Genetic Therapy of Fuchs Endothelial Corneal Dystrophy: Where Are We? A Review
Abstract
1. Introduction
2. Methods
3. Fuchs Endothelial Corneal Dystrophy
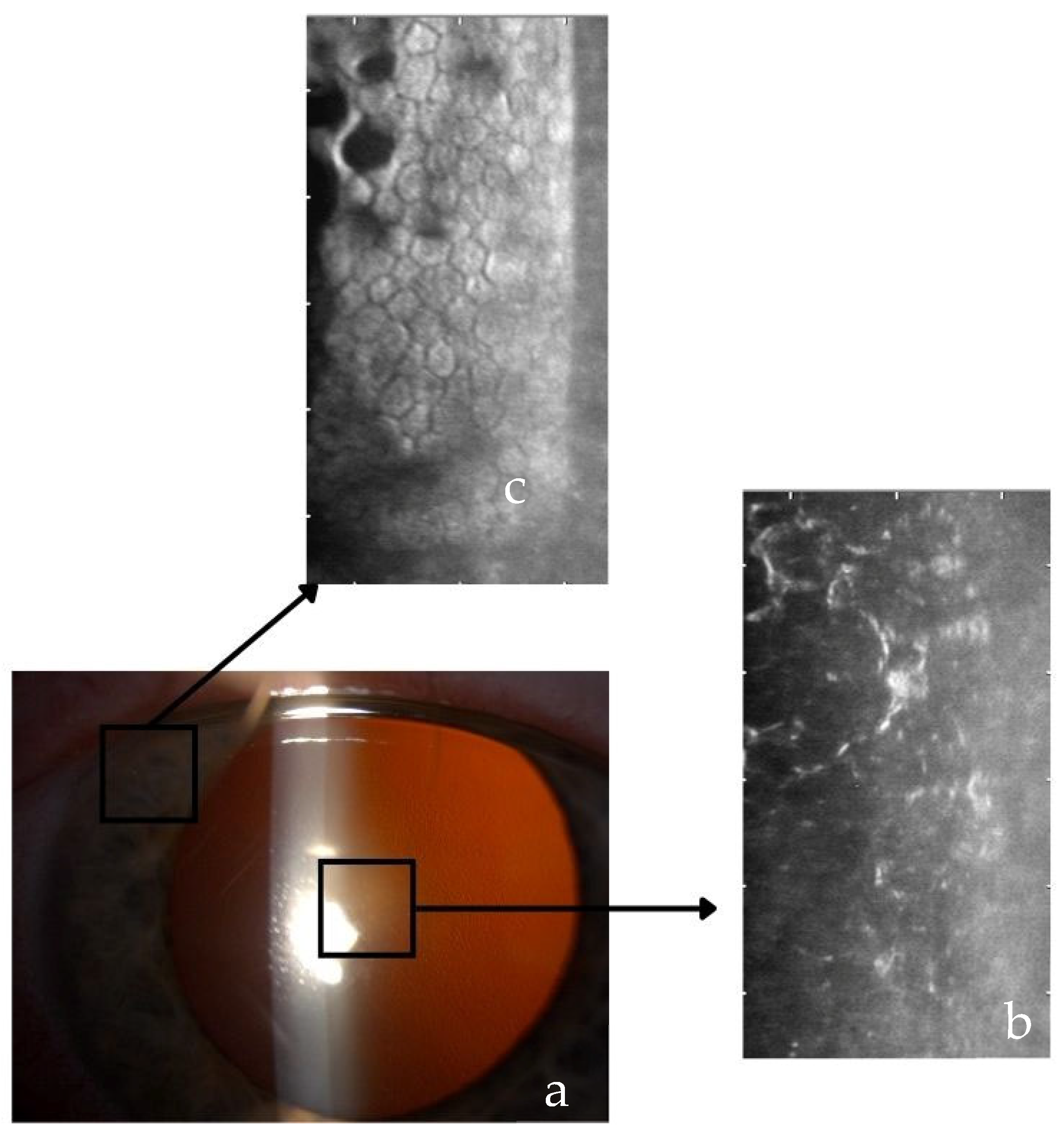
3.1. Clinical Staging and Symptomatic
3.2. Etiology of FECD
4. Genetics of FECD
4.1. Inheritance of FECD
4.2. FECD Genes
4.2.1. Early-Onset Subtype
4.2.2. Late-Onset Subtype
TCF4 Gene
SLC4A11 Gene and Other
5. Genetic Treatment for FECD
5.1. Established Treatments for FECD
5.2. Genetic Therapy Principles in FECD
5.3. Challenges of the CRISPR-Cas9 System in General
Specific Challenges of Applying CRISPR-Cas9 to TCF4
5.4. Real World Genetic Treatment for FECD
5.4.1. DT-168
5.4.2. Ad-Cas9-Col8a2gRNA
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | adeno-associated viruses |
| Cas9 | CRISPR-associated 9 |
| CECs | corneal endothelial cells |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| DMEK | Descemet membrane endothelial keratoplasty |
| DSO | descemetorhexis only |
| FECD | Fuchs endothelial corneal dystrophy |
| RPE | retinal pigment epithelium |
References
- Yi, L.Y.; Hsieh, H.H.; Lin, Z.Q.; Hung, K.F.; Sun, Y.C. Exploring the Role of ROCK Inhibition in Corneal Edema Through Crosstalk Between Epithelial and Endothelial Cells. J. Ophthalmol. 2024, 5, 9381303. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 15, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Ueno, M.; Toda, M.; Imai, K.; Tomioka, Y.; Numa, K.; Tanaka, H.; Inatomi, T.; Kameda, T.; Tsujikawa, A.; et al. Long-Term Corneal Rejuvenation after Transplantation of Cultured Human Corneal Endothelial Cells. Ophthalmology 2025, 132, 1105–1113. [Google Scholar] [CrossRef]
- Leroy, B.P.; Fischer, M.D.; Flannery, J.G.; MacLaren, R.E.; Dalkara, D.; Scholl, H.P.N.; Chung, D.C.; Spera, C.; Viriato, D.; Banhazi, J. Gene Therapy for Inherited Retinal Disease: Long-Term Durability of Effect. Ophthalmic Res. 2023, 66, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ong Tone, S.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef]
- Oie, Y.; Watanabe, S.; Nishida, K. Evaluation of Visual Quality in Patients with Fuchs Endothelial Corneal Dystrophy. Cornea 2016, 35 (Suppl. S1), S55–S58. [Google Scholar] [CrossRef]
- Bhadra, P.; Sahoo, S.; Sahu, S.K.; Priyadarshini, S.; Mohanty, A.; Das, S. Demographic profile and clinical characteristics of Fuchs’ endothelial corneal dystrophy in a tertiary eye care center. Indian J. Ophthalmol. 2023, 71, 505–509. [Google Scholar] [CrossRef]
- Watanabe, S.; Oie, Y.; Fujimoto, H.; Soma, T.; Koh, S.; Tsujikawa, M.; Maeda, N.; Nishida, K. Relationship between corneal guttae and quality of vision in patients with mild fuchs’ endothelial corneal dystrophy. Ophthalmology 2015, 122, 2103–2109. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Purcell, J.J., Jr.; Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar] [CrossRef]
