPIP2;6 is involved in root-to-shoot arsenic translocation in rice (Oryza sativa L.). Plant cell reports 2024, 43, 64. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; Kumar, K.; Chhikara, S.; Mcdermott, J.; Liu, Z.J.; Musante, C.; White, J.C.; Dhankher, O.P. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res. 2012, 21, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Kundan, K.; Mosa, K.A.; Chhikara, S.; Musante, C.; White, J.C.; Dhankher, O.P. Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta 2014, 239, 187–198. [Google Scholar]
- Modareszadeh, M.; Bahmani, R.; Kim, D.; Hwang, S. Decreases in arsenic accumulation by the plasma membrane intrinsic protein PIP2;2 in Arabidopsis and yeast. Environ. Pollut. 2021, 275, 116646. [Google Scholar] [CrossRef]
- Tian, S.; Torres, R.; Ballester, A.R.; Li, B.; Vilanova, L.; González-Candelas, L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430. [Google Scholar] [CrossRef]
- Li, G.; Chen, T.; Zhang, Z.; Li, B.; Tian, S. Roles of aquaporins in plant-pathogen interaction. Plants 2020, 9, 1134. [Google Scholar] [CrossRef]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.C.; Chen, T.; Li, B.Q.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit-Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Angel Torres, M. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.B.; Li, P.; Wang, H.; Ji, H.T.; Xie, J.Y.; Qiu, Q.L.; Shen, D.; Dong, H.S. Plant Aquaporin AtPIP1;4 Links Apoplastic H2O2 Induction to Disease Immunity Pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.J. Molecular Mochanisms that Aquaporins AtPIP1;4 and AtPIP2;4 Deploy to Faciliate H2O2 Transport and Regulate Disease Resistance; Shandong Agricultural University: Taian, China, 2022. [Google Scholar]
- Sang, S.L.; You, Z.Z.; Dong, H.S. Arabidopsis aquaporins and protein interactions and their regulatory effects on growth promotion. In Proceedings of the Annual Conference of the Chinese Society of Plant Pathology; China Agricultural Science and Technology Press: Beijing, China, 2012. [Google Scholar]
- Zhang, M.; Shi, H.T.; Li, N.N.; Tian, Y.; Peng, J.F.; Chen, X.C.; Zhang, L.Y.; Zhang, M.X.; Dong, H.S. Aquaporin OsPIP2;2 links the H2O2 signal and a membrane-anchored transcription factor to promote plant defense. Plant Physiol. 2022, 188, 2325–2341. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Chen, X.C.; Yao, X.H.; An, Y.Y.; Wang, X.; Qin, L.N.; Li, X.X.; Wang, Z.D.; Liu, S.; Sun, Z.M.; et al. Phosphorylation of a wheat aquaporin at two sites enhances both plant growth and defense. Mol. Plant 2022, 15, 1772–1789. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.Y.; Mo, X.Y.; Ji, H.T.; Bian, H.J.; Hu, Y.Q.; Majid, T.; Long, J.Y.; Pang, H.; Tao, Y.; et al. Rice aquaporin PIP1;3 and harpin Hpa1 of bacterial blight pathogen cooperate in a type III effector translocation. J. Exp. Bot. 2019, 70, 3057–3073. [Google Scholar] [CrossRef]
- You, Z.Z. Roles of the Hpa1 Protein in Disease Resistance and in Phtosynthesis After Interaction with Aquaporin PIP1;4 in Arabiaopsis; Nanjing Agricultural University: Nanjing, China, 2013. [Google Scholar]
