Transcriptional Activation Mechanisms and Target Genes of the Oncogene Product Tax of Human T-Cell Leukemia Virus Type 1
Abstract
1. Introduction
2. Roles of Tax in HTLV-1-Mediated Transformation
3. Tax Activates Host Cell Transcription Factors
3.1. cAMP Responsive Element-Binding Factor (CREB)/Activating Transcription Factor (ATF)
3.2. Nuclear Factor-Kappa B (NF-κB)
3.3. SRF
4. Cellular Target Genes of Tax
4.1. Cytokines, Their Receptors, and Cell Surface Molecules
4.2. Proto-Oncogenes, Transcription Factors, and Growth Signal Transducers
4.3. Cell Cycle Regulators
4.4. Genes Related to Apoptosis and Cell Survival
4.5. Human Telomerase Reverse Transcriptase (hTERT)
4.6. Genes Involved in Suppression of DNA Repair and Induction of Genome Instability
4.7. MicroRNAs (miRNAs)
4.8. Epigenetic Genes
4.9. Genes Involved in Cellular Metabolism
4.10. Genes Involved in Viral Transmission
4.11. Genes Involved in Invasion and Infiltration
5. Tax-Mediated Repression of Gene Expression
5.1. Tax Inactivates the Tumor Suppressor p53
5.2. Pro-Apoptotic Genes
5.3. Signal Transducers
5.4. Cell Cycle Regulators
5.5. Transcription Factors
5.6. Others
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCB1 | ATP-binding cassette sub-family B member 1 |
| AC | asymptomatic carrier |
| ALCAM | activated cell adhesion molecule |
| AP-1 | activator protein 1 |
| Apaf-1 | apoptotic protease activating factor 1 |
| APCCdc20 | Cdc20-associated anaphase promoting complex |
| ATF | activating transcription factor |
| ATL | adult T-cell leukemia/lymphoma |
| ATM | ataxia-telangiectasia mutated |
| Bax | Bcl-2-associated X protein |
| BBB | blood–brain barrier |
| BCL | B cell CLL/lymphoma |
| Bcl-xL | Bcl2-related protein, long isoform |
| BER | base excision repair |
| β-TrCP | β-transducin repeat-containing protein |
| bFGF | basic fibroblast growth factor |
| BFL1 | BCL2-related protein A1 |
| BH3 | Bcl-2 homology 3 |
| bHLH | basic helix-loop-helix |
| Bid | BH3-interacting domain death agonist |
| Bim | Bcl-2-interacting mediator of cell death |
| BIRC3 | baculoviral IAP repeat-containing 3 |
| BMP | bone morphogenetic protein |
| BRF1 | butyrate response factor 1 |
| BRG1 | BRM/SWI2-related gene 1 |
| bZIP | basic leucine zipper |
| CADM-1 | cell adhesion molecule 1 |
| CAK | CDK activating kinase |
| cAMP | cyclic adenosine monophosphate |
| CARM1 | coactivator-associated arginine methyltransferase 1 |
| Cas-L | Crk-associated substrate lymphocyte type |
| CBP | CREB binding protein |
| CCL | CC chemokine |
| CCR | C-C chemokine receptor |
| CD | cluster of differentiation |
| CDK | cyclin-dependent kinase |
| CDKN2A | cyclin-dependent kinase inhibitor 2A |
| c-FLIP | cellular FLICE-inhibitory protein |
| cMOAT | multispecific organic anion transporter, canalicular |
| CRE | cAMP responsive element |
| CREB | CRE binding factor |
| CRM1 | chromosomal region maintenance 1 |
| CRMP2 | collapsin response mediator protein 2 |
| CTCF | CCCTC-binding factor |
| CTCF-BS | CTCF binding site |
| CTD | C-terminal domain |
| CXCL | chemokine, CXC motif, ligand |
| CXCR | C-X-C chemokine receptor |
| DNA-PK | DNA-dependent protein kinase |
| DC | dendritic cell |
| DDSB | DNA double strand break |
| DG2 | disialoganglioside |
| DHH | desert hedgehog |
| DHODH | dihydroorotate dehydrogenase |
| Egr | early growth response |
| EVC | Ellis Van Creveld |
| EZH2 | enhancer of zeste homolog 2 |
| FADD | fas-associated protein with death domain |
| FasL | fas ligand |
| Fbw7 | F-box and WD repeat domain containing 7 |
| FN | fibronectin |
| Fuc-T | fucosyltransferase |
| GATA3 | GATA-binding protein 3 |
| G-CSF | granulocyte colony-stimulating factor |
| GLUT1 | glucose transporter 1 |
| GM2/GD2 synthase | β-1,4-N-acetylgalactosaminyltransferase |
| GSK3β | glycogen synthase kinase-3β |
| HAM | HTLV-1-associated myelopathy |
| HBZ | HTLV-1 basic leucine zipper factor |
| HDAC1 | histone deacetylase 1 |
| Hh | hedgehog |
| HIF-1α | hypoxia-inducible factor-1α |
| HLTF | helicase-like transcription factor |
| HR | homologous recombination |
| hTERT | human telomerase reverse transcriptase |
| HTLV-1 | human T-cell leukemia virus type 1 |
| IAP | inhibitors of apoptosis |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN | interferon |
| IGSF | immunoglobulin superfamily |
| IHH | Indian hedgehog |
| IL | interleukin |
| IκB | inhibitor of NF-κB |
| IKK | IκB kinase |
| iNOS | inducible nitric oxide synthase |
| IRF | interferon regulatory factor |
| JAG1 | jagged |
| JAK | Janus kinase |
| KID | kinase-inducible domain |
| KIX | KID interacting domain |
| KLF4 | Kruppel-like factor 4 |
| LAIR1 | leukocyte-associated immunoglobulin-like receptor 1 |
| LFA | lymphocyte function-associated antigen |
| LRP | lung resistance-related protein |
| LTR | long terminal repeat |
| MAGI | membrane-associated guanylate kinase, WW and PDZ domains-containing |
| MAP3K | mitogen-activated protein kinase kinase kinase |
| Mat1 | menage a trois homolog 1 |
| MBD2 | methyl-CpG-binding domain 2 |
| Mcl-1 | myeloid cell leukemia sequence 1 |
| MCP | monocyte chemoattractant protein |
| MDC1 | mediator of DNA damage checkpoint protein 1 |
| MDM2 | murine double minute 2 |
| MDR1 | multidrug resistance protein 1 |
| MEKK1 | MAP/ERK kinase kinase 1 |
| MHC | major histocompatibility complex, class I |
| MIP | macrophage inflammatory protein |
| miRNA | microRNA |
| MMP | matrix metalloproteinase |
| MRP1 | multidrug resistance-associated protein 1 |
| MTOC | microtubule-organizing center |
| NAP1 | nucleosome assembly protein 1 |
| NDRG | N-Myc downstream-regulated gene |
| NEMO | NF-κB essential modulator |
| NER | nucleotide excision repair |
| NF1 | neurofibromatosis type I |
| NF-κB | nuclear factor κB |
| NGF | nerve growth factor |
| NHEJ | non-homologous end joining |
| NICD | notch intra-cellular domain |
| NIK | NF-κB-inducing kinase |
| NO | nitric oxide |
| NOD | nonobese diabetic |
| NOS | nitric oxide synthase |
| NRP1 | neuropilin 1 |
| OPN | osteopontin |
| ORP4L | OSBP-related protein 4 |
| OSBP2 | oxysterol-binding protein 2 |
| p53BP1 | p53-binding protein 1 |
| PBL | peripheral blood lymphocyte |
| PBM | PDZ domain-binding motif |
| PBMC | peripheral blood mononuclear cell |
| P/CAF | p300/CBP-associated factor |
| PCNA | proliferating cell nuclear antigen |
| PDE3B | phosphodiesterase 3B |
| P-gp | permeability glycoprotein |
| PHA | phytohemagglutinin |
| PI3K | phosphatidylinositol 3-kinases |
| PI(3,4,5)P3 | phosphatidylinositol (3,4,5)-trisphosphate |
| PLA2G4C | phospholipase A2, group IVC |
| PLCG1 | phospholipase C γ 1 |
| PMA | phorbol 12-myristate 13-acetate |
| PRC2 | polycomb repressive complex 2 |
| PRMT5 | protein arginine methyltransferase 5 |
| pTCRα | pre-T-cell receptor α |
| PTHrP | parathyroid hormone-related peptide |
| P-TEFb | positive transcription elongation factor |
| PTPN6 | protein-tyrosine phosphatase, nonreceptor-type, 6 |
| PUMA | p53-upregulated modulator of apoptosis |
| pX | protein coding region X |
| RA | rheumatoid arthritis |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| RBP-Jκ | recombination signal Jκ binding protein |
| RGMa | repulsive guidance molecule A |
| RISC | RNA-induced silencing complex |
| RNF8 | ring finger protein 8 |
| RNF130 | ring-type E3 ubiquitin transferase 130 |
| ROS | reactive oxygen species |
| SCID | severe combined immunodeficiency |
| SHH | Sonic hedgehog |
| SHP-1 | Src homology-2-containing protein-tyrosine phosphatase 1 |
| SIRT1 | sirtuin-1 |
| SMYD3 | SET and MYND domain containing protein |
| SRE | serum response elements |
| SRF | serum-responsive factor |
| STAT | signal transducer and activator of transcription |
| STLV | simian T-lymphotropic virus |
| TAB2 | TAK1-binding protein 2 |
| TAK1 | TGF-β-activating kinase 1 |
| Tax | trans-activator of pX region |
| TAXBP1 | Tax binding protein 1 |
| TBK1 | TANK-binding kinase 1 |
| TCF | ternary complex factor |
| TCR | T-cell receptor |
| TF | transcription factor |
| TFIIH | transcription factor II H |
| TGF-β | transforming growth factor β |
| THEMIS | thymocyte-expressed molecule |
| TIMP-1 | tissue inhibitor of matrix metalloproteinases-1 |
| TIPS | tax-induced promoter silencing |
| TNF | tumor necrosis factor |
| TNFAIP2 | TNF-α-induced protein 2 |
| TNFRSF | tumor necrosis factor receptor superfamily |
| TORC | transducers of regulated CREB |
| TPp53INP1 | tumor protein p53-induced nuclear protein 1 |
| TRAF6 | TNF receptor-associated factor 6 |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| Treg | regulatory T-cell |
| TRX | thioredoxin |
| TSLC1 | tumor suppressor in lung cancer 1 |
| TSP | tropical spastic paraparesis |
| TSS | tax-speckle structures |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| v-onc | viral oncogene |
| WIP1 | wildtype p53-induced phosphatase 1 |
| XIAP | X-chromosome-linked inhibitor of apoptosis |
| Zap-70 | ζ-chain-associated protein kinase |
| ZNF268 | zinc finger protein 268 |
| XPO1 | exportin 1 |
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 1981, 78, 6476–6480. [Google Scholar] [CrossRef]
- Uchiyama, T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 1997, 15, 15–37. [Google Scholar] [CrossRef]
- Yoshida, M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 2005, 24, 5931–5937. [Google Scholar] [CrossRef]
- Forlani, G.; Shallak, M.; Accolla, R.S.; Romanelli, M.G. HTLV-1 infection and pathogenesis: New insights from cellular and animal models. Int. J. Mol. Sci. 2021, 22, 8001. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, K. Discovery of adult T-cell leukemia. Retrovirology 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Igakura, T.; Stinchcombe, J.C.; Goon, P.K.; Taylor, G.P.; Weber, J.N.; Griffiths, G.M.; Tanaka, Y.; Osame, M.; Bangham, C.R. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 2003, 299, 1713–1716. [Google Scholar] [CrossRef]
- Pais-Correia, A.M.; Sachse, M.; Guadagnini, S.; Robbiati, V.; Lasserre, R.; Gessain, A.; Gout, O.; Alcover, A.; Thoulouze, M.I. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 2010, 16, 83–89. [Google Scholar] [CrossRef]
- Van Prooyen, N.; Gold, H.; Andresen, V.; Schwartz, O.; Jones, K.; Ruscetti, F.; Lockett, S.; Gudla, P.; Venzon, D.; Franchini, G. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. USA 2010, 107, 20738–20743. [Google Scholar] [CrossRef]
- Pique, C.; Jones, K.S. Pathways of cell-cell transmission of HTLV-1. Front. Microbiol. 2012, 3, 378. [Google Scholar] [CrossRef]
- Gross, C.; Thoma-Kress, A.K. Molecular mechanisms of HTLV-1 cell-to-cell transmission. Viruses 2016, 8, 74. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986, 1, 1031–1032. [Google Scholar] [CrossRef]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de The, G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Bangham, C.R.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Primers 2015, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Taylor, G.P.; Jacobson, S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert. Rev. Clin. Immunol. 2014, 10, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Varmus, H. Retroviruses. Science 1988, 240, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Leib-Mosch, C.; Brack-Werner, R.; Salmons, B.; Schmidt, J.; Strauss, P.G.; Hehlmann, R.; Erfle, V. The significance of retroviruses in oncology. Onkologie 1990, 13, 405–414. [Google Scholar] [CrossRef]
- Burmeister, T. Oncogenic retroviruses in animals and humans. Rev. Med. Virol. 2001, 11, 369–380. [Google Scholar] [CrossRef]
- Seiki, M.; Hattori, S.; Hirayama, Y.; Yoshida, M. Human adult T-cell leukemia virus: Complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 1983, 80, 3618–3622. [Google Scholar] [CrossRef]
- Seiki, M.; Eddy, R.; Shows, T.B.; Yoshida, M. Nonspecific integration of the HTLV provirus genome into adult T-cell leukaemia cells. Nature 1984, 309, 640–642. [Google Scholar] [CrossRef]
- Seiki, M.; Hikikoshi, A.; Taniguchi, T.; Yoshida, M. Expression of the pX gene of HTLV-I: General splicing mechanism in the HTLV family. Science 1985, 228, 1532–1534. [Google Scholar] [CrossRef]
- Ciminale, V.; Pavlakis, G.N.; Derse, D.; Cunningham, C.P.; Felber, B.K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: Novel mRNAs and proteins produced by HTLV type I. J. Virol. 1992, 66, 1737–1745. [Google Scholar] [CrossRef]
- Bai, X.T.; Nicot, C. Overview on HTLV-1 p12, p8, p30, p13: Accomplices in persistent infection and viral pathogenesis. Front. Microbiol. 2012, 3, 400. [Google Scholar] [CrossRef] [PubMed]
- Giam, C.Z.; Semmes, O.J. HTLV-1 infection and adult T-cell leukemia/lymphoma-A tale of two proteins: Tax and HBZ. Viruses 2016, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, E.W.; Giam, C.Z. NF-κB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma. FEBS J. 2018, 285, 3324–3336. [Google Scholar] [CrossRef] [PubMed]
- Fochi, S.; Ciminale, V.; Trabetti, E.; Bertazzoni, U.; D’Agostino, D.M.; Zipeto, D.; Romanelli, M.G. NF-κB and microRNA deregulation mediated by HTLV-1 Tax and HBZ. Pathogens 2019, 8, 290. [Google Scholar] [CrossRef]
- Mohanty, S.; Harhaj, E.W. Mechanisms of oncogenesis by HTLV-1 Tax. Pathogens 2020, 9, 543. [Google Scholar] [CrossRef]
- Ernzen, K.J.; Panfil, A.R. Regulation of HTLV-1 transformation. Biosci. Rep. 2022, 42, BSR20211921. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. HTLV-1 Tax tug-of-war: Cellular senescence and death or cellular transformation. Pathogens 2024, 13, 87. [Google Scholar] [CrossRef]
- Sodroski, J.G.; Rosen, C.A.; Haseltine, W.A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science 1984, 225, 381–385. [Google Scholar] [CrossRef]
- Fujisawa, J.; Seiki, M.; Kiyokawa, T.; Yoshida, M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc. Natl. Acad. Sci. USA 1985, 82, 2277–2281. [Google Scholar] [CrossRef]
- Qu, Z.; Xiao, G. Human T-cell lymphotropic virus: A model of NF-κB-associated tumorigenesis. Viruses 2011, 3, 714–749. [Google Scholar] [CrossRef]
- Currer, R.; Van Duyne, R.; Jaworski, E.; Guendel, I.; Sampey, G.; Das, R.; Narayanan, A.; Kashanchi, F. HTLV tax: A fascinating multifunctional co-regulator of viral and cellular pathways. Front. Microbiol. 2012, 3, 406. [Google Scholar] [CrossRef]
- Fochi, S.; Mutascio, S.; Bertazzoni, U.; Zipeto, D.; Romanelli, M.G. HTLV Deregulation of the NF-κB pathway: An update on Tax and antisense proteins role. Front. Microbiol. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, R.; Berchtold, S.; Radant, I.; Alt, M.; Fleckenstein, B.; Sodroski, J.G.; Haseltine, W.A.; Ramstedt, U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 1992, 66, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ono, H.; Shimotohno, K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood 1995, 86, 4243–4249. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ono, H.; Nyunoya, H.; Shimotohno, K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene 1997, 14, 2071–2078. [Google Scholar] [CrossRef]
- Robek, M.D.; Ratner, L. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 1999, 73, 4856–4865. [Google Scholar] [CrossRef]
- Robek, M.D.; Ratner, L. Immortalization of T lymphocytes by human T-cell leukemia virus type 1 is independent of the tax-CBP/p300 interaction. J. Virol. 2000, 74, 11988–11992. [Google Scholar] [CrossRef]
- Ratner, L.; Portis, T.; Robek, M.; Harding, J.; Grossman, W. Studies of the immortalizing activity of HTLV type 1 Tax, using an infectious molecular clone and transgenic mice. AIDS Res. Hum. Retroviruses 2000, 16, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Yang, L.; Kuo, Y.L.; Ho, Y.K.; Shih, H.M.; Giam, C.Z. NF-κB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011, 7, e1002025. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Zhi, H.; DeBiaso, D.; Philip, S.; Shih, H.M.; Giam, C.Z. HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-κB and depends on chronically activated IKKα and p65/RelA. J. Virol. 2012, 86, 9474–9483. [Google Scholar] [CrossRef] [PubMed]
- Gaudray, G.; Gachon, F.; Basbous, J.; Biard-Piechaczyk, M.; Devaux, C.; Mesnard, J.M. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 2002, 76, 12813–12822. [Google Scholar] [CrossRef]
- Lemasson, I.; Lewis, M.R.; Polakowski, N.; Hivin, P.; Cavanagh, M.H.; Thebault, S.; Barbeau, B.; Nyborg, J.K.; Mesnard, J.M. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J. Virol. 2007, 81, 1543–1553. [Google Scholar] [CrossRef]
- Zhao, T.; Yasunaga, J.; Satou, Y.; Nakao, M.; Takahashi, M.; Fujii, M.; Matsuoka, M. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-κB. Blood 2009, 113, 2755–2764. [Google Scholar] [CrossRef]
- Mesnard, J.M.; Barbeau, B.; Cesaire, R.; Peloponese, J.M. Roles of HTLV-1 basic Zip factor (HBZ) in viral chronicity and leukemic transformation. Potential new therapeutic approaches to prevent and treat HTLV-1-related diseases. Viruses 2015, 7, 6490–6505. [Google Scholar] [CrossRef]
- Matsuoka, M.; Mesnard, J.M. HTLV-1 bZIP factor: The key viral gene for pathogenesis. Retrovirology 2020, 17, 2. [Google Scholar] [CrossRef]
- Giam, C.Z. HTLV-1 replication and adult T cell leukemia development. Recent. Results Cancer Res. 2021, 217, 209–243. [Google Scholar] [CrossRef]
- Zhang, W.; Nisbet, J.W.; Albrecht, B.; Ding, W.; Kashanchi, F.; Bartoe, J.T.; Lairmore, M.D. Human T-lymphotropic virus type 1 p30II regulates gene transcription by binding CREB binding protein/p300. J. Virol. 2001, 75, 9885–9895. [Google Scholar] [CrossRef]
- Nicot, C.; Dundr, M.; Johnson, J.M.; Fullen, J.R.; Alonzo, N.; Fukumoto, R.; Princler, G.L.; Derse, D.; Misteli, T.; Franchini, G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 2004, 10, 197–201. [Google Scholar] [CrossRef]
- Green, P.L. HTLV-1 p30II: Selective repressor of gene expression. Retrovirology 2004, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Andresen, V.; Pise-Masison, C.A.; Sinha-Datta, U.; Bellon, M.; Valeri, V.; Washington Parks, R.; Cecchinato, V.; Fukumoto, R.; Nicot, C.; Franchini, G. Suppression of HTLV-1 replication by Tax-mediated rerouting of the p13 viral protein to nuclear speckles. Blood 2011, 118, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Harrod, R. Silencers of HTLV-1 and HTLV-2: The pX-encoded latency-maintenance factors. Retrovirology 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Moles, R.; Sarkis, S.; Galli, V.; Omsland, M.; Purcell, D.F.J.; Yurick, D.; Khoury, G.; Pise-Masison, C.A.; Franchini, G. p30 protein: A critical regulator of HTLV-1 viral latency and host immunity. Retrovirology 2019, 16, 42. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Kogure, Y.; Kataoka, K. Genetic alterations in adult T-cell leukemia/lymphoma. Cancer Sci. 2017, 108, 1719–1725. [Google Scholar] [CrossRef]
- Sakihama, S.; Morichika, K.; Saito, R.; Miyara, M.; Miyagi, T.; Hayashi, M.; Uchihara, J.; Tomoyose, T.; Ohshiro, K.; Nakayama, S.; et al. Genetic profile of adult T-cell leukemia/lymphoma in Okinawa: Association with prognosis, ethnicity, and HTLV-1 strains. Cancer Sci. 2021, 112, 1300–1309. [Google Scholar] [CrossRef]
- Chlichlia, K.; Khazaie, K. HTLV-1 Tax: Linking transformation, DNA damage and apoptotic T-cell death. Chem. Biol. Interact. 2010, 188, 359–365. [Google Scholar] [CrossRef]