- Zhang, J.; McGhee, C.N.J.; Patel, D.V. The Molecular Basis of Fuchs’ Endothelial Corneal Dystrophy. Mol. Diagn. Ther. 2019, 23, 97–112. [Google Scholar] [CrossRef]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W., Jr. Corneal endothelial dysfunction: Evolving understanding and treatment options. Prog. Retin. Eye Res. 2021, 82, 100904. [Google Scholar] [CrossRef] [PubMed]
- Nanda, G.G.; Alone, D.P. REVIEW: Current understanding of the pathogenesis of Fuchs’ endothelial corneal dystrophy. Mol. Vis. 2019, 5, 295–310. [Google Scholar]
- Biswas, S.; Munier, F.L.; Yardley, J.; Hart-Holden, N.; Perveen, R.; Cousin, P.; Sutphin, J.E.; Noble, B.; Batterbury, M.; Kielty, C.; et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum. Mol. Genet. 2001, 10, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Fujiki, K.; Murakami, A.; Kato, T.; Chen, L.Z.; Onoe, H.; Nakayasu, K.; Sakurai, M.; Takahashi, M.; Sugiyama, K.; et al. Analysis of COL8A2 gene mutation in Japanese patients with Fuchs’ endothelial dystrophy and posterior polymorphous dystrophy. Jpn. J. Ophthalmol. 2004, 48, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, J.D.; Sundin, O.H.; Rencs, E.V.; Emmert, D.G.; Stark, W.J.; Cheng, C.J.; Schmidt, G.W. Analysis and documentation of progression of Fuchs corneal dystrophy with retroillumination photography. Cornea 2006, 25, 485–489. [Google Scholar] [CrossRef]
- Liskova, P.; Prescott, Q.; Bhattacharya, S.S.; Tuft, S.J. British family with early-onset Fuchs’ endothelial corneal dystrophy associated with p.L450W mutation in the COL8A2 gene. Br. J. Ophthalmol. 2007, 91, 1717–1718. [Google Scholar] [CrossRef]
- Mootha, V.V.; Gong, X.; Ku, H.C.; Xing, C. Association and familial segregation of CTG18.1 trinucleotide repeat expansion of TCF4 gene in Fuchs’ endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 33–42. [Google Scholar] [CrossRef]
- Wieben, E.D.; Aleff, R.A.; Tosakulwong, N.; Butz, M.L.; Highsmith, W.E.; Edwards, A.O.; Baratz, K.H. A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2-2) gene predicts Fuchs corneal dystrophy. PLoS ONE 2012, 7, e49083. [Google Scholar] [CrossRef]
- Baratz, K.H.; Tosakulwong, N.; Ryu, E.; Brown, W.L.; Branham, K.; Chen, W.; Tran, K.D.; Schmid-Kubista, K.E.; Heckenlively, J.R.; Swaroop, A.; et al. E2-2 protein and Fuchs’s corneal dystrophy. N. Engl. J. Med. 2010, 363, 1016–1024. [Google Scholar] [CrossRef]
- Afshari, N.A.; Igo RPJr Morris, N.J.; Stambolian, D.; Sharma, S.; Pulagam, V.L.; Dunn, S.; Stamler, J.F.; Truitt, B.J.; Rimmler, J.; Kuot, A.; et al. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nat. Commun. 2017, 8, 14898. [Google Scholar] [CrossRef]
- Mootha, V.V.; Hansen, B.; Rong, Z.; Mammen, P.P.; Zhou, Z.; Xing, C.; Gong, X. Fuchs’ Endothelial Corneal Dystrophy and RNA Foci in Patients with Myotonic Dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4579–4585. [Google Scholar] [CrossRef]