- Nguyen, M.X.; Moon, S.; Jung, K.H. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 2013, 238, 669–681. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.; Chen, J.; Liu, M.H.; Chen, Z.L.; Qu, L.J.; Gu, H. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Ali, Z.; Wang, C.B.; Xu, L.; Yi, J.; Xu, Z.L.; Liu, X.Q.; He, X.L.; Huang, Y.; Khan, I.; et al. Genome-Wide Sequence Characterization and Expression Analysis of Major Intrinsic Proteins in Soybean (Glycine max L.). PLoS ONE 2013, 8, e56312. [Google Scholar] [CrossRef]
- Kong, W.L.; Bendahmane, M.; Fu, X.P. Genome-Wide Identification and Characterization of Aquaporins and Their Role in the Flower Opening Processes in Carnation (Dianthus caryophyllus). Molecules 2018, 23, 1895. [Google Scholar] [CrossRef]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE 2017, 8, e79052. [Google Scholar] [CrossRef]
- Duan, M.S.; Liu, X.H.; Wang, S.Q.; Zhang, Y.H.; He, J.Y.; Liu, H.; Yu, Y.B. Cloning of CsPIP2;7 and CsPIP2;8 Genes in Tea Plant and Its Response to Drought Stress. J. Plant Genet. Resour. 2020, 21, 11. [Google Scholar]
- Wang, F. Cloning, Localization and Expression Analysis of the Plasma Membrane Aquaporin PIP1 from Glycyrrhiza uralensis Fischy; Northeast Normal University: Changchun, China, 2006. [Google Scholar]
- Endler, A.; Reiland, S.; Gerrits, B.; Schmidt, U.G.; Baginsky, S.; Martinoia, E. In vivo phosphorylation sites of barley tonoplast proteins identified by a phosphoproteomic approach. Proteomics 2009, 9, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Niittylae, T.; Fuglsang, A.T.; Palmgren, M.G.; Frommer, W.B.; Schulze, W.X. Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol. Cell. Proteom. 2007, 6, 1711–1726. [Google Scholar] [CrossRef] [PubMed]
- Prak, S.; Hem, S.; Boudet, J.; Viennois, G.; Sommerer, N.; Rossignol, M.; Maurel, C.; Santoni, V. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins. Mol. Cell. Proteom. 2008, 7, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, S.A.; Nuhse, T.S.; Ashford, D.A.; Sanders, D.; Maathuis, F.J. A proteomic and phosphoproteomic analysis of Oryza sativa plasma membrane and vacuolar membrane. Plant J. 2008, 56, 146–156. [Google Scholar] [CrossRef]
- Prado, K.; Cotelle, V.; Li, G.W.; Bellati, J.; Tang, N.; Colette, T.R.; Alexandre, M.; Santoni, V.; Maurel, C. Oscillating Aquaporin Phosphorylation and 14-3-3 Proteins Mediate the Circadian Regulation of Leaf Hydraulics. Plant Cell 2019, 31, 417–429. [Google Scholar] [CrossRef]
- Wu, F.Q.; Sheng, P.K.; Tan, J.J.; Chen, X.L.; Lu, G.W.; Ma, W.W.; Heng, Y.Q.; Lin, Q.B.; Zhu, S.S.; Wang, J.L.; et al. Plasma membrane receptor-like kinase leaf panicle 2 acts downstream of the drought and salt tolerance transcription factor to regulate drought sensitivity in rice. J. Exp. Bot. 2015, 66, 271–281. [Google Scholar] [CrossRef]
- Van Wilder, V.; Miecielica, U.; Degand, H.; Derua, R.; Waelkens, E.; Chaumont, F. Maize plasma membrane aquaporins belonging to the PIP1 and PIP2 subgroups are in vivo phosphorylated. Plant Cell Physiol. 2008, 49, 1364–1377. [Google Scholar] [CrossRef]
- Kline, K.G.; Barrett-Wilt, G.A.; Sussman, M.R. In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 15986–15991. [Google Scholar] [CrossRef]