- Nicot, C. HTLV-I Tax-mediated inactivation of cell cycle checkpoints and DNA repair pathways contribute to cellular transformation: “A Random Mutagenesis Model”. J. Cancer Sci. 2015, 2. [Google Scholar] [CrossRef]
- Boxus, M.; Twizere, J.C.; Legros, S.; Dewulf, J.F.; Kettmann, R.; Willems, L. The HTLV-1 Tax interactome. Retrovirology 2008, 5, 76. [Google Scholar] [CrossRef]
- Azran, I.; Schavinsky-Khrapunsky, Y.; Aboud, M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 2004, 1, 20. [Google Scholar] [CrossRef]
- Tanaka, A.; Takahashi, C.; Yamaoka, S.; Nosaka, T.; Maki, M.; Hatanaka, M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Greene, W.C. Type I human T cell leukemia virus tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J. Clin. Investig. 1991, 88, 1038–1042. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shibata, H.; Fujisawa, J.I.; Inoue, H.; Hakura, A.; Tsukahara, T.; Fujii, M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 1997, 71, 4445–4451. [Google Scholar] [CrossRef]
- Neuveut, C.; Low, K.G.; Maldarelli, F.; Schmitt, I.; Majone, F.; Grassmann, R.; Jeang, K.T. Human T-cell leukemia virus type 1 Tax and cell cycle progression: Role of cyclin D-cdk and p110Rb. Mol. Cell Biol. 1998, 18, 3620–3632. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, I.; Rosin, O.; Rohwer, P.; Gossen, M.; Grassmann, R. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 1998, 72, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Iwanaga, R.; Arai, M.; Huang, Y.; Matsumura, Y.; Nakamura, M. Cell type-specific E2F activation and cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. J. Biol. Chem. 2000, 275, 11154–11163. [Google Scholar] [CrossRef] [PubMed]
- Nerenberg, M.; Hinrichs, S.H.; Reynolds, R.K.; Khoury, G.; Jay, G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987, 237, 1324–1329. [Google Scholar] [CrossRef]
- Hinrichs, S.H.; Nerenberg, M.; Reynolds, R.K.; Khoury, G.; Jay, G. A transgenic mouse model for human neurofibromatosis. Science 1987, 237, 1340–1343. [Google Scholar] [CrossRef]
- Hall, A.P.; Irvine, J.; Blyth, K.; Cameron, E.R.; Onions, D.E.; Campbell, M.E. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J. Pathol. 1998, 186, 209–214. [Google Scholar] [CrossRef]
- Grossman, W.J.; Kimata, J.T.; Wong, F.H.; Zutter, M.; Ley, T.J.; Ratner, L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 1995, 92, 1057–1061. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sawa, H.; Lewis, M.J.; Orba, Y.; Sheehy, N.; Yamamoto, Y.; Ichinohe, T.; Tsunetsugu-Yokota, Y.; Katano, H.; Takahashi, H.; et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 2006, 12, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Rauch, D.; Gross, S.; Harding, J.; Niewiesk, S.; Lairmore, M.; Piwnica-Worms, D.; Ratner, L. Imaging spontaneous tumorigenesis: Inflammation precedes development of peripheral NK tumors. Blood 2009, 113, 1493–1500. [Google Scholar] [CrossRef]
- Ohsugi, T.; Kumasaka, T.; Okada, S.; Urano, T. The Tax protein of HTLV-1 promotes oncogenesis in not only immature T cells but also mature T cells. Nat. Med. 2007, 13, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Niewiesk, S. Animals models of human T cell leukemia virus type I leukemogenesis. ILAR J. 2016, 57, 3–11. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sano, K.; Ainai, A.; Suzuki, T. Application of HTLV-1 tax transgenic mice for therapeutic intervention. Adv. Biol. Regul. 2018, 68, 10–12. [Google Scholar] [CrossRef]
- El Hajj, H.; El-Sabban, M.; Hasegawa, H.; Zaatari, G.; Ablain, J.; Saab, S.T.; Janin, A.; Mahfouz, R.; Nasr, R.; Kfoury, Y.; et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 2010, 207, 2785–2792. [Google Scholar] [CrossRef]
- Mahgoub, M.; Yasunaga, J.I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef] [PubMed]
- Hleihel, R.; Skayneh, H.; de The, H.; Hermine, O.; Bazarbachi, A. Primary cells from patients with adult T cell leukemia/lymphoma depend on HTLV-1 Tax expression for NF-κB activation and survival. Blood Cancer J. 2023, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Jegado, B.; Kashanchi, F.; Dutartre, H.; Mahieux, R. STLV-1 as a model for studying HTLV-1 infection. Retrovirology 2019, 16, 41. [Google Scholar] [CrossRef]
- Hussein, O.; Mahgoub, M.; Shichijo, T.; Nakagawa, S.; Tanabe, J.; Akari, H.; Miura, T.; Matsuoka, M.; Yasunaga, J.I. Evolution of primate T-cell leukemia virus type 1 accessory genes and functional divergence of its antisense proteins. PLoS Pathog. 2025, 21, e1013158. [Google Scholar] [CrossRef]
- Miura, M.; Yasunaga, J.; Tanabe, J.; Sugata, K.; Zhao, T.; Ma, G.; Miyazato, P.; Ohshima, K.; Kaneko, A.; Watanabe, A.; et al. Characterization of simian T-cell leukemia virus type 1 in naturally infected Japanese macaques as a model of HTLV-1 infection. Retrovirology 2013, 10, 118. [Google Scholar] [CrossRef]
- Suehiro, Y.; Hasegawa, A.; Iino, T.; Sasada, A.; Watanabe, N.; Matsuoka, M.; Takamori, A.; Tanosaki, R.; Utsunomiya, A.; Choi, I.; et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br. J. Haematol. 2015, 169, 356–367. [Google Scholar] [CrossRef]
- Kannagi, M.; Hasegawa, A.; Nagano, Y.; Iino, T.; Okamura, J.; Suehiro, Y. Maintenance of long remission in adult T-cell leukemia by Tax-targeted vaccine: A hope for disease-preventive therapy. Cancer Sci. 2019, 110, 849–857. [Google Scholar] [CrossRef]
- Iino, T.; Hasegawa, A.; Matsutani, T.; Akashi, K.; Kannagi, M.; Suehiro, Y. Elimination of residual adult T-cell leukaemia clones by Tax-targeted dendritic cell vaccine. EJHaem 2025, 6, e1072. [Google Scholar] [CrossRef] [PubMed]
- Cann, A.J.; Rosenblatt, J.D.; Wachsman, W.; Shah, N.P.; Chen, I.S. Identification of the gene responsible for human T-cell leukaemia virus transcriptional regulation. Nature 1985, 318, 571–574. [Google Scholar] [CrossRef]
- Chen, I.S.; Slamon, D.J.; Rosenblatt, J.D.; Shah, N.P.; Quan, S.G.; Wachsman, W. The x gene is essential for HTLV replication. Science 1985, 229, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Seiki, M.; Sato, M.; Yoshida, M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40 chi HTLV-I. EMBO J. 1986, 5, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Shimotohno, K.; Takano, M.; Teruuchi, T.; Miwa, M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc. Natl. Acad. Sci. USA 1986, 83, 8112–8116. [Google Scholar] [CrossRef]
- Zhao, L.J.; Giam, C.Z. Interaction of the human T-cell lymphotrophic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc. Natl. Acad. Sci. USA 1991, 88, 11445–11449. [Google Scholar] [CrossRef]
- Zhao, L.J.; Giam, C.Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 1992, 89, 7070–7074. [Google Scholar] [CrossRef]
- Jeang, K.T.; Boros, I.; Brady, J.; Radonovich, M.; Khoury, G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J. Virol. 1988, 62, 4499–4509. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujisawa, J.I.; Toita, M.; Yoshida, M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc. Natl. Acad. Sci. USA 1993, 90, 610–614. [Google Scholar] [CrossRef]
- Franklin, A.A.; Kubik, M.F.; Uittenbogaard, M.N.; Brauweiler, A.; Utaisincharoen, P.; Matthews, M.A.; Dynan, W.S.; Hoeffler, J.P.; Nyborg, J.K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB). J. Biol. Chem. 1993, 268, 21225–21231. [Google Scholar] [CrossRef]
- Gachon, F.; Peleraux, A.; Thebault, S.; Dick, J.; Lemasson, I.; Devaux, C.; Mesnard, J.M. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J. Virol. 1998, 72, 8332–8337. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F.; Thebault, S.; Peleraux, A.; Devaux, C.; Mesnard, J.M. Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4). Mol. Cell Biol. 2000, 20, 3470–3481. [Google Scholar] [CrossRef]
- Baranger, A.M.; Palmer, C.R.; Hamm, M.K.; Giebler, H.A.; Brauweiler, A.; Nyborg, J.K.; Schepartz, A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature 1995, 376, 606–608. [Google Scholar] [CrossRef]
- Adya, N.; Giam, C.Z. Distinct regions in human T-cell lymphotropic virus type I tax mediate interactions with activator protein CREB and basal transcription factors. J. Virol. 1995, 69, 1834–1841. [Google Scholar] [CrossRef]
- Yin, M.J.; Gaynor, R.B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol. Cell Biol. 1996, 16, 3156–3168. [Google Scholar] [CrossRef]
- Yin, M.J.; Gaynor, R.B. HTLV-1 21 bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J. Mol. Biol. 1996, 264, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lenzmeier, B.A.; Giebler, H.A.; Nyborg, J.K. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol. Cell Biol. 1998, 18, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.G.; Matsumoto, J.; Tanaka, Y.; Shimotohno, K. SR-related protein TAXREB803/SRL300 is an important cellular factor for the transactivational function of human T-cell lymphotropic virus type 1 Tax. J. Virol. 2003, 77, 10015–10027. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.P.; Laurance, M.E.; Lundblad, J.R.; Goldman, P.S.; Shih, H.; Connor, L.M.; Marriott, S.J.; Goodman, R.H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 1996, 380, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Giebler, H.A.; Loring, J.E.; van Orden, K.; Colgin, M.A.; Garrus, J.E.; Escudero, K.W.; Brauweiler, A.; Nyborg, J.K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: A molecular mechanism of Tax transactivation. Mol. Cell Biol. 1997, 17, 5156–5164. [Google Scholar] [CrossRef]
- Kashanchi, F.; Duvall, J.F.; Kwok, R.P.; Lundblad, J.R.; Goodman, R.H.; Brady, J.N. The coactivator CBP stimulates human T-cell lymphotrophic virus type I Tax transactivation in vitro. J. Biol. Chem. 1998, 273, 34646–34652. [Google Scholar] [CrossRef]
- Geiger, T.R.; Sharma, N.; Kim, Y.M.; Nyborg, J.K. The human T-cell leukemia virus type 1 tax protein confers CBP/p300 recruitment and transcriptional activation properties to phosphorylated CREB. Mol. Cell Biol. 2008, 28, 1383–1392. [Google Scholar] [CrossRef]
- Harrod, R.; Tang, Y.; Nicot, C.; Lu, H.S.; Vassilev, A.; Nakatani, Y.; Giam, C.Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell Biol. 1998, 18, 5052–5061. [Google Scholar] [CrossRef]
- Vendel, A.C.; McBryant, S.J.; Lumb, K.J. KIX-mediated assembly of the CBP-CREB-HTLV-1 tax coactivator-activator complex. Biochemistry 2003, 42, 12481–12487. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, I.; Nyborg, J.K. Human T-cell leukemia virus type I tax repression of p73β is mediated through competition for the C/H1 domain of CBP. J. Biol. Chem. 2001, 276, 15720–15727. [Google Scholar] [CrossRef] [PubMed]
- Scoggin, K.E.; Ulloa, A.; Nyborg, J.K. The oncoprotein Tax binds the SRC-1-interacting domain of CBP/p300 to mediate transcriptional activation. Mol. Cell Biol. 2001, 21, 5520–5530. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Nyborg, J.K. Molecular characterization of HTLV-1 Tax interaction with the KIX domain of CBP/p300. J. Mol. Biol. 2007, 372, 958–969. [Google Scholar] [CrossRef]
- Siu, Y.T.; Chin, K.T.; Siu, K.L.; Yee Wai Choy, E.; Jeang, K.T.; Jin, D.Y. TORC1 and TORC2 coactivators are required for tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J. Virol. 2006, 80, 7052–7059. [Google Scholar] [CrossRef]
- Kim, Y.M.; Geiger, T.R.; Egan, D.I.; Sharma, N.; Nyborg, J.K. The HTLV-1 tax protein cooperates with phosphorylated CREB, TORC2 and p300 to activate CRE-dependent cyclin D1 transcription. Oncogene 2010, 29, 2142–2152. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ramirez, J.A.; Mick, J.E.; Giebler, H.A.; Yan, J.P.; Nyborg, J.K. Molecular characterization of the Tax-containing HTLV-1 enhancer complex reveals a prominent role for CREB phosphorylation in Tax transactivation. J. Biol. Chem. 2007, 282, 18750–18757. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, H.; Schiltz, R.L.; Pise-Masison, C.A.; Ogryzko, V.V.; Nakatani, Y.; Brady, J.N. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell Biol. 1999, 19, 8136–8145. [Google Scholar] [CrossRef]
- Lu, H.; Pise-Masison, C.A.; Fletcher, T.M.; Schiltz, R.L.; Nagaich, A.K.; Radonovich, M.; Hager, G.; Cole, P.A.; Brady, J.N. Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol. Cell Biol. 2002, 22, 4450–4462. [Google Scholar] [CrossRef]
- Sharma, N.; Nyborg, J.K. The coactivators CBP/p300 and the histone chaperone NAP1 promote transcription-independent nucleosome eviction at the HTLV-1 promoter. Proc. Natl. Acad. Sci. USA 2008, 105, 7959–7963. [Google Scholar] [CrossRef]
- Easley, R.; Carpio, L.; Guendel, I.; Klase, Z.; Choi, S.; Kehn-Hall, K.; Brady, J.N.; Kashanchi, F. Human T-lymphotropic virus type 1 transcription and chromatin-remodeling complexes. J. Virol. 2010, 84, 4755–4768. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Pise-Masison, C.A.; Linton, R.; Park, H.U.; Schiltz, R.L.; Sartorelli, V.; Brady, J.N. Tax relieves transcriptional repression by promoting histone deacetylase 1 release from the human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 2004, 78, 6735–6743. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Lavorgna, A.; Sehgal, M.; Gao, L.; Ginwala, R.; Sagar, D.; Harhaj, E.W.; Khan, Z.K. Myocyte enhancer factor (MEF)-2 plays essential roles in T-cell transformation associated with HTLV-1 infection by stabilizing complex between Tax and CREB. Retrovirology 2015, 12, 23. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Pires, B.R.B.; Silva, R.; Ferreira, G.M.; Abdelhay, E. NF-kappaB: Two Sides of the Same Coin. Genes 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Thu, Y.M.; Richmond, A. NF-κB inducing kinase: A key regulator in the immune system and in cancer. Cytokine Growth Factor. Rev. 2010, 21, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Elwood, J.; Beraud, C.; Greene, W.C. Human T-cell leukemia virus type I Tax activation of NF-κB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Mol. Cell Biol. 1994, 14, 7377–7384. [Google Scholar] [CrossRef]
- Maggirwar, S.B.; Harhaj, E.; Sun, S.C. Activation of NF-κB/Rel by Tax involves degradation of IκBα and is blocked by a proteasome inhibitor. Oncogene 1995, 11, 993–998. [Google Scholar]
- Uhlik, M.; Good, L.; Xiao, G.; Harhaj, E.W.; Zandi, E.; Karin, M.; Sun, S.C. NF-κB-inducing kinase and IκB kinase participate in human T-cell leukemia virus I Tax-mediated NF-κB activation. J. Biol. Chem. 1998, 273, 21132–21136. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirai, H.; Murakami, T.; Yoshida, M. Tax protein of HTLV-1 destabilizes the complexes of NF-κB and IκB-α and induces nuclear translocation of NF-κB for transcriptional activation. Oncogene 1995, 10, 1199–1207. [Google Scholar] [PubMed]
- Petropoulos, L.; Hiscott, J. Association between HTLV-1 Tax and IκBα is dependent on the IκBα phosphorylation state. Virology 1998, 252, 189–199. [Google Scholar] [CrossRef]
- Good, L.; Sun, S.C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 tax involves degradation of IκBβ. J. Virol. 1996, 70, 2730–2735. [Google Scholar] [CrossRef]
- Hirai, H.; Suzuki, T.; Fujisawa, J.; Inoue, J.; Yoshida, M. Tax protein of human T-cell leukemia virus type I binds to the ankyrin motifs of inhibitory factor κB and induces nuclear translocation of transcription factor NF-κB proteins for transcriptional activation. Proc. Natl. Acad. Sci. USA 1994, 91, 3584–3588. [Google Scholar] [CrossRef]
- Hirai, H.; Fujisawa, J.; Suzuki, T.; Ueda, K.; Muramatsu, M.; Tsuboi, A.; Arai, N.; Yoshida, M. Transcriptional activator Tax of HTLV-1 binds to the NF-κB precursor p105. Oncogene 1992, 7, 1737–1742. [Google Scholar] [PubMed]
- Watanabe, M.; Muramatsu, M.; Hirai, H.; Suzuki, T.; Fujisawa, J.; Yoshida, M.; Arai, K.; Arai, N. HTLV-I encoded Tax in association with NF-κB precursor p105 enhances nuclear localization of NF-κB p50 and p65 in transfected cells. Oncogene 1993, 8, 2949–2958. [Google Scholar] [PubMed]
- Rousset, R.; Desbois, C.; Bantignies, F.; Jalinot, P. Effects on NF-κB1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature 1996, 381, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, J.; Lacoste, J.; Pepin, N.; Rice, N.; Hiscott, J. Overproduction of NFKB2 (lyt-10) and c-Rel: A mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signalling pathway. Oncogene 1994, 9, 841–852. [Google Scholar]
- Murakami, T.; Hirai, H.; Suzuki, T.; Fujisawa, J.; Yoshida, M. HTLV-1 Tax enhances NF-κB2 expression and binds to the products p52 and p100, but does not suppress the inhibitory function of p100. Virology 1995, 206, 1066–1074. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirai, H.; Fujisawa, J.; Fujita, T.; Yoshida, M. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κB site and CArG box. Oncogene 1993, 8, 2391–2397. [Google Scholar]
- Suzuki, T.; Hirai, H.; Yoshida, M. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-κB p65 and c-Rel proteins bound to the NF-κB binding site and activates transcription. Oncogene 1994, 9, 3099–3105. [Google Scholar]
- Bex, F.; McDowall, A.; Burny, A.; Gaynor, R. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-κB proteins. J. Virol. 1997, 71, 3484–3497. [Google Scholar] [CrossRef]
- Bex, F.; Yin, M.J.; Burny, A.; Gaynor, R.B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol. Cell Biol. 1998, 18, 2392–2405. [Google Scholar] [CrossRef]
- Yamaoka, S.; Courtois, G.; Bessia, C.; Whiteside, S.T.; Weil, R.; Agou, F.; Kirk, H.E.; Kay, R.J.; Israel, A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 1998, 93, 1231–1240. [Google Scholar] [CrossRef]
- Geleziunas, R.; Ferrell, S.; Lin, X.; Mu, Y.; Cunningham, E.T., Jr.; Grant, M.; Connelly, M.A.; Hambor, J.E.; Marcu, K.B.; Greene, W.C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKKβ cellular kinases. Mol. Cell Biol. 1998, 18, 5157–5165. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Sun, S.C. IKK γ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 1999, 274, 22911–22914. [Google Scholar] [CrossRef]
- Chu, Z.L.; Shin, Y.A.; Yang, J.M.; DiDonato, J.A.; Ballard, D.W. IKKγ mediates the interaction of cellular IκB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 1999, 274, 15297–15300. [Google Scholar] [CrossRef]
- Jin, D.Y.; Giordano, V.; Kibler, K.V.; Nakano, H.; Jeang, K.T. Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I Tax interacts directly with IκB kinase γ. J. Biol. Chem. 1999, 274, 17402–17405. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Good, L.; Xiao, G.; Uhlik, M.; Cvijic, M.E.; Rivera-Walsh, I.; Sun, S.C. Somatic mutagenesis studies of NF-κB signaling in human T cells: Evidence for an essential role of IKK γ in NF-κB activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene 2000, 19, 1448–1456. [Google Scholar] [CrossRef]
- Sun, S.C.; Harhaj, E.W.; Xiao, G.; Good, L. Activation of I-κB kinase by the HTLV type 1 Tax protein: Mechanistic insights into the adaptor function of IKKγ. AIDS Res. Hum. Retroviruses 2000, 16, 1591–1596. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Montano, M.; Van Beneden, K.; Chen, L.F.; Greene, W.C. Human T-cell lymphotropic virus type 1 tax induction of biologically active NF-κB requires IκB kinase-1-mediated phosphorylation of RelA/p65. J. Biol. Chem. 2004, 279, 18137–18145. [Google Scholar] [CrossRef]