- Winkler, N.S.; Milone, M.; Martinez-Thompson, J.M.; Raja, H.; Aleff, R.A.; Patel, S.V.; Fautsch, M.P.; Wieben, E.D.; Baratz, K.H. Fuchs’ Endothelial Corneal Dystrophy in Patients with Myotonic Dystrophy, Type 1. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3053–3057. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Morgan, P.E.; Ramprasad, V.; Tan, D.T.; Yong, V.H.; Venkataraman, D.; Venkatraman, A.; Yam, G.H.; Nagasamy, S.; Law, R.W.; et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum. Mol. Genet. 2008, 17, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Riazuddin, S.A.; Vithana, E.N.; Seet, L.F.; Liu, Y.; Al-Saif, A.; Koh, L.W.; Heng, Y.M.; Aung, T.; Meadows, D.N.; Eghrari, A.O.; et al. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophy. Hum. Mutat. 2010, 31, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Soumittra, N.; Loganathan, S.K.; Madhavan, D.; Ramprasad, V.L.; Arokiasamy, T.; Sumathi, S.; Karthiyayini, T.; Rachapalli, S.R.; Kumaramanickavel, G.; Casey, J.R.; et al. Biosynthetic and functional defects in newly identified SLC4A11 mutants and absence of COL8A2 mutations in Fuchs endothelial corneal dystrophy. J. Hum. Genet. 2014, 59, 444–453. [Google Scholar] [CrossRef]
- Mehta, J.S.; Vithana, E.N.; Tan, D.T.; Yong, V.H.; Yam, G.H.; Law, R.W.; Chong, W.G.; Pang, C.P.; Aung, T. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 184–188. [Google Scholar] [CrossRef]
- Gupta, R.; Kumawat, B.L.; Paliwal, P.; Tandon, R.; Sharma, N.; Sen, S.; Kashyap, S.; Nag, T.C.; Vajpayee, R.B.; Sharma, A. Association of ZEB1 and TCF4 rs613872 changes with late onset Fuchs endothelial corneal dystrophy in patients from northern India. Mol. Vis. 2015, 21, 1252–1260. [Google Scholar]
- Riazuddin, S.A.; Parker, D.S.; McGlumphy, E.J.; Oh, E.C.; Iliff, B.W.; Schmedt, T.; Jurkunas, U.; Schleif, R.; Katsanis, N.; Gottsch, J.D. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am. J. Hum. Genet. 2012, 90, 533–539. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Vasanth, S.; Katsanis, N.; Gottsch, J.D. Mutations in AGBL1 cause dominant late-onset Fuchs corneal dystrophy and alter protein-protein interaction with TCF4. Am. J. Hum. Genet. 2013, 93, 758–764. [Google Scholar] [CrossRef]
- Wieben, E.D.; Aleff, R.A.; Tang, X.; Kalari, K.R.; Maguire, L.J.; Patel, S.V.; Baratz, K.H.; Fautsch, M.P. Gene expression in the corneal endothelium of Fuchs endothelial corneal dystrophy patients with and without expansion of a trinucleotide repeat in TCF4. PLoS ONE 2018, 13, e0200005. [Google Scholar] [CrossRef]
- Synowiec, E.; Wojcik, K.A.; Izdebska, J.; Binczyk, E.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Polymorphisms of the homologous recombination gene RAD51 in keratoconus and Fuchs endothelial corneal dystrophy. Dis. Markers 2013, 35, 353–362. [Google Scholar] [CrossRef]