- Qing, D.J.; Yang, Z.; Li, M.Z.; Wong, W.S.; Guo, G.Y.; Liu, S.C.; Guo, H.W.; Li, N. Quantitative and Functional Phosphoproteomic Analysis Reveals that Ethylene Regulates Water Transport via the C-terminal Phosphorylation of Aquaporin PIP2;1 in Arabidopsis. Mol. Plant 2016, 9, 158–174. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.D.; Garcia-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE 2018, 13, e0192422. [Google Scholar] [CrossRef]
- Santoni, V.; Verdoucq, L.; Sommerer, N.; Vinh, J.; Pflieger, D.; Maurel, C. Methylation of aquaporins in plant plasma membrane. Biochem. J. 2006, 400, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Vialaret, J.; Li, G.W.; Hem, S.; Prado, K.; Rossignol, M.; Maurel, C.; Santoni, V. Coordinated Post-translational Responses of Aquaporins to Abiotic and Nutritional Stimuli in Arabidopsis Roots. Mol. Cell. Proteom. 2013, 12, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Scalera, V.; Gena, P.; Mastrodonato, M.; Kitagawa, Y.; Carulli, S.; Svelto, M.; Calamita, G. Functional reconstitution of a rice aquaporin water channel, PIP1;1, by a micro-batchwise methodology. Plant Physiol. Biochem. 2014, 85, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Barone, L.M.; Shih, C.; Wasserman, B.P. Mercury-induced conformational changes and identification of conserved surface loops in plasma membrane aquaporins from higher plants : Topology of PMIP31 from β vulgaris L. J. Biol. Chem. 1997, 272, 30672–30677. [Google Scholar] [CrossRef]
- Bienert, G.P.; Cavez, D.; Besserer, A.; Berny, M.C.; Gilis, D.; Rooman, M.; Chaumont, F. A conserved cysteine residue is involved in disulfide bond formation between plant plasma membrane aquaporin monomers. Biochem. J. 2012, 445, 101–111. [Google Scholar] [CrossRef]
- Frick, A.; Jarva, M.; Ekvall, M.; Uzdavinys, P.; Nyblom, M.; Törnroth-Horsefield, S. Mercury increases water permeability of a plant aquaporin through a non-cysteine-related mechanism. Biochem. J. 2013, 454, 491–499. [Google Scholar] [CrossRef]
- Bienert, G.P.; Moller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.E.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Liu, M.X.; Yuan, X.Y.; Yang, Z.M.; Huang, B.R. Physiological Effects of Aquaporin in Regulating Drought Tolerance through Overexpressing of Festuca arundinacea Aquaporin Gene FaPIP2;1. J. Am. Soc. Hortic. Sci. 2015, 140, 404–412. [Google Scholar] [CrossRef]
- Fischer, M.; Kaldenhoff, R. On the pH Regulation of Plant Aquaporins. J. Biol. Chem. 2008, 283, 33889–33892. [Google Scholar] [CrossRef]
- Yaneff, A.; Sigaut, L.; Gómez, N.; Fandiño, C.A.; Alleva, K.; Pietrasanta, L.I.; Amodeo, G. Loop B serine of a plasma membrane aquaporin type PIP2 but not PIP1 plays a key role in pH sensing. BBA-Biomembranes 2016, 1858, 2778–2787. [Google Scholar] [CrossRef]
- Zhang, M.F.; Liu, R.L.; Liu, H.; Yang, H.B.; Li, X.; Wang, P.; Zhu, F.; Xu, R.W.; Xue, S.W.; Cheng, Y.J. Citrus NIP5;1 aquaporin regulates cell membrane water permeability and alters PIPs plasma membrane localization. Plant Mol. Biol. 2021, 106, 449–462. [Google Scholar] [CrossRef]
| PIP1 | PIP2 | Reference | ||
|---|---|---|---|---|
| Structure | C-terminal extension | Shorter | Longer | [17] |
| N-terminal extension | Longer | Shorter | [17] | |
| Function | Water channel activity | Lower | Higher | [15] |
| Distribution and gene numbers of different species | A. thaliana | 5 | 8 | [19] |
| O. sativa | 3 | 8 | [20] | |
| C. arietinum | 4 | 5 | [21] | |