- Wu, X.; Sun, S.C. Retroviral oncoprotein Tax deregulates NF-κB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 2007, 8, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Minoda, Y.; Yoshida, R.; Yoshida, H.; Iha, H.; Kobayashi, T.; Yoshimura, A.; Takaesu, G. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem. Biophys. Res. Commun. 2008, 365, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.J.; Christerson, L.B.; Yamamoto, Y.; Kwak, Y.T.; Xu, S.; Mercurio, F.; Barbosa, M.; Cobb, M.H.; Gaynor, R.B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell 1998, 93, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Sebald, A.; Mattioli, I.; Schmitz, M.L. T cell receptor-induced lipid raft recruitment of the IκB kinase complex is necessary and sufficient for NF-κB activation occurring in the cytosol. Eur. J. Immunol. 2005, 35, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, N.S.; Sun, S.C.; Harhaj, E.W. Activation of NF-κB by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of IκB kinase. J. Biol. Chem. 2007, 282, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ren, T.; Guan, H.; Jiang, Y.; Cheng, H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IκB kinases to the microdomains for persistent activation of NF-κB. J. Biol. Chem. 2009, 284, 6208–6217. [Google Scholar] [CrossRef]
- Journo, C.; Filipe, J.; About, F.; Chevalier, S.A.; Afonso, P.V.; Brady, J.N.; Flynn, D.; Tangy, F.; Israel, A.; Vidalain, P.O.; et al. NRP/Optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-κB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 2009, 5, e1000521. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Cai, S.H.; Cheng, H. Identification of TBK1 and IKKε, the non-canonical IκB kinases, as crucial pro-survival factors in HTLV-1-transformed T lymphocytes. Leuk. Res. 2016, 46, 37–44. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanaka, Y.; Gohda, J.; Inoue, J. Activation of the IκB kinase complex by HTLV-1 Tax requires cytosolic factors involved in Tax-induced polyubiquitination. J. Biochem. 2011, 150, 679–686. [Google Scholar] [CrossRef]
- Shembade, N.; Harhaj, E.W. Role of post-translational modifications of HTLV-1 Tax in NF-κB activation. World J. Biol. Chem. 2010, 1, 13–20. [Google Scholar] [CrossRef]
- Mohanty, S.; Han, T.; Choi, Y.B.; Lavorgna, A.; Zhang, J.; Harhaj, E.W. The E3/E4 ubiquitin conjugation factor UBE4B interacts with and ubiquitinates the HTLV-1 Tax oncoprotein to promote NF-κB activation. PLoS Pathog. 2020, 16, e1008504. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Zhi, H.; Bowlin, T.; Dorjbal, B.; Philip, S.; Zahoor, M.A.; Shih, H.M.; Semmes, O.J.; Schaefer, B.; Glover, J.N.; et al. HTLV-1 Tax stimulates ubiquitin E3 ligase, Ring Finger Protein 8, to assemble Lysine 63-linked polyubiquitin chains for TAK1 and IKK activation. PLoS Pathog. 2015, 11, e1005102. [Google Scholar] [CrossRef]
- Shibata, Y.; Tokunaga, F.; Goto, E.; Komatsu, G.; Gohda, J.; Saeki, Y.; Tanaka, K.; Takahashi, H.; Sawasaki, T.; Inoue, S.; et al. HTLV-1 Tax induces formation of the active macromolecular IKK complex by generating Lys63- and Met1-linked hybrid polyubiquitin chains. PLoS Pathog. 2017, 13, e1006162. [Google Scholar] [CrossRef]
- Wang, C.; Long, W.; Peng, C.; Hu, L.; Zhang, Q.; Wu, A.; Zhang, X.; Duan, X.; Wong, C.C.; Tanaka, Y.; et al. HTLV-1 Tax functions as a ubiquitin E3 ligase for direct IKK activation via synthesis of mixed-linkage polyubiquitin chains. PLoS Pathog. 2016, 12, e1005584. [Google Scholar] [CrossRef]
- Beraud, C.; Sun, S.C.; Ganchi, P.; Ballard, D.W.; Greene, W.C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: Implications for viral latency. Mol. Cell Biol. 1994, 14, 1374–1382. [Google Scholar] [CrossRef]
- Xiao, G.; Cvijic, M.E.; Fong, A.; Harhaj, E.W.; Uhlik, M.T.; Waterfield, M.; Sun, S.C. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: Evidence for the involvement of IKKα. EMBO J. 2001, 20, 6805–6815. [Google Scholar] [CrossRef]
- Xiao, G.; Fong, A.; Sun, S.C. Induction of p100 processing by NF-κB-inducing kinase involves docking IκB kinase α (IKK α) to p100 and IKKα-mediated phosphorylation. J. Biol. Chem. 2004, 279, 30099–30105. [Google Scholar] [CrossRef]
- Fong, A.; Sun, S.C. Genetic evidence for the essential role of β-transducin repeat-containing protein in the inducible processing of NF-κB2/p100. J. Biol. Chem. 2002, 277, 22111–22114. [Google Scholar] [CrossRef]
- Qu, Z.; Qing, G.; Rabson, A.; Xiao, G. Tax deregulation of NF-κB2 p100 processing involves both β-TrCP-dependent and -independent mechanisms. J. Biol. Chem. 2004, 279, 44563–44572. [Google Scholar] [CrossRef] [PubMed]
- Shuh, M.; Derse, D. Ternary complex factors and cofactors are essential for human T-cell leukemia virus type 1 tax transactivation of the serum response element. J. Virol. 2000, 74, 11394–11397. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.Y.; Marriott, S.J. Human T-cell leukemia virus type 1 Tax enhances serum response factor DNA binding and alters site selection. J. Virol. 2007, 81, 6089–6098. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Nakamura, M.; Saito, S.; Nagata, K.; Sugamura, K.; Hinuma, Y. Electroporation: Application to human lymphoid cell lines for stable introduction of a transactivator gene of human T-cell leukemia virus type I. Nucleic Acids Res. 1989, 17, 1589–1604. [Google Scholar] [CrossRef]
- Nagata, K.; Ohtani, K.; Nakamura, M.; Sugamura, K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J. Virol. 1989, 63, 3220–3226. [Google Scholar] [CrossRef]
- Miura, S.; Ohtani, K.; Numata, N.; Niki, M.; Ohbo, K.; Ina, Y.; Gojobori, T.; Tanaka, Y.; Tozawa, H.; Nakamura, M.; et al. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol. Cell Biol. 1991, 11, 1313–1325. [Google Scholar] [CrossRef]
- Nakashima, K.; Kawakami, A.; Hida, A.; Yamasaki, S.; Nakamura, H.; Kamachi, M.; Miyashita, T.; Tanaka, F.; Izumi, Y.; Tamai, M.; et al. Protection of mitochondrial perturbation by human T-lymphotropic virus type 1 tax through induction of Bcl-xL expression. J. Lab. Clin. Med. 2003, 142, 341–347. [Google Scholar] [CrossRef]
- Kawakami, A.; Nakashima, T.; Sakai, H.; Urayama, S.; Yamasaki, S.; Hida, A.; Tsuboi, M.; Nakamura, H.; Ida, H.; Migita, K.; et al. Inhibition of caspase cascade by HTLV-I tax through induction of NF-κB nuclear translocation. Blood 1999, 94, 3847–3854. [Google Scholar] [CrossRef]
- Baba, M.; Imai, T.; Yoshida, T.; Yoshie, O. Constitutive expression of various chemokine genes in human T-cell lines infected with human T-cell leukemia virus type 1: Role of the viral transactivator Tax. Int. J. Cancer 1996, 66, 124–129. [Google Scholar] [CrossRef]
- Iwata, S.; Souta-Kuribara, A.; Yamakawa, A.; Sasaki, T.; Shimizu, T.; Hosono, O.; Kawasaki, H.; Tanaka, H.; Dang, N.H.; Watanabe, T.; et al. HTLV-I Tax induces and associates with Crk-associated substrate lymphocyte type (Cas-L). Oncogene 2005, 24, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Nagakubo, D.; Shirakawa, A.K.; Nakayama, T.; Shigeta, A.; Hieshima, K.; Yamada, Y.; Yoshie, O. CXCR7 is inducible by HTLV-1 Tax and promotes growth and survival of HTLV-1-infected T cells. Int. J. Cancer 2009, 125, 2229–2235. [Google Scholar] [CrossRef]
- Lemasson, I.; Robert-Hebmann, V.; Hamaia, S.; Duc Dodon, M.; Gazzolo, L.; Devaux, C. Transrepression of lck gene expression by human T-cell leukemia virus type 1-encoded p40tax. J. Virol. 1997, 71, 1975–1983. [Google Scholar] [CrossRef]
- Yamashita, I.; Katamine, S.; Moriuchi, R.; Nakamura, Y.; Miyamoto, T.; Eguchi, K.; Nagataki, S. Transactivation of the human interleukin-6 gene by human T-lymphotropic virus type 1 Tax protein. Blood 1994, 84, 1573–1578. [Google Scholar] [CrossRef]
- Higashimura, N.; Takasawa, N.; Tanaka, Y.; Nakamura, M.; Sugamura, K. Induction of OX40, a receptor of gp34, on T cells by trans-acting transcriptional activator, Tax, of human T-cell leukemia virus type I. Jpn. J. Cancer Res. 1996, 87, 227–231. [Google Scholar] [CrossRef]
- Chen, X.; Zachar, V.; Zdravkovic, M.; Guo, M.; Ebbesen, P.; Liu, X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J. Gen. Virol. 1997, 78 Pt 12, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Fukudome, K.; Hayashi, M.; Takagi, S.; Yoshie, O. Induction of ICAM-1 and LFA-3 by Tax1 of human T-cell leukemia virus type 1 and mechanism of down-regulation of ICAM-1 or LFA-1 in adult-T-cell-leukemia cell lines. Int. J. Cancer 1995, 60, 554–561. [Google Scholar] [CrossRef]
- Mori, N.; Murakami, S.; Oda, S.; Eto, S. Human T-cell leukemia virus type I tax induces intracellular adhesion molecule-1 expression in T cells. Blood 1994, 84, 350–351. [Google Scholar] [CrossRef]
- Tanaka, Y.; Mizuguchi, M.; Takahashi, Y.; Fujii, H.; Tanaka, R.; Fukushima, T.; Tomoyose, T.; Ansari, A.A.; Nakamura, M. Human T-cell leukemia virus type-I Tax induces the expression of CD83 on T cells. Retrovirology 2015, 12, 56. [Google Scholar] [CrossRef]
- Naito, T.; Ushirogawa, H.; Fukushima, T.; Tanaka, Y.; Saito, M. EOS, an Ikaros family zinc finger transcription factor, interacts with the HTLV-1 oncoprotein Tax and is downregulated in peripheral blood mononuclear cells of HTLV-1-infected individuals, irrespective of clinical statuses. Virol. J. 2019, 16, 160. [Google Scholar] [CrossRef]
- Sasada, T.; Nakamura, H.; Masutani, H.; Ueda, S.; Sono, H.; Takabayashi, A.; Yodoi, J. Thioredoxin-mediated redox control of human T cell lymphotropic virus type I (HTLV-I) gene expression. Mol. Immunol. 2002, 38, 723–732. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Zachar, V.; Chang, C.; Ebbesen, P. Transcriptional activation of human TR3/nur77 gene expression by human T-lymphotropic virus type I Tax protein through two AP-1-like elements. J. Gen. Virol. 1999, 80 Pt 12, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Beck, Z.; Bacsi, A.; Liu, X.; Ebbesen, P.; Andirko, I.; Csoma, E.; Konya, J.; Nagy, E.; Toth, F.D. Differential patterns of human cytomegalovirus gene expression in various T-cell lines carrying human T-cell leukemia-lymphoma virus type I: Role of Tax-activated cellular transcription factors. J. Med. Virol. 2003, 71, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Nagakubo, D.; Jin, Z.; Hieshima, K.; Nakayama, T.; Shirakawa, A.K.; Tanaka, Y.; Hasegawa, H.; Hayashi, T.; Tsukasaki, K.; Yamada, Y.; et al. Expression of CCR9 in HTLV-1+ T cells and ATL cells expressing Tax. Int. J. Cancer 2007, 120, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.W.; Iha, H.; Iwanaga, Y.; Bittner, M.; Chen, Y.; Jiang, Y.; Gooden, G.; Trent, J.M.; Meltzer, P.; Jeang, K.T.; et al. Genome-wide expression changes induced by HTLV-1 Tax: Evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-κB activation. Oncogene 2001, 20, 4484–4496. [Google Scholar] [CrossRef]
- Kasai, T.; Jeang, K.T. Two discrete events, human T-cell leukemia virus type I Tax oncoprotein expression and a separate stress stimulus, are required for induction of apoptosis in T-cells. Retrovirology 2004, 1, 7. [Google Scholar] [CrossRef]
- Chen, X.; Zachar, V.; Chang, C.; Ebbesen, P.; Liu, X. Differential expression of Nur77 family members in human T-lymphotropic virus type 1-infected cells: Transactivation of the TR3/nur77 gene by Tax protein. J. Virol. 1998, 72, 6902–6906. [Google Scholar] [CrossRef]
- Chen, L.; Ma, S.; Li, B.; Fink, T.; Zachar, V.; Takahashi, M.; Cuttichia, J.; Tsui, L.C.; Ebbesen, P.; Liu, X. Transcriptional activation of immediate-early gene ETR101 by human T-cell leukaemia virus type I Tax. J. Gen. Virol. 2003, 84, 3203–3214. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, T.; Mitani, K.; Ueno, H.; Kanda, Y.; Yazaki, Y.; Hirai, H. Triple synergism of human T-lymphotropic virus type 1-encoded tax, GATA-binding protein, and AP-1 is required for constitutive expression of the interleukin-5 gene in adult T-cell leukemia cells. Mol. Cell Biol. 1997, 17, 4272–4281. [Google Scholar] [CrossRef]
- Valentin, H.; Lemasson, I.; Hamaia, S.; Casse, H.; Konig, S.; Devaux, C.; Gazzolo, L. Transcriptional activation of the vascular cell adhesion molecule-1 gene in T lymphocytes expressing human T-cell leukemia virus type 1 Tax protein. J. Virol. 1997, 71, 8522–8530. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Mukaida, N.; Ballard, D.W.; Matsushima, K.; Yamamoto, N. Human T-cell leukemia virus type I Tax transactivates human interleukin 8 gene through acting concurrently on AP-1 and nuclear factor-κB-like sites. Cancer Res. 1998, 58, 3993–4000. [Google Scholar]
- Fujii, M.; Niki, T.; Mori, T.; Matsuda, T.; Matsui, M.; Nomura, N.; Seiki, M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene 1991, 6, 1023–1029. [Google Scholar]
- Fufa, T.D.; Byun, J.S.; Wakano, C.; Fernandez, A.G.; Pise-Masison, C.A.; Gardner, K. The Tax oncogene enhances ELL incorporation into p300 and P-TEFb containing protein complexes to activate transcription. Biochem. Biophys. Res. Commun. 2015, 465, 5–11. [Google Scholar] [CrossRef]
- Siekevitz, M.; Feinberg, M.B.; Holbrook, N.; Wong-Staal, F.; Greene, W.C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc. Natl. Acad. Sci. USA 1987, 84, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Wano, Y.; Feinberg, M.; Hosking, J.B.; Bogerd, H.; Greene, W.C. Stable expression of the tax gene of type I human T-cell leukemia virus in human T cells activates specific cellular genes involved in growth. Proc. Natl. Acad. Sci. USA 1988, 85, 9733–9737. [Google Scholar] [CrossRef]
- Hoyos, B.; Ballard, D.W.; Bohnlein, E.; Siekevitz, M.; Greene, W.C. Kappa B-specific DNA binding proteins: Role in the regulation of human interleukin-2 gene expression. Science 1989, 244, 457–460. [Google Scholar] [CrossRef]
- McGuire, K.L.; Curtiss, V.E.; Larson, E.L.; Haseltine, W.A. Influence of human T-cell leukemia virus type I tax and rex on interleukin-2 gene expression. J. Virol. 1993, 67, 1590–1599. [Google Scholar] [CrossRef]
- Good, L.; Maggirwar, S.B.; Sun, S.C. Activation of the IL-2 gene promoter by HTLV-I tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 1996, 15, 3744–3750. [Google Scholar] [CrossRef]
- Curtiss, V.E.; Smilde, R.; McGuire, K.L. Requirements for interleukin 2 promoter transactivation by the Tax protein of human T-cell leukemia virus type 1. Mol. Cell Biol. 1996, 16, 3567–3575. [Google Scholar] [CrossRef]
- Cross, S.L.; Feinberg, M.B.; Wolf, J.B.; Holbrook, N.J.; Wong-Staal, F.; Leonard, W.J. Regulation of the human interleukin-2 receptor α chain promoter: Activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell 1987, 49, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Nabel, G.J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature 1988, 333, 776–778. [Google Scholar] [CrossRef]
- Ruben, S.; Poteat, H.; Tan, T.H.; Kawakami, K.; Roeder, R.; Haseltine, W.; Rosen, C.A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science 1988, 241, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.W.; Bohnlein, E.; Lowenthal, J.W.; Wano, Y.; Franza, B.R.; Greene, W.C. HTLV-I tax induces cellular proteins that activate the κB element in the IL-2 receptor α gene. Science 1988, 241, 1652–1655. [Google Scholar] [CrossRef]
- Green, J.E.; Begley, C.G.; Wagner, D.K.; Waldmann, T.A.; Jay, G. trans activation of granulocyte-macrophage colony-stimulating factor and the interleukin-2 receptor in transgenic mice carrying the human T-lymphotropic virus type 1 tax gene. Mol. Cell Biol. 1989, 9, 4731–4737. [Google Scholar] [CrossRef]
- Marriott, S.J.; Trinh, D.; Brady, J.N. Activation of interleukin-2 receptor α expression by extracellular HTLV-I Tax1 protein: A potential role in HTLV-I pathogenesis. Oncogene 1992, 7, 1749–1755. [Google Scholar]
- Ashrafi, F.; Nassiri, M.; Javadmanesh, A.; Rahimi, H.; Rezaee, S.A. Epigenetics evaluation of the oncogenic mechanisms of two closely related bovine and human deltaretroviruses: A system biology study. Microb. Pathog. 2020, 139, 103845. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Ohbo, K.; Takasawa, N.; Ishii, N.; Tanaka, N.; Nakamura, M.; Sugamura, K. Functional analysis of the human interleukin 2 receptor γ chain gene promoter. J. Biol. Chem. 1995, 270, 7479–7486. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Takasawa, N.; Ohbo, K.; Higashimura, N.; Ohtani, K.; Tanaka, Y.; Sugamura, K. HTLV-I Tax trans-activation and cell growth signaling. Leukemia 1997, 11 (Suppl. S3), 7–9. [Google Scholar] [PubMed]
- Maeda, M.; Tanabe-Shibuya, J.; Miyazato, P.; Masutani, H.; Yasunaga, J.I.; Usami, K.; Shimizu, A.; Matsuoka, M. IL-2/IL-2 receptor pathway plays a crucial role in the growth and malignant transformation of HTLV-1-infected T cells to develop adult T-cell leukemia. Front. Microbiol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Satoh, M.; Toma, H.; Sugahara, K.; Etoh, K.; Shiroma, Y.; Kiyuna, S.; Takara, M.; Matsuoka, M.; Yamaguchi, K.; Nakada, K.; et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4+25+ HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene 2002, 21, 2466–2475. [Google Scholar] [CrossRef]
- Trejo, S.R.; Fahl, W.E.; Ratner, L. The tax protein of human T-cell leukemia virus type 1 mediates the transactivation of the c-sis/platelet-derived growth factor-B promoter through interactions with the zinc finger transcription factors Sp1 and NGFI-A/Egr-1. J. Biol. Chem. 1997, 272, 27411–27421. [Google Scholar] [CrossRef]
- Fung, M.M.; Chu, Y.L.; Fink, J.L.; Wallace, A.; McGuire, K.L. IL-2- and STAT5-regulated cytokine gene expression in cells expressing the Tax protein of HTLV-1. Oncogene 2005, 24, 4624–4633. [Google Scholar] [CrossRef]
- Chen, J.; Petrus, M.; Bryant, B.R.; Phuc Nguyen, V.; Stamer, M.; Goldman, C.K.; Bamford, R.; Morris, J.C.; Janik, J.E.; Waldmann, T.A. Induction of the IL-9 gene by HTLV-I Tax stimulates the spontaneous proliferation of primary adult T-cell leukemia cells by a paracrine mechanism. Blood 2008, 111, 5163–5172. [Google Scholar] [CrossRef] [PubMed]
- Azimi, N.; Brown, K.; Bamford, R.N.; Tagaya, Y.; Siebenlist, U.; Waldmann, T.A. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-κB site. Proc. Natl. Acad. Sci. USA 1998, 95, 2452–2457. [Google Scholar] [CrossRef]
- Azimi, N.; Mariner, J.; Jacobson, S.; Waldmann, T.A. How does interleukin 15 contribute to the pathogenesis of HTLV type 1-associated myelopathy/tropical spastic paraparesis? AIDS Res. Hum. Retroviruses 2000, 16, 1717–1722. [Google Scholar] [CrossRef]
- Mariner, J.M.; Lantz, V.; Waldmann, T.A.; Azimi, N. Human T cell lymphotropic virus type I Tax activates IL-15Rα gene expression through an NF-κB site. J. Immunol. 2001, 166, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, M.; Asao, H.; Hara, T.; Higuchi, M.; Fujii, M.; Nakamura, M. Transcriptional activation of the interleukin-21 gene and its receptor gene by human T-cell leukemia virus type 1 Tax in human T-cells. J. Biol. Chem. 2009, 284, 25501–25511. [Google Scholar] [CrossRef]
- Rajaei, T.; Farajifard, H.; Rafatpanah, H.; Bustani, R.; Valizadeh, N.; Rajaei, B.; Rezaee, S.A. Role of IL-21 in HTLV-1 infections with emphasis on HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Med. Microbiol. Immunol. 2017, 206, 195–201. [Google Scholar] [CrossRef]
- Kim, S.J.; Kehrl, J.H.; Burton, J.; Tendler, C.L.; Jeang, K.T.; Danielpour, D.; Thevenin, C.; Kim, K.Y.; Sporn, M.B.; Roberts, A.B. Transactivation of the transforming growth factor β1 (TGF-β1) gene by human T lymphotropic virus type 1 tax: A potential mechanism for the increased production of TGF-β1 in adult T cell leukemia. J. Exp. Med. 1990, 172, 121–129. [Google Scholar] [CrossRef]