- Wojcik, K.A.; Synowiec, E.; Polakowski, P.; Głowacki, S.; Izdebska, J.; Lloyd, S.; Galea, D.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Polymorphism of the flap endonuclease 1 gene in keratoconus and Fuchs endothelial corneal dystrophy. Int. J. Mol. Sci. 2014, 15, 14786–14802. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, K.A.; Synowiec, E.; Polakowski, P.; Błasiak, J.; Szaflik, J.; Szaflik, J.P. Variation in DNA Base Excision Repair Genes in Fuchs Endothelial Corneal Dystrophy. Med. Sci. Monit. 2015, 21, 2809–2827. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, E.; Wojcik, K.A.; Izdebska, J.; Binczyk, E.; Szaflik, J.; Blasiak, J.; Szaflik, J.P. Polymorphism of the LIG3 gene in keratoconus and Fuchs endothelial corneal dystrophy. Cell Mol. Biol. 2015, 61, 56–63. [Google Scholar] [PubMed]
- Kuot, A.; Hewitt, A.W.; Griggs, K.; Klebe, S.; Mills, R.; Jhanji, V.; Craig, J.E.; Sharma, S.; Burdon, K.P. Association of TCF4 and CLU polymorphisms with Fuchs’ endothelial dystrophy and implication of CLU and TGFBI proteins in the disease process. Eur. J. Hum. Genet. 2012, 20, 632–638. [Google Scholar] [CrossRef]
- Kniestedt, C.; Taralczak, M.; Thiel, M.A.; Stuermer, J.; Baumer, A.; Gloor, B.P. A novel PITX2 mutation and a polymorphism in a 5-generation family with Axenfeld-Rieger anomaly and coexisting Fuchs’ endothelial dystrophy. Ophthalmology 2006, 113, e1–e8. [Google Scholar] [CrossRef]
- Li, Y.J.; Minear, M.A.; Qin, X.; Rimmler, J.; Hauser, M.A.; Allingham, R.R.; Igo, R.P.; Lass, J.H.; Iyengar, S.K.; Klintworth, G.K.; et al. Mitochondrial polymorphism A10398G and Haplogroup I are associated with Fuchs’ endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4577–4584. [Google Scholar] [CrossRef]
- Li, Q.J.; Ashraf, M.F.; Shen, D.F.; Green, W.R.; Stark, W.J.; Chan, C.C.; O’Brien, T.P. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch. Ophthalmol. 2001, 119, 1597–1604. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Jiao, X.; Li, A.; Sergeev, Y.V.; Ding, X.; Sharma, A.K.; Chan, C.C.; Medina, I.; Edwards, A.O. Mutations in KCNJ13 cause autosomal-dominant snowflake vitreoretinal degeneration. Am. J. Hum. Genet. 2008, 82, 174–180. [Google Scholar] [CrossRef]
- Foja, S.; Luther, M.; Hoffmann, K.; Rupprecht, A.; Gruenauer-Kloevekorn, C. CTG18.1 repeat expansion may reduce TCF4 gene expression in corneal endothelial cells of German patients with Fuchs’ dystrophy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1621–1631. [Google Scholar] [CrossRef]
- Fautsch, M.P.; Wieben, E.D.; Baratz, K.H.; Bhattacharyya, N.; Sadan, A.N.; Hafford-Tear, N.J.; Tuft, S.J.; Davidson, A.E. TCF4-mediated Fuchs endothelial corneal dystrophy: Insights into a common trinucleotide repeat-associated disease. Prog. Retin. Eye Res. 2021, 81, 100883. [Google Scholar] [CrossRef]