| C. sinensis | 4 | 7 | [22] | |
| Phaseolus vulgaris | 5 | 7 | [23] | |
| Coffea canephora | 3 | 4 | [24] | |
| Medicago sativa | 5 | 5 | [25] | |
| Linum usitatissimum | 5 | 11 | [26] | |
| Zea mays | 6 | 7 | [27] | |
| Gene | Species | Research Methods | Stress Condition | Specific Functions | References |
|---|---|---|---|---|---|
| AtPIP1;2 | A. thaliana | Transcription level | Drought | Downregulated in the roots | [63] |
| Salt ion | Upregulation in the roots | [63] | |||
| MaPIP2-3 MaPIP2-7 | Musa paradisiaca | Transcription level | Drought | Upregulation in the Baxijiao and Fenjiao | [64] |
| MaPIP2-6 | Musa paradisiaca | Transcription level | Drought | Reduced the drought resistance of Baxijiao and improved the drought resistance of Fenjiao | [64] |
| Overexpression in transgenic banana | Salt ion | Increased resistance to salt stress | [82] | ||
| MaPIP1;1 | Musa paradisiaca | Overexpression in Arabidopsis | Drought | Improved ion distribution, reduced membrane damage, and increased Arabidopsis resistance to drought stress | [68] |
| MusaPIP1;2 | Musa paradisiaca | Overexpression in transgenic banana | Drought | Improved cell water levels and improved drought resistance of transgenic banana plants | [67] |
| Low temperature | Positively influence tolerance to cold stress | [67] | |||
| TaAQP8 | Triticum aestivum | Overexpression in tobacco | Drought | Increased tobacco resistance to drought stress | [69] |
| OsPIP2;2 | O. sativa | Overexpression in rice protoplasts | Drought | Enhanced H2O transport and drought responses | [70] |
| SlPIP2;1, SlPIP2;7, SlPIP2;5 | Solanum lycopersicum | Transcription level | Drought | Improved water uptake by maintaining osmotic balance and improving water content | [71] |
| AtPIP2;5 | A. thaliana | Transcription level | Low temperature | Upregulated | [63] |
| OsPIP1;1 OsPIP1;2 | O. sativa | Transcription level | Low temperature t | Increased resistance to cold tolerance | [72] |
| MdPIP2;5a MdPIP2;5b | Malus domestica | Overexpression in Arabidopsis | Low temperature | Increased the tolerance of transgenic Arabidopsis to cold stress | [73] |
| Salt ion | Increased the tolerance of transgenic Arabidopsis to salt ion stress | [73] | |||
| PaPIP1-2 | Prunus armeniaca | Overexpression in Arabidopsis | Low temperature | Enhanced the growth of transgenic plants under cold stress by lowering the level of MDA, increasing Pro accumulation, and increasing SOD activity | [74] |
| RcPIP2;1 RcPIP2;2 | Rhododendron catawbiense | Overexpression in Arabidopsis | Low temperature | Lower ability to resist freeze drying | [75] |
| TaAQP7 | T. aestivum | Overexpression in tobacco | Low temperature | Stronger cold tolerance for transgenic tobacco compared to non-transgenic tobacco | [77,78] |
| OsPIP1;1 | O. sativa | Overexpression in transgenic rice | Salt ion | Enhanced salt tolerance | [81] |
| TaPIP1;5 | T. aestivum | Transcription level | Salt ion | MiR1118 primarily regulates membrane damage, ion homeostasis, and wh eat water status through TaPIP1;5 | [83] |
| CsPIP1;1 | C. sinensis | mRNA expression analysis | Salt ion | Significant upregulation in roots | [86] |
| CmoPIP1-4 | Cucurbita moschata | Overexpression in yeast | Salt ion | Conferred salt tolerance to yeast | [84] |