- Kim, S.J.; Winokur, T.S.; Lee, H.D.; Danielpour, D.; Kim, K.Y.; Geiser, A.G.; Chen, L.S.; Sporn, M.B.; Roberts, A.B.; Jay, G. Overexpression of transforming growth factor-β in transgenic mice carrying the human T-cell lymphotropic virus type I tax gene. Mol. Cell Biol. 1991, 11, 5222–5228. [Google Scholar] [CrossRef]
- Baum, P.R.; Gayle, R.B., 3rd; Ramsdell, F.; Srinivasan, S.; Sorensen, R.A.; Watson, M.L.; Seldin, M.F.; Baker, E.; Sutherland, G.R.; Clifford, K.N.; et al. Molecular characterization of murine and human OX40/OX40 ligand systems: Identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994, 13, 3992–4001. [Google Scholar] [CrossRef]
- Pankow, R.; Durkop, H.; Latza, U.; Krause, H.; Kunzendorf, U.; Pohl, T.; Bulfone-Paus, S. The HTLV-I tax protein transcriptionally modulates OX40 antigen expression. J. Immunol. 2000, 165, 263–270. [Google Scholar] [CrossRef]
- Saito, M.; Tanaka, R.; Arishima, S.; Matsuzaki, T.; Ishihara, S.; Tokashiki, T.; Ohya, Y.; Takashima, H.; Umehara, F.; Izumo, S.; et al. Increased expression of OX40 is associated with progressive disease in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Retrovirology 2013, 10, 51. [Google Scholar] [CrossRef]
- Ohtani, K.; Tsujimoto, A.; Tsukahara, T.; Numata, N.; Miura, S.; Sugamura, K.; Nakamura, M. Molecular mechanisms of promoter regulation of the gp34 gene that is trans-activated by an oncoprotein Tax of human T cell leukemia virus type I. J. Biol. Chem. 1998, 273, 14119–14129. [Google Scholar] [CrossRef] [PubMed]
- Motai, Y.; Takahashi, M.; Takachi, T.; Higuchi, M.; Hara, T.; Mizuguchi, M.; Aoyagi, Y.; Terai, S.; Tanaka, Y.; Fujii, M. Human T-cell leukemia virus type 1 (HTLV-1) Tax1 oncoprotein but not HTLV-2 Tax2 induces the expression of OX40 ligand by interacting with p52/p100 and RelB. Virus Genes 2016, 52, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, E.W.; Harhaj, N.S.; Grant, C.; Mostoller, K.; Alefantis, T.; Sun, S.C.; Wigdahl, B. Human T cell leukemia virus type I Tax activates CD40 gene expression via the NF-κB pathway. Virology 2005, 333, 145–158. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Janic, B.; Ramos, J.C.; Harrington, W.J., Jr.; Harhaj, E.W. Deregulated expression of CD40 ligand in HTLV-I infection: Distinct mechanisms of downregulation in HTLV-I-transformed cell lines and ATL patients. Virology 2007, 362, 99–108. [Google Scholar] [CrossRef]
- El-Sabban, M.E.; Merhi, R.A.; Haidar, H.A.; Arnulf, B.; Khoury, H.; Basbous, J.; Nijmeh, J.; de The, H.; Hermine, O.; Bazarbachi, A. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood 2002, 99, 3383–3389. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamaguchi, K.; Takatsuki, K.; Osame, M.; Yoshida, M. Constitutive expression of parathyroid hormone-related protein gene in human T cell leukemia virus type 1 (HTLV-1) carriers and adult T cell leukemia patients that can be trans-activated by HTLV-1 tax gene. J. Exp. Med. 1990, 172, 759–765. [Google Scholar] [CrossRef]
- Mori, N.; Ejima, E.; Prager, D. Transactivation of parathyroid hormone-related protein gene expression by human T-cell leukemia virus type I tax. Eur. J. Haematol. 1996, 56, 116–117. [Google Scholar] [CrossRef]
- Dittmer, J.; Gitlin, S.D.; Reid, R.L.; Brady, J.N. Transactivation of the P2 promoter of parathyroid hormone-related protein by human T-cell lymphotropic virus type I Tax1: Evidence for the involvement of transcription factor Ets1. J. Virol. 1993, 67, 6087–6095. [Google Scholar] [CrossRef]
- Ejima, E.; Rosenblatt, J.D.; Massari, M.; Quan, E.; Stephens, D.; Rosen, C.A.; Prager, D. Cell-type-specific transactivation of the parathyroid hormone-related protein gene promoter by the human T-cell leukemia virus type I (HTLV-I) tax and HTLV-II tax proteins. Blood 1993, 81, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Pise-Masison, C.A.; Clemens, K.E.; Choi, K.S.; Brady, J.N. Interaction of human T-cell lymphotropic virus type I Tax, Ets1, and Sp1 in transactivation of the PTHrP P2 promoter. J. Biol. Chem. 1997, 272, 4953–4958. [Google Scholar] [CrossRef] [PubMed]
- Nadella, M.V.; Dirksen, W.P.; Nadella, K.S.; Shu, S.; Cheng, A.S.; Morgenstern, J.A.; Richard, V.; Fernandez, S.A.; Huang, T.H.; Guttridge, D.; et al. Transcriptional regulation of parathyroid hormone-related protein promoter P2 by NF-κB in adult T-cell leukemia/lymphoma. Leukemia 2007, 21, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Nadella, M.V.; Shu, S.T.; Dirksen, W.P.; Thudi, N.K.; Nadella, K.S.; Fernandez, S.A.; Lairmore, M.D.; Green, P.L.; Rosol, T.J. Expression of parathyroid hormone-related protein during immortalization of human peripheral blood mononuclear cells by HTLV-1: Implications for transformation. Retrovirology 2008, 5, 46. [Google Scholar] [CrossRef]
- Yoshie, O.; Fujisawa, R.; Nakayama, T.; Harasawa, H.; Tago, H.; Izawa, D.; Hieshima, K.; Tatsumi, Y.; Matsushima, K.; Hasegawa, H.; et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 2002, 99, 1505–1511. [Google Scholar] [CrossRef]
- Yoshie, O. Expression of CCR4 in adult T-cell leukemia. Leuk. Lymphoma 2005, 46, 185–190. [Google Scholar] [CrossRef]
- Nakayama, T.; Hieshima, K.; Arao, T.; Jin, Z.; Nagakubo, D.; Shirakawa, A.K.; Yamada, Y.; Fujii, M.; Oiso, N.; Kawada, A.; et al. Aberrant expression of Fra-2 promotes CCR4 expression and cell proliferation in adult T-cell leukemia. Oncogene 2008, 27, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Suzumura, A.; Kondo, N.; Marunouchi, T. Induction of cytokines in glial cells by trans activator of human T-cell lymphotropic virus type I. FEBS Lett. 1992, 313, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Prager, D. Transactivation of the interleukin-1alpha promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood 1996, 87, 3410–3417. [Google Scholar] [CrossRef]
- Banerjee, P.; Rochford, R.; Antel, J.; Canute, G.; Wrzesinski, S.; Sieburg, M.; Feuer, G. Proinflammatory cytokine gene induction by human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 Tax in primary human glial cells. J. Virol. 2007, 81, 1690–1700. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ishikawa, C.; Tamaki, K.; Senba, M.; Fujita, J.; Mori, N. Interleukin-1α produced by human T-cell leukaemia virus type I-infected T cells induces intercellular adhesion molecule-1 expression on lung epithelial cells. J. Med. Microbiol. 2011, 60, 1750–1761. [Google Scholar] [CrossRef]
- Rauch, D.A.; Harding, J.C.; Ratner, L. IL-15 deficient tax mice reveal a role for IL-1α in tumor immunity. PLoS ONE 2014, 9, e85028. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, J.; Misago, M.; Serino, Y.; Ogawa, R.; Murakami, S.; Nakanishi, M.; Tonai, S.; Kominato, Y.; Morimoto, I.; Auron, P.E.; et al. Human T-cell leukemia virus type I Tax transactivates the promoter of human prointerleukin-1β gene through association with two transcription factors, nuclear factor-interleukin-6 and Spi-1. Blood 1997, 90, 3142–3153. [Google Scholar] [CrossRef]
- Li-Weber, M.; Giaisi, M.; Chlichlia, K.; Khazaie, K.; Krammer, P.H. Human T cell leukemia virus type I Tax enhances IL-4 gene expression in T cells. Eur. J. Immunol. 2001, 31, 2623–2632. [Google Scholar] [CrossRef]
- Blumenthal, S.G.; Aichele, G.; Wirth, T.; Czernilofsky, A.P.; Nordheim, A.; Dittmer, J. Regulation of the human interleukin-5 promoter by Ets transcription factors. Ets1 and Ets2, but not Elf-1, cooperate with GATA3 and HTLV-I Tax1. J. Biol. Chem. 1999, 274, 12910–12916. [Google Scholar] [CrossRef]
- Muraoka, O.; Kaisho, T.; Tanabe, M.; Hirano, T. Transcriptional activation of the interleukin-6 gene by HTLV-1 p40tax through an NF-κB-like binding site. Immunol. Lett. 1993, 37, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Shirakawa, F.; Shimizu, H.; Murakami, S.; Oda, S.; Yamamoto, K.; Eto, S. Transcriptional regulation of the human interleukin-6 gene promoter in human T-cell leukemia virus type I-infected T-cell lines: Evidence for the involvement of NF-κB. Blood 1994, 84, 2904–2911. [Google Scholar] [CrossRef]
- Mori, N.; Shirakawa, F.; Abe, M.; Kamo, Y.; Koyama, Y.; Murakami, S.; Shimizu, H.; Yamamoto, K.; Oda, S.; Eto, S. Human T-cell leukemia virus type I tax transactivates the interleukin-6 gene in human rheumatoid synovial cells. J. Rheumatol. 1995, 22, 2049–2054. [Google Scholar]
- Horiuchi, S.; Yamamoto, N.; Dewan, M.Z.; Takahashi, Y.; Yamashita, A.; Yoshida, T.; Nowell, M.A.; Richards, P.J.; Jones, S.A.; Yamamoto, N. Human T-cell leukemia virus type-I Tax induces expression of interleukin-6 receptor (IL-6R): Shedding of soluble IL-6R and activation of STAT3 signaling. Int. J. Cancer 2006, 119, 823–830. [Google Scholar] [CrossRef]
- Refaat, A.; Zhou, Y.; Suzuki, S.; Takasaki, I.; Koizumi, K.; Yamaoka, S.; Tabuchi, Y.; Saiki, I.; Sakurai, H. Distinct roles of transforming growth factor-β-activated kinase 1 (TAK1)-c-Rel and interferon regulatory factor 4 (IRF4) pathways in human T cell lymphotropic virus 1-transformed T helper 17 cells producing interleukin-9. J. Biol. Chem. 2011, 286, 21092–21099. [Google Scholar] [CrossRef]
- Mori, N.; Murakami, S.; Oda, S.; Prager, D.; Eto, S. Production of interleukin 8 in adult T-cell leukemia cells: Possible transactivation of the interleukin 8 gene by human T-cell leukemia virus type I tax. Cancer Res. 1995, 55, 3592–3597. [Google Scholar] [PubMed]
- Mori, N.; Gill, P.S.; Mougdil, T.; Murakami, S.; Eto, S.; Prager, D. Interleukin-10 gene expression in adult T-cell leukemia. Blood 1996, 88, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Prager, D. Interleukin-10 gene expression and adult T-cell leukemia. Leuk. Lymphoma 1998, 29, 239–248. [Google Scholar] [CrossRef]
- Ahuja, J.; Lepoutre, V.; Wigdahl, B.; Khan, Z.K.; Jain, P. Induction of pro-inflammatory cytokines by human T-cell leukemia virus type-1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells. Biomed. Pharmacother. 2007, 61, 201–208. [Google Scholar] [CrossRef]
- Chung, H.K.; Young, H.A.; Goon, P.K.; Heidecker, G.; Princler, G.L.; Shimozato, O.; Taylor, G.P.; Bangham, C.R.; Derse, D. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood 2003, 102, 4130–4136. [Google Scholar] [CrossRef]
- Waldele, K.; Schneider, G.; Ruckes, T.; Grassmann, R. Interleukin-13 overexpression by tax transactivation: A potential autocrine stimulus in human T-cell leukemia virus-infected lymphocytes. J. Virol. 2004, 78, 6081–6090. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, K.; Schneider, G.; Grassmann, R. Stimulation of interleukin-13 expression by human T-cell leukemia virus type 1 oncoprotein Tax via a dually active promoter element responsive to NF-κB and NFAT. J. Gen. Virol. 2008, 89, 2788–2798. [Google Scholar] [CrossRef]
- Dodon, M.D.; Li, Z.; Hamaia, S.; Gazzolo, L. Tax protein of human T-cell leukaemia virus type 1 induces interleukin 17 gene expression in T cells. J. Gen. Virol. 2004, 85, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, A.; Matsuoka, M.; Harhaj, E.W. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-κB activation and T-cell transformation. PLoS Pathog. 2014, 10, e1004418. [Google Scholar] [CrossRef]
- Araya, N.; Sato, T.; Ando, H.; Tomaru, U.; Yoshida, M.; Coler-Reilly, A.; Yagishita, N.; Yamauchi, J.; Hasegawa, A.; Kannagi, M.; et al. HTLV-1 induces a Th1-like state in CD4+CCR4+ T cells. J. Clin. Investig. 2014, 124, 3431–3442. [Google Scholar] [CrossRef]
- Mendez, E.; Kawanishi, T.; Clemens, K.; Siomi, H.; Soldan, S.S.; Calabresi, P.; Brady, J.; Jacobson, S. Astrocyte-specific expression of human T-cell lymphotropic virus type 1 (HTLV-1) Tax: Induction of tumor necrosis factor α and susceptibility to lysis by CD8+ HTLV-1-specific cytotoxic T cells. J. Virol. 1997, 71, 9143–9149. [Google Scholar] [CrossRef]
- Cowan, E.P.; Alexander, R.K.; Daniel, S.; Kashanchi, F.; Brady, J.N. Induction of tumor necrosis factor α in human neuronal cells by extracellular human T-cell lymphotropic virus type 1 Tax. J. Virol. 1997, 71, 6982–6989. [Google Scholar] [CrossRef]
- Tzagarakis-Foster, C.; Geleziunas, R.; Lomri, A.; An, J.; Leitman, D.C. Estradiol represses human T-cell leukemia virus type 1 Tax activation of tumor necrosis factor-α gene transcription. J. Biol. Chem. 2002, 277, 44772–44777. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.L.; Millet, I.; Ruddle, N.H. The lymphotoxin promoter is stimulated by HTLV-I tax activation of NF-κB in human T-cell lines. Cytokine 1993, 5, 372–378. [Google Scholar] [CrossRef]
- Tschachler, E.; Bohnlein, E.; Felzmann, S.; Reitz, M.S., Jr. Human T-lymphotropic virus type I tax regulates the expression of the human lymphotoxin gene. Blood 1993, 81, 95–100. [Google Scholar] [CrossRef]
- Nimer, S.D.; Gasson, J.C.; Hu, K.; Smalberg, I.; Williams, J.L.; Chen, I.S.; Rosenblatt, J.D. Activation of the GM-CSF promoter by HTLV-I and -II tax proteins. Oncogene 1989, 4, 671–676. [Google Scholar]
- Himes, S.R.; Coles, L.S.; Katsikeros, R.; Lang, R.K.; Shannon, M.F. HTLV-1 tax activation of the GM-CSF and G-CSF promoters requires the interaction of NF-κB with other transcription factor families. Oncogene 1993, 8, 3189–3197. [Google Scholar]
- Miyazato, A.; Kawakami, K.; Iwakura, Y.; Saito, A. Chemokine synthesis and cellular inflammatory changes in lungs of mice bearing p40tax of human T-lymphotropic virus type 1. Clin. Exp. Immunol. 2000, 120, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Ueda, A.; Ikeda, S.; Yamasaki, Y.; Yamada, Y.; Tomonaga, M.; Morikawa, S.; Geleziunas, R.; Yoshimura, T.; Yamamoto, N. Human T-cell leukemia virus type I tax activates transcription of the human monocyte chemoattractant protein-1 gene through two nuclear factor-κB sites. Cancer Res. 2000, 60, 4939–4945. [Google Scholar]
- Saito, M.; Sejima, H.; Naito, T.; Ushirogawa, H.; Matsuzaki, T.; Matsuura, E.; Tanaka, Y.; Nakamura, T.; Takashima, H. The CC chemokine ligand (CCL) 1, upregulated by the viral transactivator Tax, can be downregulated by minocycline: Possible implications for long-term treatment of HTLV-1-associated myelopathy/tropical spastic paraparesis. Virol. J. 2017, 14, 234. [Google Scholar] [CrossRef]
- Sharma, V.; May, C.C. Human T-cell lymphotrophic virus type-I tax gene induces secretion of human macrophage inflammatory protein-1α. Biochem. Biophys. Res. Commun. 1999, 262, 429–432. [Google Scholar] [CrossRef]
- Napolitano, M.; Modi, W.S.; Cevario, S.J.; Gnarra, J.R.; Seuanez, H.N.; Leonard, W.J. The gene encoding the Act-2 cytokine. Genomic structure, HTLV-I/Tax responsiveness of 5′ upstream sequences, and chromosomal localization. J. Biol. Chem. 1991, 266, 17531–17536. [Google Scholar] [CrossRef]
- Sharma, V.; Lorey, S.L. Autocrine role of macrophage inflammatory protein-1 β in human T-cell lymphotropic virus type-I tax-transfected Jurkat T-cells. Biochem. Biophys. Res. Commun. 2001, 287, 910–913. [Google Scholar] [CrossRef]
- Mori, N.; Krensky, A.M.; Ohshima, K.; Tomita, M.; Matsuda, T.; Ohta, T.; Yamada, Y.; Tomonaga, M.; Ikeda, S.; Yamamoto, N. Elevated expression of CCL5/RANTES in adult T-cell leukemia cells: Possible transactivation of the CCL5 gene by human T-cell leukemia virus type I tax. Int. J. Cancer 2004, 111, 548–557. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Sugita, S.; Yamamoto, K.; Imanishi, D.; Kohno, T.; Tomonaga, M.; Matsuyama, T. Human T cell leukemia virus type-I Tax activates human macrophage inflammatory protein-3α/CCL20 gene transcription via the NF-κB pathway. Int. Immunol. 2002, 14, 147–155. [Google Scholar] [CrossRef]
- Hieshima, K.; Nagakubo, D.; Nakayama, T.; Shirakawa, A.K.; Jin, Z.; Yoshie, O. Tax-inducible production of CC chemokine ligand 22 by human T cell leukemia virus type 1 (HTLV-1)-infected T cells promotes preferential transmission of HTLV-1 to CCR4-expressing CD4+ T cells. J. Immunol. 2008, 180, 931–939. [Google Scholar] [CrossRef]
- Naito, T.; Yasunaga, J.I.; Mitobe, Y.; Shirai, K.; Sejima, H.; Ushirogawa, H.; Tanaka, Y.; Nakamura, T.; Hanada, K.; Fujii, M.; et al. Distinct gene expression signatures induced by viral transactivators of different HTLV-1 subgroups that confer a different risk of HAM/TSP. Retrovirology 2018, 15, 72. [Google Scholar] [CrossRef]
- Souza, F.D.S.; Freitas, N.L.; Gomes, Y.C.P.; Torres, R.C.; Echevarria-Lima, J.; da Silva-Filho, I.L.; Leite, A.; de Lima, M.; da Silva, M.T.T.; Araujo, A.Q.C.; et al. Following the clues: Usefulness of biomarkers of neuroinflammation and neurodegeneration in the investigation of HTLV-1-associated myelopathy progression. Front. Immunol. 2021, 12, 737941. [Google Scholar] [CrossRef]
- Arai, M.; Ohashi, T.; Tsukahara, T.; Murakami, T.; Hori, T.; Uchiyama, T.; Yamamoto, N.; Kannagi, M.; Fujii, M. Human T-cell leukemia virus type 1 Tax protein induces the expression of lymphocyte chemoattractant SDF-1/PBSF. Virology 1998, 241, 298–303. [Google Scholar] [CrossRef]
- Moriuchi, M.; Moriuchi, H.; Fauci, A.S. HTLV type I Tax activation of the CXCR4 promoter by association with nuclear respiratory factor 1. AIDS Res. Hum. Retroviruses 1999, 15, 821–827. [Google Scholar] [CrossRef]
- Araya, N.; Yamagishi, M.; Nakashima, M.; Asahara, N.; Kiyohara, K.; Aratani, S.; Yagishita, N.; Horibe, E.; Ishizaki, I.; Watanabe, T.; et al. Virus-induced RGMa expression drives neurodegeneration in HTLV-1-associated myelopathy. JCI Insight 2025, 10, e184530. [Google Scholar] [CrossRef]
- Valentin, H.; Hamaia, S.; Konig, S.; Gazzolo, L. Vascular cell adhesion molecule-1 induced by human T-cell leukaemia virus type 1 Tax protein in T-cells stimulates proliferation of human T-lymphocytes. J. Gen. Virol. 2001, 82, 831–835. [Google Scholar] [CrossRef]
- Murakami, Y. Involvement of a cell adhesion molecule, TSLC1/IGSF4, in human oncogenesis. Cancer Sci. 2005, 96, 543–552. [Google Scholar] [CrossRef]
- Hartsough, E.J.; Weiss, M.B.; Heilman, S.A.; Purwin, T.J.; Kugel, C.H., 3rd; Rosenbaum, S.R.; Erkes, D.A.; Tiago, M.; HooKim, K.; Chervoneva, I.; et al. CADM1 is a TWIST1-regulated suppressor of invasion and survival. Cell Death Dis. 2019, 10, 281. [Google Scholar] [CrossRef]
- Nakahata, S.; Morishita, K. CADM1/TSLC1 is a novel cell surface marker for adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2012, 52, 17–22. [Google Scholar] [CrossRef]
- Zhang, J.; Yamada, O.; Matsushita, Y.; Chagan-Yasutan, H.; Hattori, T. Transactivation of human osteopontin promoter by human T-cell leukemia virus type 1-encoded Tax protein. Leuk. Res. 2010, 34, 763–768. [Google Scholar] [CrossRef]
- Zhang, J.; Yamada, O.; Kida, S.; Matsushita, Y.; Yamaoka, S.; Chagan-Yasutan, H.; Hattori, T. Identification of CD44 as a downstream target of noncanonical NF-κB pathway activated by human T-cell leukemia virus type 1-encoded Tax protein. Virology 2011, 413, 244–252. [Google Scholar] [CrossRef]
- Chagan-Yasutan, H.; Tsukasaki, K.; Takahashi, Y.; Oguma, S.; Harigae, H.; Ishii, N.; Zhang, J.; Fukumoto, M.; Hattori, T. Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk. Res. 2011, 35, 1484–1490. [Google Scholar] [CrossRef]
- Wu, M.; Wong, H.Y.; Lin, J.L.; Moliner, A.; Schwarz, H. Induction of CD137 expression by viral genes reduces T cell costimulation. J. Cell Physiol. 2019, 234, 21076–21088. [Google Scholar] [CrossRef]