- Xing, C.; Gong, X.; Hussain, I.; Khor, C.C.; Tan, D.T.; Aung, T.; Mehta, J.S.; Vithana, E.N.; Mootha, V.V. Transethnic replication of association of CTG18.1 repeat expansion of TCF4 gene with Fuchs’ corneal dystrophy in Chinese implies common causal variant. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7073–7078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasanth, S.; Eghrari, A.O.; Gapsis, B.C.; Wang, J.; Haller, N.F.; Stark, W.J.; Katsanis, N.; Riazuddin, S.A.; Gottsch, J.D. Expansion of CTG18.1 Trinucleotide Repeat in TCF4 Is a Potent Driver of Fuchs’ Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- Tsedilina, T.R.; Sharova, E.; Iakovets, V.; Skorodumova, L.O. Systematic review of SLC4A11, ZEB1, LOXHD1, and AGBL1 variants in the development of Fuchs’ endothelial corneal dystrophy. Front. Med. 2023, 10, 1153122. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.S.; Møller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivelä, T.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.; Kim, E.K.; et al. IC3D classification of corneal dystrophies-edition 2. Cornea 2015, 34, 117–159, Erratum in Cornea 2015, 34, e32; Erratum in Cornea 2022, 41, e23. [Google Scholar] [CrossRef]
- Du, J.; Aleff, R.A.; Soragni, E.; Kalari, K.; Nie, J.; Tang, X.; Davila, J.; Kocher, J.P.; Patel, S.V.; Gottesfeld, J.M.; et al. RNA toxicity and missplicing in the common eye disease fuchs endothelial corneal dystrophy. J. Biol. Chem. 2015, 290, 5979–5990. [Google Scholar] [CrossRef]
- Wieben, E.D.; Aleff, R.A.; Tang, X.; Butz, M.L.; Kalari, K.R.; Highsmith, E.W.; Jen, J.; Vasmatzis, G.; Patel, S.V.; Maguire, L.J.; et al. Trinucleotide Repeat Expansion in the Transcription Factor 4 (TCF4) Gene Leads to Widespread mRNA Splicing Changes in Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 343–352. [Google Scholar] [CrossRef]
- Gurnani, B.; Somani, A.N.; Moshirfar, M.; Patel, B.C. Fuchs Endothelial Dystrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Malhotra, D.; Loganathan, S.K.; Chiu, A.M.; Lukowski, C.M.; Casey, J.R. Human Corneal Expression of SLC4A11, a Gene Mutated in Endothelial Corneal Dystrophies. Sci. Rep. 2019, 9, 9681. [Google Scholar] [CrossRef]
- Tourabaly, M.; Knoeri, J.; Georgeon, C.; Borderie, V. Review of the Literature: Surgery Indications for Fuchs’ Endothelial Corneal Dystrophy. J. Clin. Med. 2025, 14, 2365. [Google Scholar] [CrossRef]
- Hurley, D.J.; Murtagh, P.; Guerin, M. Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (UT-DSAEK) versus Descemet Membrane Endothelial Keratoplasty (DMEK)-a systematic review and meta-analysis. Eye 2023, 37, 3026–3032. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 6, CD012097. [Google Scholar] [CrossRef]