| ZmPIP1;1 | Z. mays | Overexpression in Arabidopsis | Salt ion | NaCl treatment induced ZmPIP1;1 expression in roots and leaves, transgenic Arabidopsis plants also exhibited enhanced tolerance to salt stress. | [85] |
| VvPIP2;1 | Vitis vinifera | Transcription level | Salt ion | Increased the transcription of VvPIP2;1 | [87] |
| EsPIP2;1 | Eutrema salsugineum | Overexpression in Arabidopsis | Salt ion | Enhanced the salt stress tolerance of Arabidopsis | [89] |
| CfPIP2;1 | Cucurbita ficifolia | Overexpression in Arabidopsis | Salt ion | Increased the germination rate of seeds subjected to high salt stress | [90] |
| HvPIP2;1 | Hordeum vulgare | Overexpression In transgenic rice | Salt ion | Decreased salt tolerance | [91] |
| HvPIP1;3 HvPIP1;4 | H. vulgare | Overexpression in yeast | Pseudo metal ion (B) | Conferred boron transport function | [92] |
| AtPIP1;2 AtPIP2;1 AtPIP2;2 | A. thaliana | Transcription level | Pseudo metal ion (B) | Reduced water flow to the shoots, prevented excess boron accumulation in plant tissues | [93] |
| OsPIP2;6 | O. sativa | Overexpression in Arabidopsis and RNAi-mediated approach | Pseudo metal ion (B and As) | Yielded high boron tolerance in transgenic Arabidopsis and transported of arsenic from roots to stems, reduced arsenic accumulation in rice | [95] |
| OsPIP1;3 OsPIP2;4 OsPIP2;7 | O. sativa | Transcription level | Pseudo metal ion (B and As) | Involved in the transport of boron arsenate (As III) and provided tolerance to As(III) and boron toxicity | [96,97] |
| AtPIP2;2 | A.thaliana | Overexpression in Arabidopsis and yeast | Pseudo metal ion (As) | Increased the tolerance of yeast and Arabidopsis overexpressing As(III), reduced As(II) levels in yeast | [98] |
| AtPIP1;4 AtPIP2;4 | A. thaliana | Overexpression in Arabidopsis | Plant diseases | Enhanced resistance to Pseudomonas syringae | [105,106] |
| OsPIP2;2 | O. sativa | Protein interaction | Plant diseases | By regulating OsmaMYB nuclear translocation to inhance the resistance to bacterial blight, sheath blight, and blast disease. | [110] |
| OsPIP1;2 | O. sativa | Protein interaction | Plant diseases | Suggested its involvement in rice’s immune response to Xanthomonas oryzae pv. oryzae | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Guo, Y.; Ling, Q.; Guo, Z.; Lei, Y.; Feng, X.; Wu, J.; Zhang, N. Advances in the Structure, Function, and Regulatory Mechanism of Plant Plasma Membrane Intrinsic Proteins. Genes 2025, 16, 10. https://doi.org/10.3390/genes16010010
Li X, Guo Y, Ling Q, Guo Z, Lei Y, Feng X, Wu J, Zhang N. Advances in the Structure, Function, and Regulatory Mechanism of Plant Plasma Membrane Intrinsic Proteins. Genes. 2025; 16(1):10. https://doi.org/10.3390/genes16010010
Chicago/Turabian StyleLi, Xueting, Yirong Guo, Qiuping Ling, Zhejun Guo, Yawen Lei, Xiaomin Feng, Jiayun Wu, and Nannan Zhang. 2025. "Advances in the Structure, Function, and Regulatory Mechanism of Plant Plasma Membrane Intrinsic Proteins" Genes 16, no. 1: 10. https://doi.org/10.3390/genes16010010
APA StyleLi, X., Guo, Y., Ling, Q., Guo, Z., Lei, Y., Feng, X., Wu, J., & Zhang, N. (2025). Advances in the Structure, Function, and Regulatory Mechanism of Plant Plasma Membrane Intrinsic Proteins. Genes, 16(1), 10. https://doi.org/10.3390/genes16010010