- Kitajima, I.; Kawahara, K.; Hanyu, N.; Shin, H.; Tokioka, T.; Soejima, Y.; Tsutsui, J.; Ozawa, M.; Shimayama, T.; Maruyama, I. Enhanced E-cadherin expression and increased calcium-dependent cell-cell adhesion in human T-cell leukemia virus type I Tax-expressing PC12 cells. J. Cell Sci. 1996, 109 Pt 3, 609–617. [Google Scholar] [CrossRef]
- Pichler, K.; Kattan, T.; Gentzsch, J.; Kress, A.K.; Taylor, G.P.; Bangham, C.R.; Grassmann, R. Strong induction of 4-1BB, a growth and survival promoting costimulatory receptor, in HTLV-1-infected cultured and patients’ T cells by the viral Tax oncoprotein. Blood 2008, 111, 4741–4751. [Google Scholar] [CrossRef]
- Masamoto, I.; Yoshimitsu, M.; Kuroki, A.; Horai, S.; Ezinne, C.C.; Kozako, T.; Hachiman, M.; Kamada, Y.; Baba, M.; Arima, N. Clinical significance of CD70 expression on T cells in human T-lymphotropic virus type-1 carriers and adult T cell leukemia/lymphoma patients. Leuk. Lymphoma 2016, 57, 685–691. [Google Scholar] [CrossRef]
- Ishikawa, C.; Kawakami, H.; Uchihara, J.N.; Senba, M.; Mori, N. CD69 overexpression by human T-cell leukemia virus type 1 Tax transactivation. Biochim. Biophys. Acta 2013, 1833, 1542–1552. [Google Scholar] [CrossRef]
- Sawada, M.; Suzumura, A.; Yoshida, M.; Marunouchi, T. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J. Virol. 1990, 64, 4002–4006. [Google Scholar] [CrossRef]
- Green, J.E. trans activation of nerve growth factor in transgenic mice containing the human T-cell lymphotropic virus type I tax gene. Mol. Cell Biol. 1991, 11, 4635–4641. [Google Scholar] [CrossRef]
- Joshi, J.B.; Dave, H.P. Transactivation of the proenkephalin gene promoter by the Tax1 protein of human T-cell lymphotropic virus type I. Proc. Natl. Acad. Sci. USA 1992, 89, 1006–1010. [Google Scholar] [CrossRef]
- Low, K.G.; Chu, H.M.; Schwartz, P.M.; Daniels, G.M.; Melner, M.H.; Comb, M.J. Novel interactions between human T-cell leukemia virus type I Tax and activating transcription factor 3 at a cyclic AMP-responsive element. Mol. Cell Biol. 1994, 14, 4958–4974. [Google Scholar] [CrossRef]
- Fu, W.; Shah, S.R.; Jiang, H.; Hilt, D.C.; Dave, H.P.; Joshi, J.B. Transactivation of proenkephalin gene by HTLV-1 tax1 protein in glial cells: Involvement of Fos/Jun complex at an AP-1 element in the proenkephalin gene promoter. J. Neurovirol 1997, 3, 16–27. [Google Scholar] [CrossRef]
- Alexandre, C.; Verrier, B. Four regulatory elements in the human c-fos promoter mediate transactivation by HTLV-1 Tax protein. Oncogene 1991, 6, 543–551. [Google Scholar]
- Iwakura, Y.; Tosu, M.; Yoshida, E.; Saijo, S.; Nakayama-Yamada, J.; Itagaki, K.; Asano, M.; Siomi, H.; Hatanaka, M.; Takeda, T.; et al. Augmentation of c-fos and c-jun expression in transgenic mice carrying the human T-cell leukemia virus type-I tax gene. Virus Genes. 1995, 9, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.M.; Nimer, S.D.; Rosenblatt, J.D.; Gasson, J.C. HTLV-I and HTLV-II tax trans-activate the human EGR-1 promoter through different cis-acting sequences. Oncogene 1992, 7, 2125–2130. [Google Scholar]
- Fujii, M.; Tsuchiya, H.; Chuhjo, T.; Akizawa, T.; Seiki, M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes. Dev. 1992, 6, 2066–2076. [Google Scholar] [CrossRef]
- Li, B.; Fink, T.; Ebbesen, P.; Liu, X.D.; Zachar, V. Expression of butyrate response factor 1 in HTLV-1-transformed cells and its transactivation by tax protein. Arch. Virol. 2003, 148, 1787–1804. [Google Scholar] [CrossRef]
- Duyao, M.P.; Kessler, D.J.; Spicer, D.B.; Bartholomew, C.; Cleveland, J.L.; Siekevitz, M.; Sonenshein, G.E. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 tax is mediated by NFκB. J. Biol. Chem. 1992, 267, 16288–16291. [Google Scholar] [CrossRef]
- Gazon, H.; Barbeau, B.; Mesnard, J.M.; Peloponese, J.M., Jr. Hijacking of the AP-1 signaling pathway during development of ATL. Front. Microbiol. 2017, 8, 2686. [Google Scholar] [CrossRef]
- Fujii, M.; Iwai, K.; Oie, M.; Fukushi, M.; Yamamoto, N.; Kannagi, M.; Mori, N. Activation of oncogenic transcription factor AP-1 in T cells infected with human T cell leukemia virus type 1. AIDS Res. Hum. Retroviruses 2000, 16, 1603–1606. [Google Scholar] [CrossRef]
- Mori, N.; Fujii, M.; Iwai, K.; Ikeda, S.; Yamasaki, Y.; Hata, T.; Yamada, Y.; Tanaka, Y.; Tomonaga, M.; Yamamoto, N. Constitutive activation of transcription factor AP-1 in primary adult T-cell leukemia cells. Blood 2000, 95, 3915–3921. [Google Scholar]
- Iwai, K.; Mori, N.; Oie, M.; Yamamoto, N.; Fujii, M. Human T-cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology 2001, 279, 38–46. [Google Scholar] [CrossRef]
- Huang, Q.; Niu, Z.; Han, J.; Liu, X.; Lv, Z.; Li, H.; Yuan, L.; Li, X.; Sun, S.; Wang, H.; et al. HTLV-1 Tax upregulates early growth response protein 1 through nuclear factor-κB signaling. Oncotarget 2017, 8, 51123–51133. [Google Scholar] [CrossRef] [PubMed]
- Arima, N.; Molitor, J.A.; Smith, M.R.; Kim, J.H.; Daitoku, Y.; Greene, W.C. Human T-cell leukemia virus type I Tax induces expression of the Rel-related family of κB enhancer-binding proteins: Evidence for a pretranslational component of regulation. J. Virol. 1991, 65, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Senba, M.; Hashimoto, T.; Imaizumi, A.; Mori, N. Expression and significance of Pim-3 kinase in adult T-cell leukemia. Eur. J. Haematol. 2017, 99, 495–504. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Feedback loop regulation between Pim kinases and Tax keeps human T-cell leukemia virus type 1 viral replication in check. J. Virol. 2022, 96, e0196021. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Targeting Pim kinases in hematological cancers: Molecular and clinical review. Mol. Cancer 2023, 22, 18. [Google Scholar] [CrossRef]
- Migone, T.S.; Lin, J.X.; Cereseto, A.; Mulloy, J.C.; O’Shea, J.J.; Franchini, G.; Leonard, W.J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 1995, 269, 79–81. [Google Scholar] [CrossRef]
- Takemoto, S.; Mulloy, J.C.; Cereseto, A.; Migone, T.S.; Patel, B.K.; Matsuoka, M.; Yamaguchi, K.; Takatsuki, K.; Kamihira, S.; White, J.D.; et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 13897–13902. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Fujii, M.; Tsukahara, T.; Arai, M.; Ohashi, T.; Wakao, H.; Kannagi, M.; Yamamoto, N. Human T-cell leukemia virus type 1 Tax protein induces the expression of STAT1 and STAT5 genes in T-cells. Oncogene 1999, 18, 2667–2675. [Google Scholar] [CrossRef]
- Sharma, S.; Mamane, Y.; Grandvaux, N.; Bartlett, J.; Petropoulos, L.; Lin, R.; Hiscott, J. Activation and regulation of interferon regulatory factor 4 in HTLV type 1-infected T lymphocytes. AIDS Res. Hum. Retroviruses 2000, 16, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Grandvaux, N.; Mamane, Y.; Genin, P.; Azimi, N.; Waldmann, T.; Hiscott, J. Regulation of IFN regulatory factor 4 expression in human T cell leukemia virus-I-transformed T cells. J. Immunol. 2002, 169, 3120–3130. [Google Scholar] [CrossRef] [PubMed]
- Cherian, M.A.; Olson, S.; Sundaramoorthi, H.; Cates, K.; Cheng, X.; Harding, J.; Martens, A.; Challen, G.A.; Tyagi, M.; Ratner, L.; et al. An activating mutation of interferon regulatory factor 4 (IRF4) in adult T-cell leukemia. J. Biol. Chem. 2018, 293, 6844–6858. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Barnes, B.J.; Mori, N. Constitutive expression of IRF-5 in HTLV-1-infected T cells. Int. J. Oncol. 2015, 47, 361–369. [Google Scholar] [CrossRef]
- Shimizu, T.; Kawakita, S.; Li, Q.H.; Fukuhara, S.; Fujisawa, J. Human T-cell leukemia virus type 1 Tax protein stimulates the interferon-responsive enhancer element via NF-κB activity. FEBS Lett. 2003, 539, 73–77. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Mori, S.; Yoshida, M.; Kishimoto, T.; Inoue, K.; Yamamoto, T.; Toyoshima, K. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc. Natl. Acad. Sci. USA 1989, 86, 6538–6542. [Google Scholar] [CrossRef]
- Uchiumi, F.; Semba, K.; Yamanashi, Y.; Fujisawa, J.; Yoshida, M.; Inoue, K.; Toyoshima, K.; Yamamoto, T. Characterization of the promoter region of the src family gene lyn and its trans activation by human T-cell leukemia virus type I-encoded p40tax. Mol. Cell Biol. 1992, 12, 3784–3795. [Google Scholar] [CrossRef]
- Mills, G.B.; Arima, N.; May, C.; Hill, M.; Schmandt, R.; Li, J.; Miyamoto, N.G.; Greene, W.C. Neither the LCK nor the FYN kinases are obligatory for IL-2-mediated signal transduction in HTLV-I-infected human T cells. Int. Immunol. 1992, 4, 1233–1243. [Google Scholar] [CrossRef]
- Letafati, A.; Bahavar, A.; Tabarraei, A.; Norouzi, M.; Amiri, A.; Mozhgani, S.H. Human T-cell lymphotropic virus type 1 (HTLV-1) grip on T-cells: Investigating the viral tapestry of activation. Infect. Agent. Cancer 2024, 19, 23. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Song, J.R.; Zhao, M.J. NR4A1 regulates cerebral ischemia-induced brain injury by regulating neuroinflammation through interaction with NF-κB/p65. Biochem. Biophys. Res. Commun. 2019, 518, 59–65. [Google Scholar] [CrossRef]
- Takahashi, R.; Yamagishi, M.; Nakano, K.; Yamochi, T.; Yamochi, T.; Fujikawa, D.; Nakashima, M.; Tanaka, Y.; Uchimaru, K.; Utsunomiya, A.; et al. Epigenetic deregulation of Ellis Van Creveld confers robust Hedgehog signaling in adult T-cell leukemia. Cancer Sci. 2014, 105, 1160–1169. [Google Scholar] [CrossRef]
- Yamazaki, S. The Nuclear NF-κB Regulator IκBξ: Updates on Its Molecular Functions and Pathophysiological Roles. Cells 2024, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Senba, M.; Cutler, S.J.; Ralph, S.J.; Xiao, G.; Mori, N. Human T cell leukemia virus type I tax-induced IκB-ξ modulates tax-dependent and tax-independent gene expression in T cells. Neoplasia 2013, 15, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Mozhgani, S.H.; Roohinezhad, R.; Emami, S.H.; Emami, M.H.; Solooki, S.; Fattah Hesari, M.; Doroozeh, N.; Norouzi, M. Leukemia-related signaling pathways among HTLV-1-derived adult T cell leukemia/lymphoma and asymptomatic carriers in comparison to normal group. AIDS Res. Hum. Retroviruses 2025, 41, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Mori, N. FX1, a BCL6 inhibitor, reactivates BCL6 target genes and suppresses HTLV-1-infected T cells. Investig. New Drugs 2022, 40, 245–254. [Google Scholar] [CrossRef]
- Ishikawa, C.; Mori, N. Exportin-1 is critical for cell proliferation and survival in adult T cell leukemia. Investig. New Drugs 2022, 40, 718–727. [Google Scholar] [CrossRef]
- Zhong, W.; Cao, X.; Pan, G.; Niu, Q.; Feng, X.; Xu, M.; Li, M.; Huang, Y.; Yi, Q.; Yan, D. ORP4L is a prerequisite for the induction of T-cell leukemogenesis associated with human T-cell leukemia virus 1. Blood 2022, 139, 1052–1065. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Increased H19/miR-675 expression in adult T-cell leukemia is associated with a unique Notch signature pathway. Int. J. Mol. Sci. 2024, 25, 5130. [Google Scholar] [CrossRef]
- Bellon, M.; Moles, R.; Chaib-Mezrag, H.; Pancewicz, J.; Nicot, C. JAG1 overexpression contributes to Notch1 signaling and the migration of HTLV-1-transformed ATL cells. J. Hematol. Oncol. 2018, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Pancewicz, J.; Taylor, J.M.; Datta, A.; Baydoun, H.H.; Waldmann, T.A.; Hermine, O.; Nicot, C. Notch signaling contributes to proliferation and tumor formation of human T-cell leukemia virus type 1-associated adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 16619–16624. [Google Scholar] [CrossRef]
- Yeh, C.H.; Bellon, M.; Pancewicz-Wojtkiewicz, J.; Nicot, C. Oncogenic mutations in the FBXW7 gene of adult T-cell leukemia patients. Proc. Natl. Acad. Sci. USA 2016, 113, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Yeh, C.h.; Bai, X.T.; Nicot, C. The HTLV-I oncoprotein Tax inactivates the tumor suppressor FBXW7. J. Virol. 2024, 98, e0040524. [Google Scholar] [CrossRef]
- Cheng, W.; Zheng, T.; Wang, Y.; Cai, K.; Wu, W.; Zhao, T.; Xu, R. Activation of Notch1 signaling by HTLV-1 Tax promotes proliferation of adult T-cell leukemia cells. Biochem. Biophys. Res. Commun. 2019, 512, 598–603. [Google Scholar] [CrossRef]
- Miyake-Nishijima, R.; Iwata, S.; Saijo, S.; Kobayashi, H.; Kobayashi, S.; Souta-Kuribara, A.; Hosono, O.; Kawasaki, H.; Tanaka, H.; Ikeda, E.; et al. Role of Crk-associated substrate lymphocyte type in the pathophysiology of rheumatoid arthritis in tax transgenic mice and in humans. Arthritis Rheum. 2003, 48, 1890–1900. [Google Scholar] [CrossRef]
- Katayose, T.; Iwata, S.; Oyaizu, N.; Hosono, O.; Yamada, T.; Dang, N.H.; Hatano, R.; Tanaka, H.; Ohnuma, K.; Morimoto, C. The role of Cas-L/NEDD9 as a regulator of collagen-induced arthritis in a murine model. Biochem. Biophys. Res. Commun. 2015, 460, 1069–1075. [Google Scholar] [CrossRef]
- Ohashi, M.; Sakurai, M.; Higuchi, M.; Mori, N.; Fukushi, M.; Oie, M.; Coffey, R.J.; Yoshiura, K.; Tanaka, Y.; Uchiyama, M.; et al. Human T-cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi-PDZ domain protein, MAGI-3. Virology 2004, 320, 52–62. [Google Scholar] [CrossRef]
- Shoji, T.; Higuchi, M.; Kondo, R.; Takahashi, M.; Oie, M.; Tanaka, Y.; Aoyagi, Y.; Fujii, M. Identification of a novel motif responsible for the distinctive transforming activity of human T-cell leukemia virus (HTLV) type 1 Tax1 protein from HTLV-2 Tax2. Retrovirology 2009, 6, 83. [Google Scholar] [CrossRef]
- Hirata, A.; Higuchi, M.; Niinuma, A.; Ohashi, M.; Fukushi, M.; Oie, M.; Akiyama, T.; Tanaka, Y.; Gejyo, F.; Fujii, M. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology 2004, 318, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Arpin-Andre, C.; Mesnard, J.M. The PDZ domain-binding motif of the human T cell leukemia virus type 1 tax protein induces mislocalization of the tumor suppressor hScrib in T cells. J. Biol. Chem. 2007, 282, 33132–33141. [Google Scholar] [CrossRef]
- Makokha, G.N.; Takahashi, M.; Higuchi, M.; Saito, S.; Tanaka, Y.; Fujii, M. Human T-cell leukemia virus type 1 Tax protein interacts with and mislocalizes the PDZ domain protein MAGI-1. Cancer Sci. 2013, 104, 313–320. [Google Scholar] [CrossRef]
- Tsubata, C.; Higuchi, M.; Takahashi, M.; Oie, M.; Tanaka, Y.; Gejyo, F.; Fujii, M. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein is essential for the interleukin 2 independent growth induction of a T-cell line. Retrovirology 2005, 2, 46. [Google Scholar] [CrossRef]
- Peres, E.; Blin, J.; Ricci, E.P.; Artesi, M.; Hahaut, V.; Van den Broeke, A.; Corbin, A.; Gazzolo, L.; Ratner, L.; Jalinot, P.; et al. PDZ domain-binding motif of Tax sustains T-cell proliferation in HTLV-1-infected humanized mice. PLoS Pathog. 2018, 14, e1006933. [Google Scholar] [CrossRef] [PubMed]
- Cherian, M.A.; Baydoun, H.H.; Al-Saleem, J.; Shkriabai, N.; Kvaratskhelia, M.; Green, P.; Ratner, L. Akt Pathway Activation by Human T-cell Leukemia Virus Type 1 Tax Oncoprotein. J. Biol. Chem. 2015, 290, 26270–26281. [Google Scholar] [CrossRef]
- Adachi, Y.; Kitahara-Ozawa, A.; Sugamura, K.; Lee, W.J.; Yodoi, J.; Maki, M.; Murachi, T.; Hatanaka, M. Expression of calpain II gene in human hematopoietic system cells infected with human T-cell leukemia virus type I. J. Biol. Chem. 1992, 267, 19373–19378. [Google Scholar] [CrossRef] [PubMed]
- Morford, L.A.; Forrest, K.; Logan, B.; Overstreet, L.K.; Goebel, J.; Brooks, W.H.; Roszman, T.L. Calpain II colocalizes with detergent-insoluble rafts on human and Jurkat T-cells. Biochem. Biophys. Res. Commun. 2002, 295, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef]
- Ohtani, K. Implication of transcription factor E2F in regulation of DNA replication. Front. Biosci. 1999, 4, D793–D804. [Google Scholar] [CrossRef]
- Zhou, Y.; Nakajima, R.; Shirasawa, M.; Fikriyanti, M.; Zhao, L.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Expanding Roles of the E2F-RB-p53 Pathway in Tumor Suppression. Biology 2023, 12, 1511. [Google Scholar] [CrossRef]
- Kent, L.N.; Leone, G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer 2019, 19, 326–338. [Google Scholar] [CrossRef]
- Iwanaga, R.; Ohtani, K.; Hayashi, T.; Nakamura, M. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 2001, 20, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.; Clark, E.; Chong, S.; Molina, C.; Mozafari, F.; Mahieux, R.; Fujii, M.; Azimi, N.; Kashanchi, F. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J. Virol. 1999, 73, 9917–9927. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Fujii, M.; Hinz, M.; Nakayama, K.; Yamada, Y.; Ikeda, S.; Yamasaki, Y.; Kashanchi, F.; Tanaka, Y.; Tomonaga, M.; et al. Activation of cyclin D1 and D2 promoters by human T-cell leukemia virus type I tax protein is associated with IL-2-independent growth of T cells. Int. J. Cancer 2002, 99, 378–385. [Google Scholar] [CrossRef]
- Lemasson, I.; Thebault, S.; Sardet, C.; Devaux, C.; Mesnard, J.M. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16INK4A-negative T-cell line. J. Biol. Chem. 1998, 273, 23598–23604. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ohtani, K.; Iwanaga, R.; Matsumura, Y.; Nakamura, M. Direct trans-activation of the human cyclin D2 gene by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 2001, 20, 1094–1102. [Google Scholar] [CrossRef]
- Iwanaga, R.; Ozono, E.; Fujisawa, J.; Ikeda, M.A.; Okamura, N.; Huang, Y.; Ohtani, K. Activation of the cyclin D2 and cdk6 genes through NF-κB is critical for cell-cycle progression induced by HTLV-I Tax. Oncogene 2008, 27, 5635–5642. [Google Scholar] [CrossRef]
- Fisher, R.P. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J. Cell Sci. 2005, 118, 5171–5180. [Google Scholar] [CrossRef]
- Fisher, R.P.; Morgan, D.O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 1994, 78, 713–724. [Google Scholar] [CrossRef]
- Yee, A.; Nichols, M.A.; Wu, L.; Hall, F.L.; Kobayashi, R.; Xiong, Y. Molecular cloning of CDK7-associated human MAT1, a cyclin-dependent kinase-activating kinase (CAK) assembly factor. Cancer Res. 1995, 55, 6058–6062. [Google Scholar]
- Akhtar, M.S.; Heidemann, M.; Tietjen, J.R.; Zhang, D.W.; Chapman, R.D.; Eick, D.; Ansari, A.Z. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol. Cell 2009, 34, 387–393. [Google Scholar] [CrossRef]
- Glover-Cutter, K.; Larochelle, S.; Erickson, B.; Zhang, C.; Shokat, K.; Fisher, R.P.; Bentley, D.L. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol. Cell Biol. 2009, 29, 5455–5464. [Google Scholar] [CrossRef]
- Shirasawa, M.; Nakajima, R.; Zhou, Y.; Zhao, L.; Fikriyanti, M.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Activation of the CDK7 gene, coding for the catalytic subunit of the cyclin-dependent kinase (CDK)-activating kinase (CAK) and general transcription factor II H, by the trans-activator protein Tax of Human T-Cell Leukemia Virus yype-1. Genes 2024, 15, 1080. [Google Scholar] [CrossRef]
- Fujinaga, K.; Huang, F.; Peterlin, B.M. P-TEFb: The master regulator of transcription elongation. Mol. Cell 2023, 83, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Strobel, S.; Fleckenstein, B.; Kress, A.K. The transcription elongation factor ELL2 is specifically upregulated in HTLV-1-infected T-cells and is dependent on the viral oncoprotein Tax. Virology 2014, 464–465, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, S.; Strobel, S.; Mann, M.C.; Sticht, H.; Fleckenstein, B.; Thoma-Kress, A.K. Characterizing the Interaction between the HTLV-1 transactivator Tax-1 with transcription elongation factor ELL2 and its impact on viral transactivation. Int. J. Mol. Sci. 2021, 22, 13597. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Ono, H.; Shimotohno, K. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: Possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene 1996, 12, 1645–1652. [Google Scholar]