- Gorovoy, M.S. DMEK Complications. Cornea 2014, 33, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Mechels, K.B.; Greenwood, M.D.; Sudhagoni, R.G.; Berdahl, J.P. Influences on rebubble rate in Descemet’s membrane endothelial keratoplasty. Clin. Ophthalmol. 2017, 11, 2139–2144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerra, F.P.; Anshu, A.; Price, M.O.; Giebel, A.W.; Price, F.W. Descemet’s membrane endothelial keratoplasty: Prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology 2011, 118, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Machin, H.; Sutton, G.; Baird, P.N. Examining Corneal Tissue Exportation Fee and Its Impact on Equitable Allocation. Cornea 2022, 41, 390–395. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Schrittenlocher, S.; Bachmann, B.; Tiurbe, A.M.; Tuac, O.; Velten, K.; Schmidt, D.; Cursiefen, C. Impact of preoperative visual acuity on Descemet Membrane Endothelial Keratoplasty (DMEK) outcome. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 321–329. [Google Scholar] [CrossRef]
- Friedrich, M.; Son, H.S.; Lind, J.; Hammer, M.; Chychko, L.; Yildirim, T.M.; Auffarth, G.U.; Augustin, V.A. Preoperative edema severity affects outcomes after Descemet membrane endothelial keratoplasty for Fuchs endothelial corneal dystrophy: A cohort study. Eye Vis. 2025, 12, 9. [Google Scholar] [CrossRef]
- Garcerant, D.; Hirnschall, N.; Toalster, N.; Zhu, M.; Wen, L.; Moloney, G. Descemet’s stripping without endothelial keratoplasty. Curr. Opin. Ophthalmol. 2019, 30, 275–285. [Google Scholar] [CrossRef]
- Borkar, D.S.; Veldman, P.; Colby, K.A. Treatment of Fuchs Endothelial Dystrophy by Descemet Stripping without Endothelial Keratoplasty. Cornea 2016, 35, 1267–1273. [Google Scholar] [CrossRef]
- Williams, K.A.; Irani, Y.D. Gene Therapy and Gene Editing for the Corneal Dystrophies. Asia Pac. J. Ophthalmol. 2016, 5, 312–316. [Google Scholar] [CrossRef]
- Tetsch, L. The adaptive bacterial immune system CRISPR-Cas and its therapeutic potential. Med. Monatsschr. Pharm. 2017, 40, 17–23. [Google Scholar] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Cabral, T.; DiCarlo, J.E.; Justus, S.; Sengillo, J.D.; Xu, Y.; Tsang, S.H. CRISPR applications in ophthalmologic genome surgery. Curr. Opin. Ophthalmol. 2017, 28, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Aleman, T.S.; Jayasundera, K.T.; Ashimatey, B.S.; Kim, K.; Rashid, A.; Jaskolka, M.C.; Myers, R.L.; Lam, B.L.; Bailey, S.T.; et al. Gene Editing for CEP290-Associated Retinal Degeneration. N. Engl. J. Med. 2024, 390, 1972–1984. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860, Erratum in Lancet 2017, 390, 848. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishiguchi, K.M.; Fujita, K.; Miya, F.; Katayama, S.; Nakazawa, T. Single AAV-mediated mutation replacement genome editing in limited number of photoreceptors restores vision in mice. Nat. Commun. 2020, 11, 482. [Google Scholar] [CrossRef]
- Li, F.; Wing, K.; Wang, J.H.; Luu, C.D.; Bender, J.A.; Chen, J.; Wang, Q.; Lu, Q.; Nguyen Tran, M.T.; Young, K.M.; et al. Comparison of CRISPR/Cas Endonucleases for in vivo Retinal Gene Editing. Front. Cell. Neurosci. 2020, 14, 570917. [Google Scholar] [CrossRef]
- Gallego, C.; Gonçalves, M.A.F.V.; Wijnholds, J. Novel Therapeutic Approaches for the Treatment of Retinal Degenerative Diseases: Focus on CRISPR/Cas-Based Gene Editing. Front. Neurosci. 2020, 14, 838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, D.; Zhang, J.; Xu, J.; Chen, Y.E. Recent Advances in Improving Gene-Editing Specificity through CRISPR-Cas9 Nuclease Engineering. Cells 2022, 11, 2186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, W.; Luo, T.; Mao, L.; Wang, M. Spatiotemporal Delivery of CRISPR/Cas9 Genome Editing Machinery Using Stimuli-Responsive Vehicles. Angew. Chem. Int. Ed. Engl. 2021, 60, 8596–8606. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Noori, H.; Jahandideh, A.; Haeri Moghaddam, N.; Kamrani Mousavi, S.M.; Nourizadeh, H.; Saeedi, S.; Karimi, M.; Hamblin, M.R. Smart Strategies for Precise Delivery of CRISPR/Cas9 in Genome Editing. ACS Appl. Bio Mater. 2022, 5, 413–437. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Zheng, Z.; Nguyen, N.T.; Liebers, M.; Topkar, V.V.; Thapar, V.; Wyvekens, N.; Khayter, C.; Iafrate, A.J.; Le, L.P.; et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015, 33, 187–197. [Google Scholar] [CrossRef]
- Quinn, J.; Musa, A.; Kantor, A.; McClements, M.E.; Cehajic-Kapetanovic, J.; MacLaren, R.E.; Xue, K. Genome-Editing Strategies for Treating Human Retinal Degenerations. Hum. Gene Ther. 2021, 32, 247–259. [Google Scholar] [CrossRef]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Klermund, J.; Rhiel, M.; Kocher, T.; Chmielewski, K.O.; Bischof, J.; Andrieux, G.; El Gaz, M.; Hainzl, S.; Boerries, M.; Cornu, T.I.; et al. On- and off-target effects of paired CRISPR-Cas nickase in primary human cells. Mol. Ther. 2024, 32, 1298–1310. [Google Scholar] [CrossRef]
- Sundaresan, Y.; Yacoub, S.; Kodati, B.; Amankwa, C.E.; Raola, A.; Zode, G. Therapeutic applications of CRISPR/Cas9 gene editing technology for the treatment of ocular diseases. FEBS J. 2023, 290, 5248–5269. [Google Scholar] [CrossRef]
- Koo, T.; Lee, J.; Kim, J.S. Measuring and Reducing Off-Target Activities of Programmable Nucleases Including CRISPR-Cas9. Mol. Cells 2015, 38, 475–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Juárez, J.; Rodríguez-Uribe, G.; Borooah, S. Toward the Treatment of Inherited Diseases of the Retina Using CRISPR-Based Gene Editing. Front. Med. 2021, 8, 698521. [Google Scholar] [CrossRef] [PubMed]
- Müller Paul, H.; Istanto, D.D.; Heldenbrand, J.; Hudson, M.E. CROPSR: An automated platform for complex genome-wide CRISPR gRNA design and validation. BMC Bioinform. 2022, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Monteys, A.M.; Ebanks, S.A.; Keiser, M.S.; Davidson, B.L. CRISPR/Cas9 Editing of the Mutant Huntingtin Allele In Vitro and In Vivo. Mol. Ther. 2017, 25, 12–23. [Google Scholar] [CrossRef]