- de La Fuente, C.; Deng, L.; Santiago, F.; Arce, L.; Wang, L.; Kashanchi, F. Gene expression array of HTLV type 1-infected T cells: Up-regulation of transcription factors and cell cycle genes. AIDS Res. Hum. Retroviruses 2000, 16, 1695–1700. [Google Scholar] [CrossRef]
- de La Fuente, C.; Santiago, F.; Chong, S.Y.; Deng, L.; Mayhood, T.; Fu, P.; Stein, D.; Denny, T.; Coffman, F.; Azimi, N.; et al. Overexpression of p21waf1 in human T-cell lymphotropic virus type 1-infected cells and its association with cyclin A/cdk2. J. Virol. 2000, 74, 7270–7283. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Farhadi, A.; Wang, X.F.; Robb, M.L.; Birx, D.L.; Kim, J.H. Human T-cell leukemia virus type 1 Tax activates cyclin-dependent kinase inhibitor p21/Waf1/Cip1 expression through a p53-independent mechanism: Inhibition of cdk2. Int. J. Cancer 2003, 107, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhi, H.; Liu, M.; Kuo, Y.L.; Giam, C.Z. Induction of p21CIP1/WAF1 expression by human T-lymphotropic virus type 1 Tax requires transcriptional activation and mRNA stabilization. Retrovirology 2009, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- LaBaer, J.; Garrett, M.D.; Stevenson, L.F.; Slingerland, J.M.; Sandhu, C.; Chou, H.S.; Fattaey, A.; Harlow, E. New functional activities for the p21 family of CDK inhibitors. Genes. Dev. 1997, 11, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Olivier, P.; Diehl, J.A.; Fero, M.; Roussel, M.F.; Roberts, J.M.; Sherr, C.J. The p21Cip1 and p27Kip1 CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999, 18, 1571–1583. [Google Scholar] [CrossRef]
- Kehn, K.; Deng, L.; de la Fuente, C.; Strouss, K.; Wu, K.; Maddukuri, A.; Baylor, S.; Rufner, R.; Pumfery, A.; Bottazzi, M.E.; et al. The role of cyclin D2 and p21/waf1 in human T-cell leukemia virus type 1 infected cells. Retrovirology 2004, 1, 6. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakahata, S.; Hamasaki, M.; Saito, Y.; Kawano, Y.; Hidaka, T.; Yamashita, K.; Umeki, K.; Taki, T.; Taniwaki, M.; et al. Downregulation of CDKN1A in adult T-cell leukemia/lymphoma despite overexpression of CDKN1A in human T-lymphotropic virus 1-infected cell lines. J. Virol. 2010, 84, 6966–6977. [Google Scholar] [CrossRef]
- Haller, K.; Ruckes, T.; Schmitt, I.; Saul, D.; Derow, E.; Grassmann, R. Tax-dependent stimulation of G1 phase-specific cyclin-dependent kinases and increased expression of signal transduction genes characterize HTLV type 1-transformed T cells. AIDS Res. Hum. Retroviruses 2000, 16, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Haller, K.; Wu, Y.; Derow, E.; Schmitt, I.; Jeang, K.T.; Grassmann, R. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell Biol. 2002, 22, 3327–3338. [Google Scholar] [CrossRef]
- Kehn, K.; Fuente Cde, L.; Strouss, K.; Berro, R.; Jiang, H.; Brady, J.; Mahieux, R.; Pumfery, A.; Bottazzi, M.E.; Kashanchi, F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene 2005, 24, 525–540. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitao, S.; Matsushime, H.; Yoshida, M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996, 15, 1607–1614. [Google Scholar] [CrossRef]
- Low, K.G.; Dorner, L.F.; Fernando, D.B.; Grossman, J.; Jeang, K.T.; Comb, M.J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J. Virol. 1997, 71, 1956–1962. [Google Scholar] [CrossRef]
- Suzuki, T.; Narita, T.; Uchida-Toita, M.; Yoshida, M. Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology 1999, 259, 384–391. [Google Scholar] [CrossRef]
- Lemoine, F.J.; Marriott, S.J. Accelerated G1 phase progression induced by the human T cell leukemia virus type I (HTLV-I) Tax oncoprotein. J. Biol. Chem. 2001, 276, 31851–31857. [Google Scholar] [CrossRef]
- Liang, M.H.; Geisbert, T.; Yao, Y.; Hinrichs, S.H.; Giam, C.Z. Human T-lymphotropic virus type 1 oncoprotein tax promotes S-phase entry but blocks mitosis. J. Virol. 2002, 76, 4022–4033. [Google Scholar] [CrossRef]
- Liu, B.; Hong, S.; Tang, Z.; Yu, H.; Giam, C.Z. HTLV-I Tax directly binds the Cdc20-associated anaphase-promoting complex and activates it ahead of schedule. Proc. Natl. Acad. Sci. USA 2005, 102, 63–68. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Good, L.; Xiao, G.; Sun, S.C. Gene expression profiles in HTLV-I-immortalized T cells: Deregulated expression of genes involved in apoptosis regulation. Oncogene 1999, 18, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Kannagi, M.; Ohashi, T.; Kato, H.; Arai, M.; Nunez, G.; Iwanaga, Y.; Yamamoto, N.; Ohtani, K.; Nakamura, M.; et al. Induction of Bcl-xL expression by human T-cell leukemia virus type 1 Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 1999, 73, 7981–7987. [Google Scholar] [CrossRef]
- Nicot, C.; Mahieux, R.; Takemoto, S.; Franchini, G. Bcl-XL is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood 2000, 96, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mathews Griner, L.A.; Ju, W.; Duveau, D.Y.; Guha, R.; Petrus, M.N.; Wen, B.; Maeda, M.; Shinn, P.; Ferrer, M.; et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 2015, 112, 12480–12485. [Google Scholar] [CrossRef]
- Macaire, H.; Riquet, A.; Moncollin, V.; Biemont-Trescol, M.C.; Duc Dodon, M.; Hermine, O.; Debaud, A.L.; Mahieux, R.; Mesnard, J.M.; Pierre, M.; et al. Tax protein-induced expression of antiapoptotic Bfl-1 protein contributes to survival of human T-cell leukemia virus type 1 (HTLV-1)-infected T-cells. J. Biol. Chem. 2012, 287, 21357–21370. [Google Scholar] [CrossRef] [PubMed]
- Waldele, K.; Silbermann, K.; Schneider, G.; Ruckes, T.; Cullen, B.R.; Grassmann, R. Requirement of the human T-cell leukemia virus (HTLV-1) tax-stimulated HIAP-1 gene for the survival of transformed lymphocytes. Blood 2006, 107, 4491–4499. [Google Scholar] [CrossRef]
- Zane, L.; Sibon, D.; Legras, C.; Lachuer, J.; Wierinckx, A.; Mehlen, P.; Delfau-Larue, M.H.; Gessain, A.; Gout, O.; Pinatel, C.; et al. Clonal expansion of HTLV-1 positive CD8+ cells relies on cIAP-2 but not on c-FLIP expression. Virology 2010, 407, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Yamada, Y.; Hata, T.; Ikeda, S.; Yamasaki, Y.; Tomonaga, M.; Yamamoto, N. Expression of survivin in HTLV-I-infected T-cell lines and primary ATL cells. Biochem. Biophys. Res. Commun. 2001, 282, 1110–1113. [Google Scholar] [CrossRef]
- Kamihira, S.; Yamada, Y.; Hirakata, Y.; Tomonaga, M.; Sugahara, K.; Hayashi, T.; Dateki, N.; Harasawa, H.; Nakayama, K. Aberrant expression of caspase cascade regulatory genes in adult T-cell leukaemia: Survivin is an important determinant for prognosis. Br. J. Haematol. 2001, 114, 63–69. [Google Scholar] [CrossRef]
- Kawakami, H.; Tomita, M.; Matsuda, T.; Ohta, T.; Tanaka, Y.; Fujii, M.; Hatano, M.; Tokuhisa, T.; Mori, N. Transcriptional activation of survivin through the NF-κB pathway by human T-cell leukemia virus type I tax. Int. J. Cancer 2005, 115, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.; Fas, S.C.; Giaisi, M.; Bleumink, M.; Merling, A.; Stumpf, C.; Baumann, S.; Holtkotte, D.; Bosch, V.; Krammer, P.H.; et al. HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP). Blood 2006, 107, 3933–3939. [Google Scholar] [CrossRef]
- Okamoto, K.; Fujisawa, J.; Reth, M.; Yonehara, S. Human T-cell leukemia virus type-I oncoprotein Tax inhibits Fas-mediated apoptosis by inducing cellular FLIP through activation of NF-κB. Genes. Cells 2006, 11, 177–191. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Shi, J.; Zhang, Y.; Liu, S.; Liu, Y.; Zheng, D. Human T-cell leukemia virus type 1 Tax-deregulated autophagy pathway and c-FLIP expression contribute to resistance against death receptor-mediated apoptosis. J. Virol. 2014, 88, 2786–2798. [Google Scholar] [CrossRef]
- Daniel, S.; Arvelo, M.B.; Patel, V.I.; Longo, C.R.; Shrikhande, G.; Shukri, T.; Mahiou, J.; Sun, D.W.; Mottley, C.; Grey, S.T.; et al. A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation. Blood 2004, 104, 2376–2384. [Google Scholar] [CrossRef]
- Laherty, C.D.; Perkins, N.D.; Dixit, V.M. Human T cell leukemia virus type I Tax and phorbol 12-myristate 13-acetate induce expression of the A20 zinc finger protein by distinct mechanisms involving nuclear factor κB. J. Biol. Chem. 1993, 268, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Hamano, A.; Mochida, K.; Kakeya, A.; Uno, M.; Tsuruyama, E.; Ichikawa, H.; Tokunaga, F.; Utsunomiya, A.; Watanabe, T.; et al. A20 targets caspase-8 and FADD to protect HTLV-I-infected cells. Leukemia 2016, 30, 716–727. [Google Scholar] [CrossRef]
- Choi, Y.B.; Harhaj, E.W. HTLV-1 tax stabilizes MCL-1 via TRAF6-dependent K63-linked polyubiquitination to promote cell survival and transformation. PLoS Pathog. 2014, 10, e1004458. [Google Scholar] [CrossRef]
- Chuang, S.E.; Doong, S.L.; Lin, M.T.; Cheng, A.L. Tax of the human T-lymphotropic virus type I transactivates promoter of the MDR-1 gene. Biochem. Biophys. Res. Commun. 1997, 238, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Gant, T.W.; Cann, A.J. The mechanism of trans-activation of the MDR1 gene by human T-cell leukemia virus. Biochem. Biophys. Res. Commun. 1998, 249, 397–404. [Google Scholar] [CrossRef]
- Lau, A.; Nightingale, S.; Taylor, G.P.; Gant, T.W.; Cann, A.J. Enhanced MDR1 gene expression in human T-cell leukemia virus-I-infected patients offers new prospects for therapy. Blood 1998, 91, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, Y.; Terashi, K.; Yamaguchi, A.; Kawamata, N.; Tokito, Y.; Mori, H.; Umehara, M.; Yoshiyama, T.; Ohtsubo, H.; Arimura, K.; et al. Human T-cell lymphotropic virus type I Tax activates lung resistance-related protein expression in leukemic clones established from an adult T-cell leukemia patient. Exp. Hematol. 2002, 30, 340–345. [Google Scholar] [CrossRef]
- Chlichlia, K.; Busslinger, M.; Peter, M.E.; Walczak, H.; Krammer, P.H.; Schirrmacher, V.; Khazaie, K. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene 1997, 14, 2265–2272. [Google Scholar] [CrossRef]
- Rivera-Walsh, I.; Waterfield, M.; Xiao, G.; Fong, A.; Sun, S.C. NF-κB signaling pathway governs TRAIL gene expression and human T-cell leukemia virus-I Tax-induced T-cell death. J. Biol. Chem. 2001, 276, 40385–40388. [Google Scholar] [CrossRef]
- Gabet, A.S.; Mortreux, F.; Charneau, P.; Riou, P.; Duc-Dodon, M.; Wu, Y.; Jeang, K.T.; Wattel, E. Inactivation of hTERT transcription by Tax. Oncogene 2003, 22, 3734–3741. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Datta, U.; Horikawa, I.; Michishita, E.; Datta, A.; Sigler-Nicot, J.C.; Brown, M.; Kazanji, M.; Barrett, J.C.; Nicot, C. Transcriptional activation of hTERT through the NF-κB pathway in HTLV-I-transformed cells. Blood 2004, 104, 2523–2531. [Google Scholar] [CrossRef]
- Matsumura-Arioka, Y.; Ohtani, K.; Hara, T.; Iwanaga, R.; Nakamura, M. Identification of two distinct elements mediating activation of telomerase (hTERT) gene expression in association with cell growth in human T cells. Int. Immunol. 2005, 17, 207–215. [Google Scholar] [CrossRef]
- Hara, T.; Matsumura-Arioka, Y.; Ohtani, K.; Nakamura, M. Role of human T-cell leukemia virus type I Tax in expression of the human telomerase reverse transcriptase (hTERT) gene in human T-cells. Cancer Sci. 2008, 99, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Zane, L.; Sibon, D.; Capraro, V.; Galia, P.; Karam, M.; Delfau-Larue, M.H.; Gilson, E.; Gessain, A.; Gout, O.; Hermine, O.; et al. HTLV-1 positive and negative T cells cloned from infected individuals display telomerase and telomere genes deregulation that predominate in activated but untransformed CD4+ T cells. Int. J. Cancer 2012, 131, 821–833. [Google Scholar] [CrossRef]
- Bellon, M.; Yuan, Y.; Nicot, C. Transcription independent stimulation of telomerase enzymatic activity by HTLV-I Tax through stimulation of IKK. J. Cancer Sci. 2021, 8. [Google Scholar] [CrossRef]
- Miyake, H.; Suzuki, T.; Hirai, H.; Yoshida, M. Trans-activator Tax of human T-cell leukemia virus type 1 enhances mutation frequency of the cellular genome. Virology 1999, 253, 155–161. [Google Scholar] [CrossRef]
- Philpott, S.M.; Buehring, G.C. Defective DNA repair in cells with human T-cell leukemia/bovine leukemia viruses: Role of tax gene. J. Natl. Cancer Inst. 1999, 91, 933–942. [Google Scholar] [CrossRef]
- Lemoine, F.J.; Marriott, S.J. Genomic instability driven by the human T-cell leukemia virus type I (HTLV-I) oncoprotein, Tax. Oncogene 2002, 21, 7230–7234. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, H.H.; Bai, X.T.; Shelton, S.; Nicot, C. HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS ONE 2012, 7, e42226. [Google Scholar] [CrossRef] [PubMed]
- Ressler, S.; Morris, G.F.; Marriott, S.J. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 1997, 71, 1181–1190. [Google Scholar] [CrossRef]
- Kao, S.Y.; Marriott, S.J. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 1999, 73, 4299–4304. [Google Scholar] [CrossRef]
- Lemoine, F.J.; Kao, S.Y.; Marriott, S.J. Suppression of DNA repair by HTLV type 1 Tax correlates with Tax trans-activation of proliferating cell nuclear antigen gene expression. AIDS Res. Hum. Retroviruses 2000, 16, 1623–1627. [Google Scholar] [CrossRef]
- Edwards, D.C.; Marriott, S.J. Human T-cell leukemia virus type 1 Tax relieves repression of proliferating cell nuclear antigen gene expression. J. Virol. 2008, 82, 11714–11722. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Nunokawa, Y.; Yamada, Y.; Ikeda, S.; Tomonaga, M.; Yamamoto, N. Expression of human inducible nitric oxide synthase gene in T-cell lines infected with human T-cell leukemia virus type-I and primary adult T-cell leukemia cells. Blood 1999, 94, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, T.; Matsuzaki, H.; Nagasaki, A.; Hata, H.; Yoshida, M.; Matsuoka, M.; Kuribayashi, N.; Kimura, T.; Harada, N.; Takatsuki, K.; et al. Detection of inducible nitric oxide synthase (iNOS) mRNA by RT-PCR in ATL patients and HTLV-I infected cell lines: Clinical features and apoptosis by NOS inhibitor. Leukemia 1999, 13, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Nakamura, T.; Shirabe, S.; Ueki, Y.; Nishiura, Y.; Furuya, T.; Tsujino, A.; Nakane, S.; Eguchi, K.; Nagataki, S. Up-regulation of iNOS mRNA expression and increased production of NO in human monoblast cell line, U937 transfected by HTLV-I tax gene. Immunobiology 1997, 197, 513–521. [Google Scholar] [CrossRef]
- Chaib-Mezrag, H.; Lemacon, D.; Fontaine, H.; Bellon, M.; Bai, X.T.; Drac, M.; Coquelle, A.; Nicot, C. Tax impairs DNA replication forks and increases DNA breaks in specific oncogenic genome regions. Mol. Cancer 2014, 13, 205. [Google Scholar] [CrossRef]
- Baydoun, H.H.; Cherian, M.A.; Green, P.; Ratner, L. Inducible nitric oxide synthase mediates DNA double strand breaks in human T-cell leukemia virus type 1-induced leukemia/lymphoma. Retrovirology 2015, 12, 71. [Google Scholar] [CrossRef]
- Kinjo, T.; Ham-Terhune, J.; Peloponese, J.M., Jr.; Jeang, K.T. Induction of reactive oxygen species by human T-cell leukemia virus type 1 tax correlates with DNA damage and expression of cellular senescence marker. J. Virol. 2010, 84, 5431–5437. [Google Scholar] [CrossRef]
- Takahashi, M.; Higuchi, M.; Makokha, G.N.; Matsuki, H.; Yoshita, M.; Tanaka, Y.; Fujii, M. HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 2013, 122, 715–725. [Google Scholar] [CrossRef]
- Marriott, S.J.; Semmes, O.J. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 2005, 24, 5986–5995. [Google Scholar] [CrossRef]
- He, Y.; Pasupala, N.; Zhi, H.; Dorjbal, B.; Hussain, I.; Shih, H.M.; Bhattacharyya, S.; Biswas, R.; Miljkovic, M.; Semmes, O.J.; et al. NF-κB-induced R-loop accumulation and DNA damage select for nucleotide excision repair deficiencies in adult T cell leukemia. Proc. Natl. Acad. Sci. USA 2021, 118, e2005568118. [Google Scholar] [CrossRef] [PubMed]
- Giam, C.Z.; Pasupala, N. NF-κB-induced R-loops and genomic instability in HTLV-1-infected and adult T-cell leukemia cells. Viruses 2022, 14, 877. [Google Scholar] [CrossRef]
- Dayaram, T.; Lemoine, F.J.; Donehower, L.A.; Marriott, S.J. Activation of WIP1 phosphatase by HTLV-1 Tax mitigates the cellular response to DNA damage. PLoS ONE 2013, 8, e55989. [Google Scholar] [CrossRef]
- Zane, L.; Yasunaga, J.; Mitagami, Y.; Yedavalli, V.; Tang, S.W.; Chen, C.Y.; Ratner, L.; Lu, X.; Jeang, K.T. Wip1 and p53 contribute to HTLV-1 Tax-induced tumorigenesis. Retrovirology 2012, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Haoudi, A.; Daniels, R.C.; Wong, E.; Kupfer, G.; Semmes, O.J. Human T-cell leukemia virus-I tax oncoprotein functionally targets a subnuclear complex involved in cellular DNA damage-response. J. Biol. Chem. 2003, 278, 37736–37744. [Google Scholar] [CrossRef] [PubMed]
- Chandhasin, C.; Ducu, R.I.; Berkovich, E.; Kastan, M.B.; Marriott, S.J. Human T-cell leukemia virus type 1 tax attenuates the ATM-mediated cellular DNA damage response. J. Virol. 2008, 82, 6952–6961. [Google Scholar] [CrossRef]
- Park, H.U.; Jeong, J.H.; Chung, J.H.; Brady, J.N. Human T-cell leukemia virus type 1 Tax interacts with Chk1 and attenuates DNA-damage induced G2 arrest mediated by Chk1. Oncogene 2004, 23, 4966–4974. [Google Scholar] [CrossRef]
- Park, H.U.; Jeong, S.J.; Jeong, J.H.; Chung, J.H.; Brady, J.N. Human T-cell leukemia virus type 1 Tax attenuates γ-irradiation-induced apoptosis through physical interaction with Chk2. Oncogene 2006, 25, 438–447. [Google Scholar] [CrossRef]
- Boxus, M.; Twizere, J.C.; Legros, S.; Kettmann, R.; Willems, L. Interaction of HTLV-1 Tax with minichromosome maintenance proteins accelerates the replication timing program. Blood 2012, 119, 151–160. [Google Scholar] [CrossRef]
- Suzuki, T.; Uchida-Toita, M.; Andoh, T.; Yoshida, M. HTLV-1 tax oncoprotein binds to DNA topoisomerase I and inhibits its catalytic activity. Virology 2000, 270, 291–298. [Google Scholar] [CrossRef]
- Yoshida, M.; Suzuki, T. HTLV type 1 Tax oncoprotein binds to DNA topoisomerase I and inhibits its catalytic activity. AIDS Res. Hum. Retroviruses 2000, 16, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Guo, X.; Ho, Y.K.; Pasupala, N.; Engstrom, H.A.A.; Semmes, O.J.; Giam, C.Z. RNF8 dysregulation and down-regulation during HTLV-1 infection promote genomic instability in adult T-cell leukemia. PLoS Pathog. 2020, 16, e1008618. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tomari, Y. RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochim. Biophys. Acta 2016, 1859, 71–81. [Google Scholar] [CrossRef]
- Michlewski, G.; Caceres, J.F. Post-transcriptional control of miRNA biogenesis. RNA 2019, 25, 1–16. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, K.; Corradin, A.; Zanovello, P.; Amadori, A.; Bronte, V.; Ciminale, V.; D’Agostino, D.M. Role of microRNAs in HTLV-1 infection and transformation. Mol. Asp. Med. 2010, 31, 367–382. [Google Scholar] [CrossRef]
- Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010, 17, 193–199. [Google Scholar] [CrossRef]
- Misso, G.; Di Martino, M.T.; De Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gulla, A.; Tagliaferri, P.; Tassone, P.; et al. Mir-34: A new weapon against cancer? Mol. Ther. Nucleic Acids 2014, 3, e194. [Google Scholar] [CrossRef]
- Schmid, G.; Notaro, S.; Reimer, D.; Abdel-Azim, S.; Duggan-Peer, M.; Holly, J.; Fiegl, H.; Rossler, J.; Wiedemair, A.; Concin, N.; et al. Expression and promotor hypermethylation of miR-34a in the various histological subtypes of ovarian cancer. BMC Cancer 2016, 16, 102. [Google Scholar] [CrossRef]
- Sharma, V.K.; Raimondi, V.; Ruggero, K.; Pise-Masison, C.A.; Cavallari, I.; Silic-Benussi, M.; Ciminale, V.; D’Agostino, D.M. Expression of miR-34a in T-cells infected by human T-lymphotropic virus 1. Front. Microbiol. 2018, 9, 832. [Google Scholar] [CrossRef]
- Pichler, K.; Schneider, G.; Grassmann, R. MicroRNA miR-146a and further oncogenesis-related cellular microRNAs are dysregulated in HTLV-1-transformed T lymphocytes. Retrovirology 2008, 5, 100. [Google Scholar] [CrossRef]
- Tomita, M.; Tanaka, Y.; Mori, N. MicroRNA miR-146a is induced by HTLV-1 tax and increases the growth of HTLV-1-infected T-cells. Int. J. Cancer 2012, 130, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kong, X.; Lv, L.; Gao, J. MiR-155 targets TP53INP1 to regulate liver cancer stem cell acquisition and self-renewal. FEBS Lett. 2015, 589, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.L.; Yasunaga, J.; Bennasser, Y.; Dusetti, N.; Harris, D.; Ahmad, N.; Matsuoka, M.; Jeang, K.T. Roles for microRNAs, miR-93 and miR-130b, and tumor protein 53-induced nuclear protein 1 tumor suppressor in cell growth dysregulation by human T-cell lymphotrophic virus 1. Cancer Res. 2008, 68, 8976–8985. [Google Scholar] [CrossRef]
- Van Duyne, R.; Guendel, I.; Klase, Z.; Narayanan, A.; Coley, W.; Jaworski, E.; Roman, J.; Popratiloff, A.; Mahieux, R.; Kehn-Hall, K.; et al. Localization and sub-cellular shuttling of HTLV-1 tax with the miRNA machinery. PLoS ONE 2012, 7, e40662. [Google Scholar] [CrossRef]
- Yamagishi, M.; Fujikawa, D.; Watanabe, T.; Uchimaru, K. HTLV-1-mediated epigenetic pathway to adult T-cell leukemia-lymphoma. Front. Microbiol. 2018, 9, 1686. [Google Scholar] [CrossRef] [PubMed]
- Ratner, L. Epigenetic regulation of human T-cell leukemia virus gene expression. Microorganisms 2021, 10, 84. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamashita, S.; Ureshino, H.; Kamachi, K.; Kurahashi, Y.; Fukuda-Kurahashi, Y.; Yoshida, N.; Hattori, N.; Nakamura, H.; Sato, A.; et al. Targeting aberrant DNA hypermethylation as a driver of ATL leukemogenesis by using the new oral demethylating agent OR-2100. Blood 2020, 136, 871–884. [Google Scholar] [CrossRef]
- Nosaka, K.; Maeda, M.; Tamiya, S.; Sakai, T.; Mitsuya, H.; Matsuoka, M. Increasing methylation of the CDKN2A gene is associated with the progression of adult T-cell leukemia. Cancer Res. 2000, 60, 1043–1048. [Google Scholar]
- Taniguchi, A.; Nemoto, Y.; Yokoyama, A.; Kotani, N.; Imai, S.; Shuin, T.; Daibata, M. Promoter methylation of the bone morphogenetic protein-6 gene in association with adult T-cell leukemia. Int. J. Cancer 2008, 123, 1824–1831. [Google Scholar] [CrossRef]
- Yasunaga, J.; Taniguchi, Y.; Nosaka, K.; Yoshida, M.; Satou, Y.; Sakai, T.; Mitsuya, H.; Matsuoka, M. Identification of aberrantly methylated genes in association with adult T-cell leukemia. Cancer Res. 2004, 64, 6002–6009. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Gao, W.W.; Chan, C.P.; Cheng, Y.; Deng, J.J.; Yuen, K.S.; Iha, H.; Jin, D.Y. SIRT1 Suppresses Human T-Cell Leukemia Virus Type 1 Transcription. J. Virol. 2015, 89, 8623–8631. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, K.; Yamamoto, K.; Misawa, A.; Miyake, A.; Ishida, T.; Tanaka, Y.; Mochizuki, M.; Watanabe, T. SUV39H1 interacts with HTLV-1 Tax and abrogates Tax transactivation of HTLV-1 LTR. Retrovirology 2006, 3, 5. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishida, T.; Nakano, K.; Yamagishi, M.; Yamochi, T.; Tanaka, Y.; Furukawa, Y.; Nakamura, Y.; Watanabe, T. SMYD3 interacts with HTLV-1 Tax and regulates subcellular localization of Tax. Cancer Sci. 2011, 102, 260–266. [Google Scholar] [CrossRef]
- Satou, Y.; Miyazato, P.; Ishihara, K.; Yaguchi, H.; Melamed, A.; Miura, M.; Fukuda, A.; Nosaka, K.; Watanabe, T.; Rowan, A.G.; et al. The retrovirus HTLV-1 inserts an ectopic CTCF-binding site into the human genome. Proc. Natl. Acad. Sci. USA 2016, 113, 3054–3059. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Joseph, A.; Castro, V.; Chen-Liaw, A.; Skidmore, Z.; Ueno, T.; Fujisawa, J.I.; Rauch, D.A.; Challen, G.A.; Martinez, M.P.; et al. Epigenomic regulation of human T-cell leukemia virus by chromatin-insulator CTCF. PLoS Pathog. 2021, 17, e1009577. [Google Scholar] [CrossRef]
- Melamed, A.; Yaguchi, H.; Miura, M.; Witkover, A.; Fitzgerald, T.W.; Birney, E.; Bangham, C.R. The human leukemia virus HTLV-1 alters the structure and transcription of host chromatin in cis. Elife 2018, 7, e36245. [Google Scholar] [CrossRef]
- Yaguchi, H.; Melamed, A.; Ramanayake, S.; Kiik, H.; Witkover, A.; Bangham, C.R.M. The impact of HTLV-1 expression on the 3D structure and expression of host chromatin. PLoS Pathog. 2024, 20, e1011716. [Google Scholar] [CrossRef]
- Ego, T.; Tanaka, Y.; Shimotohno, K. Interaction of HTLV-1 Tax and methyl-CpG-binding domain 2 positively regulates the gene expression from the hypermethylated LTR. Oncogene 2005, 24, 1914–1923. [Google Scholar] [CrossRef]
- Rank, G.; Cerruti, L.; Simpson, R.J.; Moritz, R.L.; Jane, S.M.; Zhao, Q. Identification of a PRMT5-dependent repressor complex linked to silencing of human fetal globin gene expression. Blood 2010, 116, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Panfil, A.R.; Al-Saleem, J.; Howard, C.M.; Mates, J.M.; Kwiek, J.J.; Baiocchi, R.A.; Green, P.L. PRMT5 is upregulated in HTLV-1-mediated T-cell transformation and selective inhibition alters viral gene expression and infected cell survival. Viruses 2015, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Ernzen, K.; Melvin, C.; Yu, L.; Phelps, C.; Niewiesk, S.; Green, P.L.; Panfil, A.R. The PRMT5 inhibitor EPZ015666 is effective against HTLV-1-transformed T-cell lines in vitro and in vivo. Front. Microbiol. 2023, 14, 1101544. [Google Scholar] [CrossRef]
- Fujikawa, D.; Nakagawa, S.; Hori, M.; Kurokawa, N.; Soejima, A.; Nakano, K.; Yamochi, T.; Nakashima, M.; Kobayashi, S.; Tanaka, Y.; et al. Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood 2016, 127, 1790–1802. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, K.; Wang, B.; Chu, K.; Liu, J.; Zhang, J.; Fang, J.; Zhao, T. EZH2 activates HTLV-1 bZIP factor-mediated TGF-β signaling in adult T-cell leukemia. J. Med. Virol. 2024, 96, e70025. [Google Scholar] [CrossRef]
- Ichikawa, T.; Nakahata, S.; Fujii, M.; Iha, H.; Shimoda, K.; Morishita, K. The regulation of NDRG2 expression during ATLL development after HTLV-1 infection. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2633–2646. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lu, H.; Cho, W.K.; Park, H.U.; Pise-Masison, C.; Brady, J.N. Coactivator-associated arginine methyltransferase 1 enhances transcriptional activity of the human T-cell lymphotropic virus type 1 long terminal repeat through direct interaction with Tax. J. Virol. 2006, 80, 10036–10044. [Google Scholar] [CrossRef]
- Hayati, R.F.; Nakajima, R.; Zhou, Y.; Shirasawa, M.; Zhao, L.; Fikriyanti, M.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; et al. Trans-activation of the coactivator-associated arginine methyltransferase 1 (Carm1) gene by the oncogene product Tax of human T-cell leukemia virus type 1. Genes 2024, 15, 698. [Google Scholar] [CrossRef]
- Furukawa, K.; Akagi, T.; Nagata, Y.; Yamada, Y.; Shimotohno, K.; Cheung, N.K.; Shiku, H.; Furukawa, K. GD2 ganglioside on human T-lymphotropic virus type I-infected T cells: Possible activation of β-1,4-N-acetylgalactosaminyltransferase gene by p40tax. Proc. Natl. Acad. Sci. USA 1993, 90, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Yodoi, J.; Tursz, T. ADF, a growth-promoting factor derived from adult T cell leukemia and homologous to thioredoxin: Involvement in lymphocyte immortalization by HTLV-I and EBV. Adv. Cancer Res. 1991, 57, 381–411. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.; Lundman, P.; Carlsson, M.; Bhavani, K.; Srinivasa, B.R.; Kjellstrom, G.; Nilsson, K.; Holmgren, A. A CD4+ T cell line-secreted factor, growth promoting for normal and leukemic B cells, identified as thioredoxin. Int. Immunol. 1995, 7, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, N.; Tagaya, Y.; Wakasugi, H.; Mitsui, A.; Maeda, M.; Yodoi, J.; Tursz, T. Adult T-cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc. Natl. Acad. Sci. USA 1990, 87, 8282–8286. [Google Scholar] [CrossRef] [PubMed]
- Los, M.; Khazaie, K.; Schulze-Osthoff, K.; Baeuerle, P.A.; Schirrmacher, V.; Chlichlia, K. Human T cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J. Immunol. 1998, 161, 3050–3055. [Google Scholar] [CrossRef]
- Masutani, H.; Hirota, K.; Sasada, T.; Ueda-Taniguchi, Y.; Taniguchi, Y.; Sono, H.; Yodoi, J. Transactivation of an inducible anti-oxidative stress protein, human thioredoxin by HTLV-I Tax. Immunol. Lett. 1996, 54, 67–71. [Google Scholar] [CrossRef]
- Ishikawa, C.; Mori, N. Pivotal role of dihydroorotate dehydrogenase as a therapeutic target in adult T-cell leukemia. Eur. J. Haematol. 2024, 113, 99–109. [Google Scholar] [CrossRef]
- Fukudome, K.; Furuse, M.; Fukuhara, N.; Orita, S.; Imai, T.; Takagi, S.; Nagira, M.; Hinuma, Y.; Yoshie, O. Strong induction of ICAM-1 in human T cells transformed by human T-cell-leukemia virus type 1 and depression of ICAM-1 or LFA-1 in adult T-cell-leukemia-derived cell lines. Int. J. Cancer 1992, 52, 418–427. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hayashi, M.; Takagi, S.; Yoshie, O. Differential transactivation of the intercellular adhesion molecule 1 gene promoter by Tax1 and Tax2 of human T-cell leukemia viruses. J. Virol. 1996, 70, 8508–8517. [Google Scholar] [CrossRef]
- Owen, S.M.; Rudolph, D.L.; Dezzutti, C.S.; Shibata, N.; Naik, S.; Caughman, S.W.; Lal, R.B. Transcriptional activation of the intercellular adhesion molecule 1 (CD54) gene by human T lymphotropic virus types I and II Tax is mediated through a palindromic response element. AIDS Res. Hum. Retroviruses 1997, 13, 1429–1437. [Google Scholar] [CrossRef]
- Nejmeddine, M.; Negi, V.S.; Mukherjee, S.; Tanaka, Y.; Orth, K.; Taylor, G.P.; Bangham, C.R. HTLV-1-Tax and ICAM-1 act on T-cell signal pathways to polarize the microtubule-organizing center at the virological synapse. Blood 2009, 114, 1016–1025. [Google Scholar] [CrossRef]
- Hiyoshi, M.; Takahashi, N.; Eltalkhawy, Y.M.; Noyori, O.; Lotfi, S.; Panaampon, J.; Okada, S.; Tanaka, Y.; Ueno, T.; Fujisawa, J.I.; et al. M-Sec induced by HTLV-1 mediates an efficient viral transmission. PLoS Pathog. 2021, 17, e1010126. [Google Scholar] [CrossRef]
- Percher, F.; Curis, C.; Peres, E.; Artesi, M.; Rosewick, N.; Jeannin, P.; Gessain, A.; Gout, O.; Mahieux, R.; Ceccaldi, P.E.; et al. HTLV-1-induced leukotriene B4 secretion by T cells promotes T cell recruitment and virus propagation. Nat. Commun. 2017, 8, 15890. [Google Scholar] [CrossRef]
- Moriuchi, M.; Moriuchi, H. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J. Immunol. 2001, 166, 4231–4236. [Google Scholar] [CrossRef]
- Moriuchi, M.; Moriuchi, H. Induction of lactoferrin gene expression in myeloid or mammary gland cells by human T-cell leukemia virus type 1 (HTLV-1) tax: Implications for milk-borne transmission of HTLV-1. J. Virol. 2006, 80, 7118–7126. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Pelletier, I.; Ouellet, M.; Vargas, A.; Tremblay, M.J.; Sato, S.; Barbeau, B. Induction of galectin-1 expression by HTLV-I Tax and its impact on HTLV-I infectivity. Retrovirology 2008, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Hammes, S.R.; Kuwabara, I.; Greene, W.C.; Liu, F.T. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the β-galactoside-binding lectin, galectin-3. Am. J. Pathol. 1996, 148, 1661–1670. [Google Scholar] [PubMed]
- Albrecht, B.; Collins, N.D.; Burniston, M.T.; Nisbet, J.W.; Ratner, L.; Green, P.L.; Lairmore, M.D. Human T-lymphotropic virus type 1 open reading frame I p12I is required for efficient viral infectivity in primary lymphocytes. J. Virol. 2000, 74, 9828–9835. [Google Scholar] [CrossRef]
- Curis, C.; Percher, F.; Jeannin, P.; Montange, T.; Chevalier, S.A.; Seilhean, D.; Cartier, L.; Couraud, P.O.; Gout, O.; Gessain, A.; et al. Human T-lymphotropic virus type 1-induced overexpression of activated leukocyte cell adhesion molecule (ALCAM) facilitates trafficking of infected lymphocytes through the blood-brain barrier. J. Virol. 2016, 90, 7303–7312. [Google Scholar] [CrossRef]
- Lilienbaum, A.; Duc Dodon, M.; Alexandre, C.; Gazzolo, L.; Paulin, D. Effect of human T-cell leukemia virus type I tax protein on activation of the human vimentin gene. J. Virol. 1990, 64, 256–263. [Google Scholar] [CrossRef]
- Lilienbaum, A.; Paulin, D. Activation of the human vimentin gene by the Tax human T-cell leukemia virus. I. Mechanisms of regulation by the NF-κB transcription factor. J. Biol. Chem. 1993, 268, 2180–2188. [Google Scholar] [CrossRef]
- Hiraiwa, N.; Hiraiwa, M.; Kannagi, R. Human T-cell leukemia virus-1 encoded Tax protein transactivates α 1-->3 fucosyltransferase Fuc-T VII, which synthesizes sialyl Lewis X, a selectin ligand expressed on adult T-cell leukemia cells. Biochem. Biophys. Res. Commun. 1997, 231, 183–186. [Google Scholar] [CrossRef]
- Hiraiwa, N.; Yabuta, T.; Yoritomi, K.; Hiraiwa, M.; Tanaka, Y.; Suzuki, T.; Yoshida, M.; Kannagi, R. Transactivation of the fucosyltransferase VII gene by human T-cell leukemia virus type 1 Tax through a variant cAMP-responsive element. Blood 2003, 101, 3615–3621. [Google Scholar] [CrossRef]
- Nakachi, S.; Nakazato, T.; Ishikawa, C.; Kimura, R.; Mann, D.A.; Senba, M.; Masuzaki, H.; Mori, N. Human T-cell leukemia virus type 1 tax transactivates the matrix metalloproteinase 7 gene via JunD/AP-1 signaling. Biochim. Biophys. Acta 2011, 1813, 731–741. [Google Scholar] [CrossRef]
- Mori, N.; Sato, H.; Hayashibara, T.; Senba, M.; Hayashi, T.; Yamada, Y.; Kamihira, S.; Ikeda, S.; Yamasaki, Y.; Morikawa, S.; et al. Human T-cell leukemia virus type I Tax transactivates the matrix metalloproteinase-9 gene: Potential role in mediating adult T-cell leukemia invasiveness. Blood 2002, 99, 1341–1349. [Google Scholar] [CrossRef]
- Uchijima, M.; Sato, H.; Fujii, M.; Seiki, M. Tax proteins of human T-cell leukemia virus type 1 and 2 induce expression of the gene encoding erythroid-potentiating activity (tissue inhibitor of metalloproteinases-1, TIMP-1). J. Biol. Chem. 1994, 269, 14946–14950. [Google Scholar] [CrossRef]
- Kress, A.K.; Kalmer, M.; Rowan, A.G.; Grassmann, R.; Fleckenstein, B. The tumor marker Fascin is strongly induced by the Tax oncoprotein of HTLV-1 through NF-κB signals. Blood 2011, 117, 3609–3612. [Google Scholar] [CrossRef]
- Mohr, C.F.; Gross, C.; Bros, M.; Reske-Kunz, A.B.; Biesinger, B.; Thoma-Kress, A.K. Regulation of the tumor marker Fascin by the viral oncoprotein Tax of human T-cell leukemia virus type 1 (HTLV-1) depends on promoter activation and on a promoter-independent mechanism. Virology 2015, 485, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P.; Collette, Y.; Marignier, R.; Vuaillat, C.; Rogemond, V.; Davoust, N.; Malcus, C.; Cavagna, S.; Gessain, A.; Machuca-Gayet, I.; et al. A role for the neuronal protein collapsin response mediator protein 2 in T lymphocyte polarization and migration. J. Immunol. 2005, 175, 7650–7660. [Google Scholar] [CrossRef] [PubMed]
- Varrin-Doyer, M.; Nicolle, A.; Marignier, R.; Cavagna, S.; Benetollo, C.; Wattel, E.; Giraudon, P. Human T lymphotropic virus type 1 increases T lymphocyte migration by recruiting the cytoskeleton organizer CRMP2. J. Immunol. 2012, 188, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Lee, B.H.; Park, R.W.; Kim, I.S. Transactivation of fibronectin promoter by HTLV-I Tax through NF-κB pathway. Biochem. Biophys. Res. Commun. 2000, 276, 579–586. [Google Scholar] [CrossRef]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Pise-Masison, C.A.; Choi, K.S.; Radonovich, M.; Dittmer, J.; Kim, S.J.; Brady, J.N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 1998, 72, 1165–1170. [Google Scholar] [CrossRef]
- Suzuki, T.; Uchida-Toita, M.; Yoshida, M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene 1999, 18, 4137–4143. [Google Scholar] [CrossRef]
- Ariumi, Y.; Kaida, A.; Lin, J.Y.; Hirota, M.; Masui, O.; Yamaoka, S.; Taya, Y.; Shimotohno, K. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 2000, 19, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Pise-Masison, C.A.; Mahieux, R.; Jiang, H.; Ashcroft, M.; Radonovich, M.; Duvall, J.; Guillerm, C.; Brady, J.N. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-κB pathway and is dependent on p53 phosphorylation. Mol. Cell Biol. 2000, 20, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Pise-Masison, C.A.; Mahieux, R.; Radonovich, M.; Jiang, H.; Brady, J.N. Human T-lymphotropic virus type I Tax protein utilizes distinct pathways for p53 inhibition that are cell type-dependent. J. Biol. Chem. 2001, 276, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Radonovich, M.; Brady, J.N.; Pise-Masison, C.A. HTLV-I Tax induces a novel interaction between p65/RelA and p53 that results in inhibition of p53 transcriptional activity. Blood 2004, 104, 1490–1497. [Google Scholar] [CrossRef]
- Jeong, S.J.; Pise-Masison, C.A.; Radonovich, M.F.; Park, H.U.; Brady, J.N. A novel NF-κB pathway involving IKKβ and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-κB transcriptional activity. J. Biol. Chem. 2005, 280, 10326–10332. [Google Scholar] [CrossRef]
- Jeong, S.J.; Pise-Masison, C.A.; Radonovich, M.F.; Park, H.U.; Brady, J.N. Activated AKT regulates NF-κB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene 2005, 24, 6719–6728. [Google Scholar] [CrossRef]
- Brauweiler, A.; Garrus, J.E.; Reed, J.C.; Nyborg, J.K. Repression of bax gene expression by the HTLV-1 Tax protein: Implications for suppression of apoptosis in virally infected cells. Virology 1997, 231, 135–140. [Google Scholar] [CrossRef]
- Muhleisen, A.; Giaisi, M.; Kohler, R.; Krammer, P.H.; Li-Weber, M. Tax contributes apoptosis resistance to HTLV-1-infected T cells via suppression of Bid and Bim expression. Cell Death Dis. 2014, 5, e1575. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Sze, A.; Bel Hadj, S.; Chiang, C.; Steel, C.; Han, X.; Routy, J.P.; Lin, R.; Hiscott, J.; van Grevenynghe, J. HTLV-1 Tax-mediated inhibition of FOXO3a activity is critical for the persistence of terminally differentiated CD4+ T cells. PLoS Pathog. 2014, 10, e1004575. [Google Scholar] [CrossRef]
- Bopp, T.; Becker, C.; Klein, M.; Klein-Hessling, S.; Palmetshofer, A.; Serfling, E.; Heib, V.; Becker, M.; Kubach, J.; Schmitt, S.; et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 2007, 204, 1303–1310. [Google Scholar] [CrossRef]
- Bielenberg, M.; Kurelic, R.; Frantz, S.; Nikolaev, V.O. A mini-review: Phosphodiesterases in charge to balance intracellular cAMP during T-cell activation. Front. Immunol. 2024, 15, 1365484. [Google Scholar] [CrossRef]
- Kress, A.K.; Schneider, G.; Pichler, K.; Kalmer, M.; Fleckenstein, B.; Grassmann, R. Elevated cyclic AMP levels in T lymphocytes transformed by human T-cell lymphotropic virus type 1. J. Virol. 2010, 84, 8732–8742. [Google Scholar] [CrossRef]
- Weil, R.; Levraud, J.P.; Dodon, M.D.; Bessia, C.; Hazan, U.; Kourilsky, P.; Israel, A. Altered expression of tyrosine kinases of the Src and Syk families in human T-cell leukemia virus type 1-infected T-cell lines. J. Virol. 1999, 73, 3709–3717. [Google Scholar] [CrossRef]
- Cheng, J.; Kydd, A.R.; Nakase, K.; Noonan, K.M.; Murakami, A.; Tao, H.; Dwyer, M.; Xu, C.; Zhu, Q.; Marasco, W.A. Negative regulation of the SH2-homology containing protein-tyrosine phosphatase-1 (SHP-1) P2 promoter by the HTLV-1 Tax oncoprotein. Blood 2007, 110, 2110–2120. [Google Scholar] [CrossRef]
- Nakase, K.; Cheng, J.; Zhu, Q.; Marasco, W.A. Mechanisms of SHP-1 P2 promoter regulation in hematopoietic cells and its silencing in HTLV-1-transformed T cells. J. Leukoc. Biol. 2009, 85, 165–174. [Google Scholar] [CrossRef]
- Feigenbaum, L.; Fujita, K.; Collins, F.S.; Jay, G. Repression of the NF1 gene by Tax may explain the development of neurofibromas in human T-lymphotropic virus type 1 transgenic mice. J. Virol. 1996, 70, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Kibler, K.V.; Jeang, K.T. CREB/ATF-dependent repression of cyclin A by human T-cell leukemia virus type 1 Tax protein. J. Virol. 2001, 75, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Beauvois, A.; Gazon, H.; Chauhan, P.S.; Jamakhani, M.; Jacques, J.R.; Thiry, M.; Dejardin, E.; Valentin, E.D.; Twizere, J.C.; Peloponese, J.M.; et al. The helicase-like transcription factor redirects the autophagic flux and restricts human T cell leukemia virus type 1 infection. Proc. Natl. Acad. Sci. USA 2023, 120, e2216127120. [Google Scholar] [CrossRef]
- Suzuki, S.; Zhou, Y.; Refaat, A.; Takasaki, I.; Koizumi, K.; Yamaoka, S.; Tabuchi, Y.; Saiki, I.; Sakurai, H. Human T cell lymphotropic virus 1 manipulates interferon regulatory signals by controlling the TAK1-IRF3 and IRF4 pathways. J. Biol. Chem. 2010, 285, 4441–4446. [Google Scholar] [CrossRef]
- Diani, E.; Avesani, F.; Bergamo, E.; Cremonese, G.; Bertazzoni, U.; Romanelli, M.G. HTLV-1 Tax protein recruitment into IKKε and TBK1 kinase complexes enhances IFN-I expression. Virology 2015, 476, 92–99. [Google Scholar] [CrossRef]
- Yuen, C.K.; Chan, C.P.; Fung, S.Y.; Wang, P.H.; Wong, W.M.; Tang, H.V.; Yuen, K.S.; Chan, C.P.; Jin, D.Y.; Kok, K.H. Suppression of type I interferon production by human T-cell leukemia virus type 1 oncoprotein Tax through inhibition of IRF3 phosphorylation. J. Virol. 2016, 90, 3902–3912. [Google Scholar] [CrossRef]
- Wang, D.; Guo, M.X.; Hu, H.M.; Zhao, Z.Z.; Qiu, H.L.; Shao, H.J.; Zhu, C.G.; Xue, L.; Shi, Y.B.; Li, W.X. Human T-cell leukemia virus type 1 oncoprotein tax represses ZNF268 expression through the cAMP-responsive element-binding protein/activating transcription factor pathway. J. Biol. Chem. 2008, 283, 16299–16308. [Google Scholar] [CrossRef]
- Yamagata, T.; Nishida, J.; Tanaka, S.; Sakai, R.; Mitani, K.; Yoshida, M.; Taniguchi, T.; Yazaki, Y.; Hirai, H. A novel interferon regulatory factor family transcription factor, ICSAT/Pip/LSIRF, that negatively regulates the activity of interferon-regulated genes. Mol. Cell Biol. 1996, 16, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Takachi, T.; Takahashi, M.; Takahashi-Yoshita, M.; Higuchi, M.; Obata, M.; Mishima, Y.; Okuda, S.; Tanaka, Y.; Matsuoka, M.; Saitoh, A.; et al. Human T-cell leukemia virus type 1 Tax oncoprotein represses the expression of the BCL11B tumor suppressor in T-cells. Cancer Sci. 2015, 106, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Gilli, S.C.; Salles, T.S.; Saad, S.T. Decreased GATA3 mRNA expression in human T-cell lymphotropic virus type 1 (HTLV-1) infection. Scand. J. Infect. Dis. 2000, 32, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Gilli, S.C.; Salles, T.S.; Saad, S.T. Regulation of the GATA3 promoter by human T-cell lymphotropic virus type I Tax protein. J. Cell Biochem. 2004, 93, 1178–1187. [Google Scholar] [CrossRef]
- Riou, P.; Bex, F.; Gazzolo, L. The human T cell leukemia/lymphotropic virus type 1 Tax protein represses MyoD-dependent transcription by inhibiting MyoD-binding to the KIX domain of p300. A potential mechanism for Tax-mediated repression of the transcriptional activity of basic helix-loop-helix factors. J. Biol. Chem. 2000, 275, 10551–10560. [Google Scholar] [CrossRef]
- Colgin, M.A.; Nyborg, J.K. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J. Virol. 1998, 72, 9396–9399. [Google Scholar] [CrossRef] [PubMed]
- Nicot, C.; Mahieux, R.; Opavsky, R.; Cereseto, A.; Wolff, L.; Brady, J.N.; Franchini, G. HTLV-I Tax transrepresses the human c-Myb promoter independently of its interaction with CBP or p300. Oncogene 2000, 19, 2155–2164. [Google Scholar] [CrossRef]
- Nicot, C.; Opavsky, R.; Mahieux, R.; Johnson, J.M.; Brady, J.N.; Wolff, L.; Franchini, G. Tax oncoprotein trans-represses endogenous B-myb promoter activity in human T cells. AIDS Res. Hum. Retroviruses 2000, 16, 1629–1632. [Google Scholar] [CrossRef]
- Nicot, C.; Mahieux, R.; Pise-Masison, C.; Brady, J.; Gessain, A.; Yamaoka, S.; Franchini, G. Human T-cell lymphotropic virus type 1 Tax represses c-Myb-dependent transcription through activation of the NF-κB pathway and modulation of coactivator usage. Mol. Cell Biol. 2001, 21, 7391–7402. [Google Scholar] [CrossRef]
- Sobol, R.W.; Horton, J.K.; Kuhn, R.; Gu, H.; Singhal, R.K.; Prasad, R.; Rajewsky, K.; Wilson, S.H. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 1996, 379, 183–186. [Google Scholar] [CrossRef]
- Jeang, K.T.; Widen, S.G.; Semmes, O.J.t.; Wilson, S.H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human β-polymerase gene. Science 1990, 247, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Uittenbogaard, M.N.; Armstrong, A.P.; Chiaramello, A.; Nyborg, J.K. Human T-cell leukemia virus type I Tax protein represses gene expression through the basic helix-loop-helix family of transcription factors. J. Biol. Chem. 1994, 269, 22466–22469. [Google Scholar] [CrossRef] [PubMed]
- Wencker, M.; Sausse, C.; Derse, D.; Gazzolo, L.; Duc Dodon, M. Human T-cell leukemia virus type 1 Tax protein down-regulates pre-T-cell receptor α gene transcription in human immature thymocytes. J. Virol. 2007, 81, 301–308. [Google Scholar] [CrossRef]
- Rahman, S.; Quann, K.; Pandya, D.; Singh, S.; Khan, Z.K.; Jain, P. HTLV-1 Tax mediated downregulation of miRNAs associated with chromatin remodeling factors in T cells with stably integrated viral promoter. PLoS ONE 2012, 7, e34490. [Google Scholar] [CrossRef]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.M.; Blot, V.; Janvier, S.; Arnulf, B.; van Endert, P.M.; Heveker, N.; Pique, C.; et al. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef]
- Lambert, S.; Bouttier, M.; Vassy, R.; Seigneuret, M.; Petrow-Sadowski, C.; Janvier, S.; Heveker, N.; Ruscetti, F.W.; Perret, G.; Jones, K.S.; et al. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 2009, 113, 5176–5185. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Petrow-Sadowski, C.; Bertolette, D.C.; Huang, Y.; Ruscetti, F.W. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar] [CrossRef] [PubMed]
- Pinon, J.D.; Klasse, P.J.; Jassal, S.R.; Welson, S.; Weber, J.; Brighty, D.W.; Sattentau, Q.J. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 2003, 77, 9922–9930. [Google Scholar] [CrossRef] [PubMed]

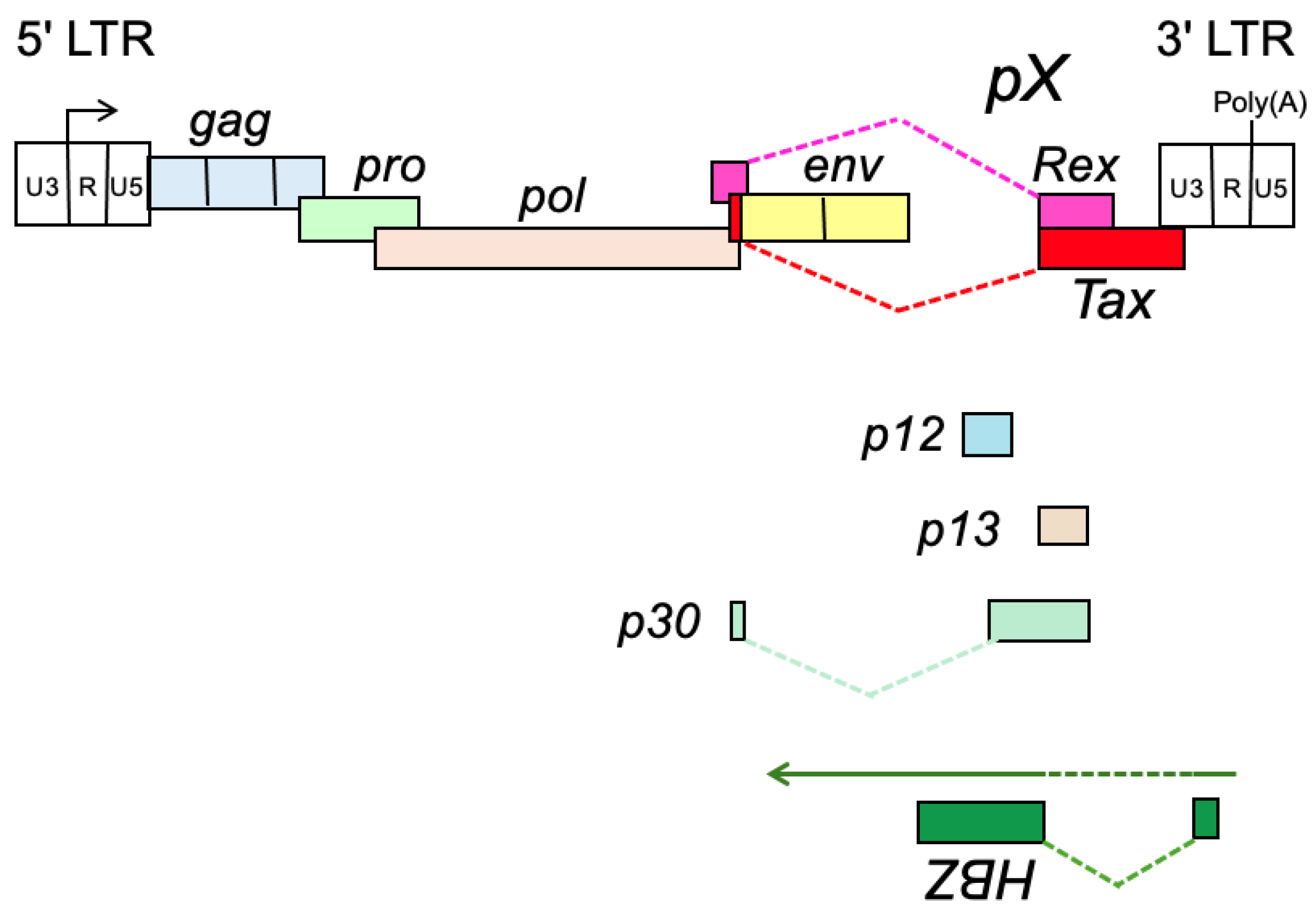

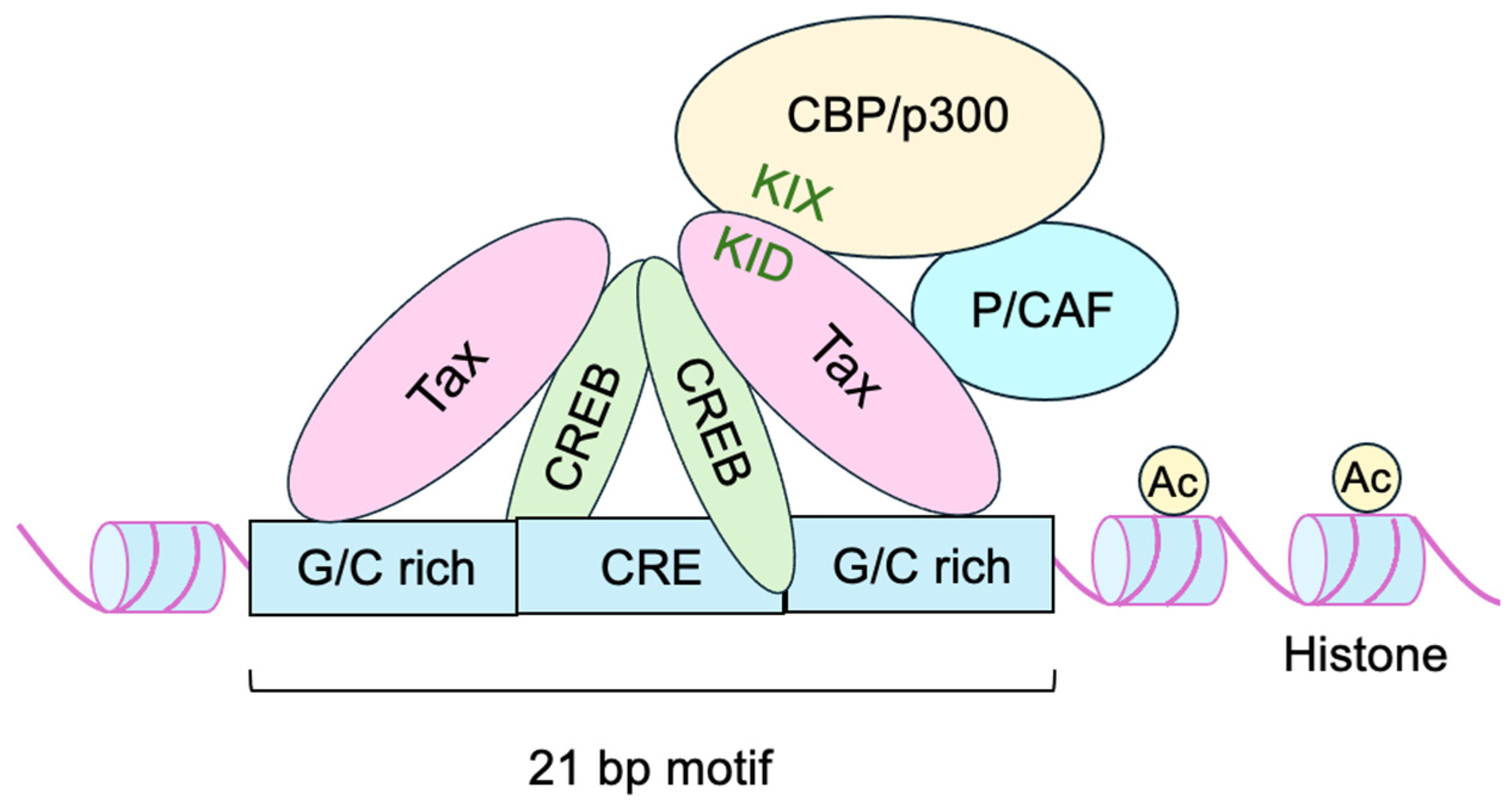
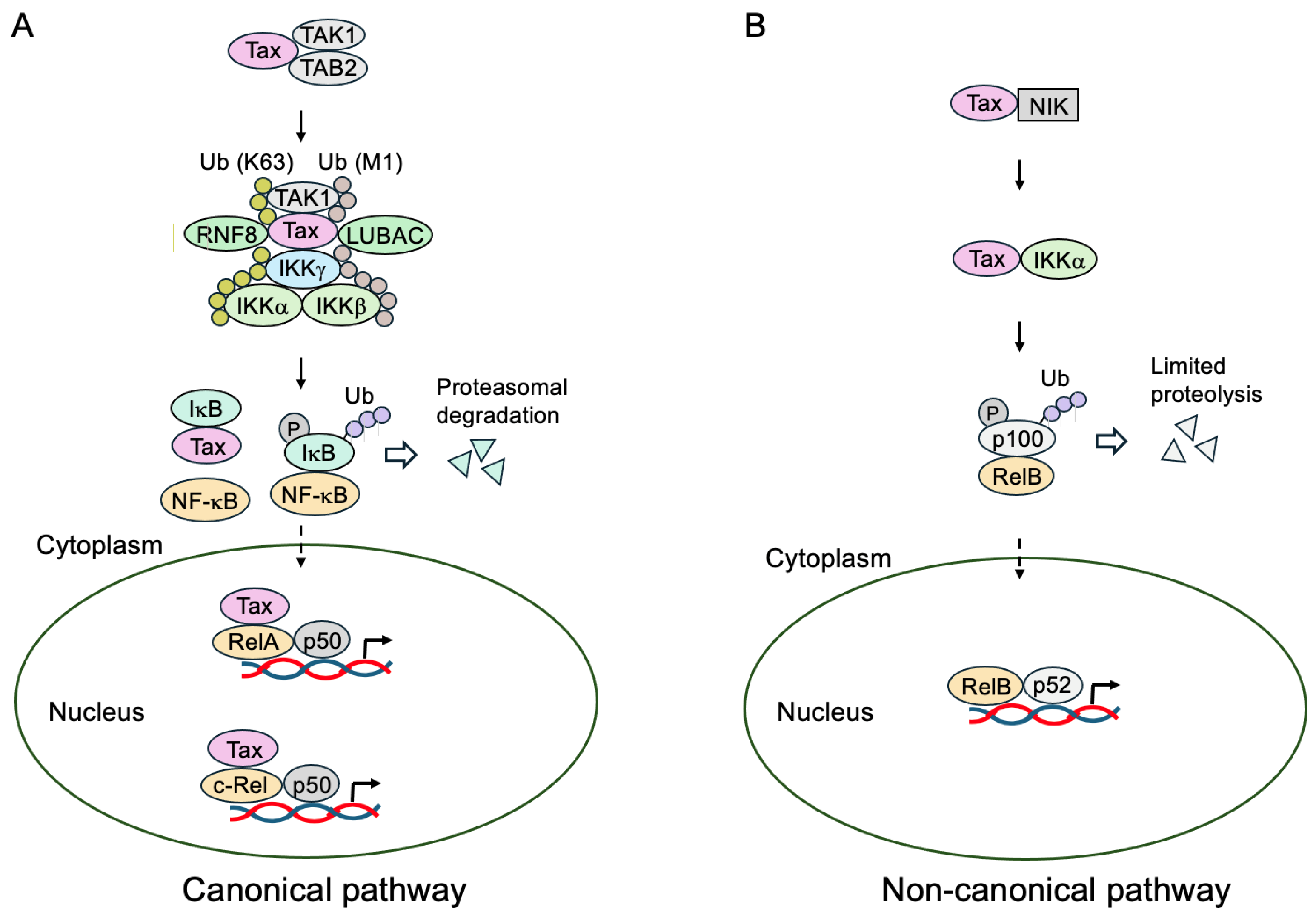
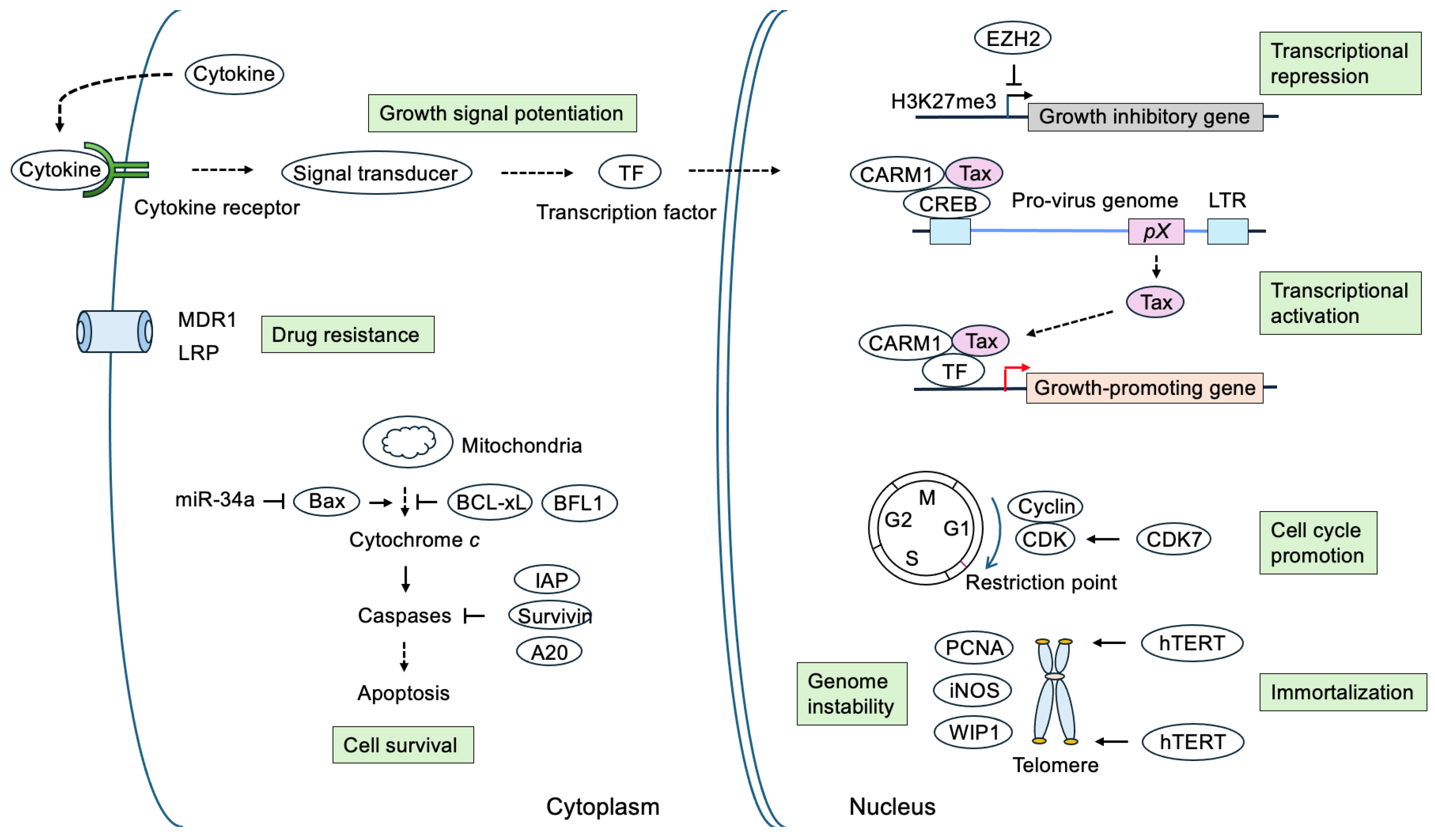
| Gene | Gene Description | References |
|---|---|---|
| Interleukins and those receptors | ||
| IL-1α | Interleukin-1α | [254,255,256,257,258] |
| IL-1β | Interleukin-1β | [256,259] |
| IL-2 | Interleukin-2 | [208,209,210,211,212,213] |
| IL-2Rα | Interleukin-2 receptor α (CD25, Tac antigen) | [208,209,214,215,216,217,218,219] |
| IL-2Rγ | Interleukin-2 receptor γ (CD132, common γ chain) | [222] |
| IL-4 | Interleukin-4 | [260] |
| IL-5 | Interleukin-5 | [203,227,261] |
| IL-6 | Interleukin-6 | [188,254,256,262,263,264,266] |
| IL-6R | Interleukin-6 receptor | [265] |
| IL-8 | Interleukin-8 | [205,267] |
| IL-9 | Interleukin-9 | [227,266] |
| IL-10 | Interleukin-10 | [83,268] |
| IL-12 | Interleukin-12 | [270] |
| IL-13 | Interleukin-13 | [227,271,272,273] |
| IL-15 | Interleukin-15 | [229] |
| IL-15R | Interleukin-15 receptor | [231] |
| IL-17 | Interleukin-17 | [274] |
| IL17R | Interleukin-17 receptor | [275] |
| IL-21 | Interleukin-21 | [232] |
| IL-21R | Interleukin-21 receptor | [232] |
| Chemokines and those receptors | ||
| CCL1 | C-C motif chemokine ligand 1 | [286] |
| CCL2 (MCP-1) | C-C motif chemokine ligand 2 (monocyte chemoattractant protein-1) | [270,284,285] |
| CCL3 (MIP-1α) | C-C motif chemokine ligand 3 (macrophage inflammatory protein 1α) | [184,284,285,287] |
| CCL4 (MIP-1β) | C-C motif chemokine ligand 4 (macrophage inflammatory protein 1β) | [288,289] |
| CCL5 (RANTES) | C-C motif chemokine ligand 5 (regulated on activation, normal T cell expressed and secreted) | [284,290] |
| CCL7 (MCP-3) | C-C motif chemokine ligand 7 (monocyte chemoattractant protein-3) | [270] |
| CCR9 | C-C chemokine receptor type 9 | [198] |
| CCL11 | C-C motif chemokine ligand 11 (Eotaxin) | [270] |
| CCL20 (MIP-3α) | C-C motif chemokine ligand 20 (macrophage inflammatory protein 3α) | [291] |
| CCL22 | C-C motif chemokine ligand 22 | [292] |
| CXCR4 | C-X-C chemokine receptor type 4 | [296] |
| CXCR7 | C-X-C chemokine receptor type 7 | [186] |
| CXCL10 (IP-10) | C-X-C motif chemokine ligand 10 (interferon γ-induced protein 10) | [184,284,293] |
| CXCL12 (SDF-1) (PBSF) | C-X-C motif chemokine ligand 12 (stromal cell-derived factor 1) (pre-B cell growth-stimulating factor) | [295] |
| XCL1 | Chemokine, C motif, and ligand 1 | [184] |
| Growth factors | ||
| c-sis | Platelet-derived growth factor subunit B | [226] |
| TGF-β1 | Transforming growth factor β1 | [234,235] |
| VEGF | Vascular endothelial growth factor | [243] |
| GM-CSF (CSF2) | Granulocyte–macrophage colony-stimulating factor (colony-stimulating factor 2) | [209,218,282,283] |
| G-CSF | Granulocyte colony-stimulating factor | [283] |
| NGF | Nerve growth factor | [311] |
| Costimulatory molecules | ||
| gp34 | Tumor necrosis factor ligand superfamily member 4 (CD252) | [181,239,240] |
| OX40 | Cluster of differentiation 134 | [189,237] |
| CD40 | Cluster of differentiation 40 | [241] |
| CD40L | CD40 ligand | [242] |
| Tumor necrosis factor-related | ||
| TNF-α | Tumor necrosis factor α | [256,270,277,278,279] |
| TNF-β | Tumor necrosis factor β (Lymphotoxin) | [256,280,281] |
| TNFRSF9/4–1BB/CD137/ILA | Tumor necrosis factor receptor superfamily member 9 | [307] |
| CD70 | Tumor necrosis factor ligand superfamily member 7 | [308] |
| Cell adhesion molecules | ||
| VCAM-1 | Vascular cell adhesion molecule 1 (CD106) | [204,298] |
| E-cadherin | [306] | |
| Others | ||
| PTHrP | Parathyroid hormone-related peptide | [245,246,247,248] |
| IFN-γ | Interferon γ | [270,271] |
| OPN | Osteopontin | [302] |
| CD44 | Cluster of differentiation 44 | [303] |
| LFA-3 (CD58) | Lymphocyte function-associated antigen, type 3 (cluster of differentiation 58) | [191] |
| CD69 | Cluster of differentiation 69 | [309] |
| CD83 | Cluster of differentiation 83 | [193] |
| CD137 | Cluster of differentiation 137 | [305] |
| MHC class I | Major histocompatibility complex, class I | [310] |
| proenkephalin | [312,313,314] | |
| RGMa | Repulsive guidance molecule A | [297] |
| Gene | Gene Description | References |
|---|---|---|
| Proto-oncogenes | ||
| c-Fos | v-Fos FBJ murine osteosarcoma viral oncogene homolog | [180,315,316] |
| c-Jun | v-jun avian sarcoma virus 17 oncogene homolog | [206,316] |
| c-Myc | v-Myc avian myelocytomatosis viral oncogene homolog | [320] |
| c-Rel | v-Rel avian reticuloendotheliosis viral oncogene homolog | [134,326] |
| Transcription factors | ||
| Fra-1 | Fos-related antigen 1 | [206] |
| JunD | [206] | |
| Egr-1 | Early growth response 1 | [206,317,318] |
| Egr-2 | Early growth response 2 | [206,318] |
| ETR101 | Immediate-early response gene 2 (IER2) | [202] |
| BRF1 (ZFP36L1) | Butyrate response factor 1 (Zinc finger protein 36-like 1) | [319] |
| p105 | Nuclear factor kappa-B, subunit 1 (NFKB1) | [326] |
| STAT1 | Signal transducer and activator of transcription 1 | [332] |
| STAT5 | Signal transducer and activator of transcription 5 | [332] |
| IRF-4 | Interferon regulatory factor 4 | [333] |
| IRF-5 | Interferon regulatory factor 5 | [336] |
| Nur77 | Nuclear receptor sub-family 4, group A, member 1 (NR4A1) | [196,201] |
| BCL6 | B-cell/CLL lymphoma 6 | [347] |
| Signal transducers | ||
| Pim-1 | Serine/threonine protein kinase PIM1 | [327] |
| Pim-3 | Serine/threonine protein kinase PIM3 | [327,328] |
| Lyn | Lyn protooncogene, Src family tyrosine kinase | [339] |
| JAG1 | Jagged 1 | [351] |
| Cas-L | Crk-associated substrate lymphocyte type | [185] |
| MAGI3 | Membrane-associated guanylate kinase, WW and PDZ domains-containing 3 | [358] |
| Others | ||
| EVC | Ellis Van Creveld | [343] |
| IκB-ζ | Inhibitor of NF-κB-ζ | [345] |
| Gene | Gene Description | References |
|---|---|---|
| CCND1 | Cyclin D1 | [117,376] |
| CCND2 | Cyclin D2 | [374,375,377,378] |
| E2F1 | E2F transcription factor 1 | [373,376] |
| CDK6 | Cyclin-dependent kinase 6 | [378] |
| CDK7 | Cyclin-dependent kinase 7 | [384] |
| ELL2 | Elongation factor, RNA polymerase II, 2 | [386] |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A CDK-interacting protein 1 (CIP1) Wildtype p53-activated fragment 1 (WAF1) p21 | [389,391,392] |
| Gene | Gene Description | References |
|---|---|---|
| Bcl-xL | Bcl2-related protein, long isoform | [407,408] |
| BFL1 | Bcl2-related protein A1 | [410] |
| BIRC3 | Baculoviral IAP repeat-containing 3 (cIAP2, HIAP-1) | [411,412] |
| XIAP | X-chromosome-linked inhibitor of apoptosis | [183] |
| Survivin | Baculoviral IAP repeat-containing 5 (BIRC5) | [415] |
| c-FLIP | Cellular FLICE-inhibitory protein | [416,417,418] |
| A20 | Tumor necrosis factor-α-induced protein 3 (TNFAIP3) | [420] |
| MDR1 | Multidrug resistance protein 1 ATP-binding cassette sub-family B member 1 (ABCB1)Ppermeability glycoprotein (P-glycoprotein, P-gp) | [423,424,425] |
| LRP | Lung resistance-related protein | [426] |
| FasL | Fas ligand | [190] |
| TRAIL | TNF-related apoptosis-inducing ligand | [428] |
| Gene | Gene Description | References |
|---|---|---|
| PCNA | Proliferating cell nuclear antigen | [439,440,441] |
| iNOS | Inducible nitric oxide synthase | [443,445] |
| WIP1 | Wildtype p53-induced phosphatase 1 Protein phosphatase, magnesium/manganese-dependent, 1D (PPM1D) | [453] |
| Gene | Target Genes | Reference |
|---|---|---|
| miR-146a | [472] | |
| miR-155 | Tumor protein p53-inducible nuclear protein 1 (TP53INP1) | [473] |
| Gene | Gene Description | References |
|---|---|---|
| EZH2 | Enhancer of zeste homolog 2 | [494,495] |
| CARM1 | Coactivator-associated arginine methyltransferase 1 Protein arginine methyltransferase 4 (PRMT4) | [498] |
| Gene | Gene Description | Reference |
|---|---|---|
| GM2/GD2 synthase | β-1,4-N-acetylgalactosaminyltransferase | [499] |
| TRX | Thioredoxin | [505] |
| DHODH | Dihydroorotate dehydrogenase | [506] |
| Gene | Gene Description | References |
|---|---|---|
| ICAM-1 | Intercellular adhesion molecule 1 (CD54) | [191,192,508,509] |
| TNFAIP2 | TNF-α-induced protein 2 (M-Sec) | [511] |
| PLA2G4C | Phospholipase A2, group IVC | [512] |
| Lactoferrin | Lactotransferrin (LTF) | [514] |
| Galectin-1 | Lectin, galactoside-binding, soluble, 1 (LGALS1) | [515] |
| Galectin-3 | Lectin, galactoside-binding, soluble, 3 (LGALS3) | [516] |
| Gene | Gene Description | References |
|---|---|---|
| ALCAM | Activated cell adhesion molecule | [518] |
| Vimentin | [519,520] | |
| FucT VII | Fucosyltransferase 7 | [521,522] |
| MMP-7 | Matrix metalloproteinase 7 | [523] |
| MMP-9 | Matrix metalloproteinase 9 | [524] |
| TIMP-1 | Tissue inhibitors of matrix metalloproteinases-1 | [525] |
| Fascin | Actin-bundling protein | [526,527] |
| CRMP2 | Collapsin response mediator protein 2 | [529] |
| FN | Fibronectin | [530] |
| Gene | Gene Description | References |
|---|---|---|
| Pro-apoptotic genes | ||
| Bax | Bcl-2-associated X protein | [540] |
| Bim | Bcl-2-interacting mediator of cell death | [541,542] |
| Bid | BH3-interacting domain death agonist | [541] |
| PUMA | p53-upregulated modulator of apoptosis Bcl2-binding component 3 (BBC3) | [542] |
| Signal transducers | ||
| PDE3B | Phosphodiesterase 3B | [545] |
| Lck | Lymphocyte-specific protein-tyrosine kinase | [187] |
| Zap-70 | Zeta-chain-associated protein kinase Syk-related tyrosine kinase (SRK) | [546] |
| SHP-1 | Src homology-2-containing protein-tyrosine phosphatase 1 Tyrosine-protein phosphatase non-receptor type 6 (PTPN6) | [547] |
| NF1 | Neurofibromatosis type I | [549] |
| Cell cycle regulators | ||
| p18Ink4c | Cyclin-dependent kinase inhibitor 2C (CDKN2C) | [402] |
| CCNA2 | Cyclin A | [550] |
| Transcription factors | ||
| HLTF | Helicase-like transcription factor | [551] |
| ZNF268 | Zinc finger protein 268 | [555] |
| BCL11B | B-cell CLL/Lymphoma 11B | [557] |
| c-Myb | v-Myb avian myeloblastosis viral oncogene homolog | [562,563,564] |
| B-Myb | v-Myb avian myeloblastosis viral oncogene homolog-like 2 | [563] |
| Others | ||
| Pol β | DNA polymerase β | [566,567] |
| Type I IFN | Type I Interferon | [554] |
| pTCRα | Pre-T-cell receptor α | [568] |
| Target genes | ||
| miR-149 | p300 and P/CAF | [569] |
| miR-873 | p300 and P/CAF | [569] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirasawa, M.; Nakajima, R.; Zhou, Y.; Fikriyanti, M.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Transcriptional Activation Mechanisms and Target Genes of the Oncogene Product Tax of Human T-Cell Leukemia Virus Type 1. Genes 2025, 16, 1221. https://doi.org/10.3390/genes16101221
Shirasawa M, Nakajima R, Zhou Y, Fikriyanti M, Iwanaga R, Bradford AP, Kurayoshi K, Araki K, Ohtani K. Transcriptional Activation Mechanisms and Target Genes of the Oncogene Product Tax of Human T-Cell Leukemia Virus Type 1. Genes. 2025; 16(10):1221. https://doi.org/10.3390/genes16101221
Chicago/Turabian StyleShirasawa, Mashiro, Rinka Nakajima, Yaxuan Zhou, Mariana Fikriyanti, Ritsuko Iwanaga, Andrew P. Bradford, Kenta Kurayoshi, Keigo Araki, and Kiyoshi Ohtani. 2025. "Transcriptional Activation Mechanisms and Target Genes of the Oncogene Product Tax of Human T-Cell Leukemia Virus Type 1" Genes 16, no. 10: 1221. https://doi.org/10.3390/genes16101221
APA StyleShirasawa, M., Nakajima, R., Zhou, Y., Fikriyanti, M., Iwanaga, R., Bradford, A. P., Kurayoshi, K., Araki, K., & Ohtani, K. (2025). Transcriptional Activation Mechanisms and Target Genes of the Oncogene Product Tax of Human T-Cell Leukemia Virus Type 1. Genes, 16(10), 1221. https://doi.org/10.3390/genes16101221