- Christie, K.A.; Robertson, L.J.; Conway, C.; Blighe, K.; DeDionisio, L.A.; Chao-Shern, C.; Kowalczyk, A.M.; Marshall, J.; Turnbull, D.; Nesbit, M.A.; et al. Mutation-Independent Allele-Specific Editing by CRISPR-Cas9, a Novel Approach to Treat Autosomal Dominant Disease. Mol. Ther. 2020, 28, 1846–1857. [Google Scholar] [CrossRef]
- Oyama, Y.; Ito, S.; Yuasa, T.; Ueda, M.; Chiba, S.; Nakagawa, T.; Izumi, A.; Ikawa, M.; Koizumi, N.; Okumura, N. Generation of a Mouse Model of Fuchs Endothelial Corneal Dystrophy by Knock-in of CTG Trinucleotide Repeat Expansion in the TCF4 Gene. Investig. Ophthalmol. Vis. Sci. 2025, 66, 18. [Google Scholar] [CrossRef]
- Okumura, N.; Hayashi, R.; Nakano, M.; Yoshii, K.; Tashiro, K.; Sato, T.; Blake, D.J.; Aleff, R.; Butz, M.; Highsmith, E.W.; et al. Effect of Trinucleotide Repeat Expansion on the Expression of TCF4 mRNA in Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 779–786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rong, Z.; Gong, X.; Hulleman, J.D.; Corey, D.R.; Mootha, V.V. Trinucleotide Repeat-Targeting dCas9 as a Therapeutic Strategy for Fuchs’ Endothelial Corneal Dystrophy. Transl. Vis. Sci. Technol. 2020, 9, 47. [Google Scholar] [CrossRef]
- Jun, A.S.; Ranganathan, V.; Zack, D. CRISPR/CAS9-Based Treatments. WO2017004616A1, 5 January 2017. Available online: https://patentimages.storage.googleapis.com/f1/03/93/8e4d244e564db4/WO2017004616A1.pdf (accessed on 1 August 2025).
- Powers, A.; Cheung, K.; Osgood, N.; Kudwa, A.; Shepard, P.; Datta, S.; Safadi, M.; Sinha, S.; Kerr, J.; Zhang, C.; et al. Pharmacological and molecular features of DT-168, a topical GeneTACTM small molecule being developed as potential treatment for Fuchs Endothelial Corneal Dystrophy caused by CTG repeat expansions in the TCF4 gene. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1333. [Google Scholar]
- Fuchs Endothelial Corneal Dystrophy Program. Available online: https://www.designtx.com/our-programs/#fuchs-endothelial-corneal-dystrophy (accessed on 1 August 2025).
- Uehara, H.; Zhang, X.; Pereira, F.; Narendran, S.; Choi, S.; Bhuvanagiri, S.; Liu, J.; Kumar, S.R.; Bohner, A.; Carroll, L.; et al. Start codon disruption with CRISPR/Cas9 prevents murine Fuchs’ endothelial corneal dystrophy. eLife 2021, 10, e55637. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, B.; Li, X.; Li, M.; Wang, Y.; Dan, H.; Zhou, J.; Wei, Y.; Ge, K.; Li, P.; et al. The application and progression of CRISPR/Cas9 technology in ophthalmological diseases. Eye 2023, 37, 607–617. [Google Scholar] [CrossRef]
- Gowda, D.A.A.; Birappa, G.; Rajkumar, S.; Ajaykumar, C.B.; Srikanth, B.; Kim, S.L.; Singh, V.; Jayachandran, A.; Lee, J.; Ramakrishna, S. Recent progress in CRISPR/Cas9 system for eye disorders. Prog. Mol. Biol. Transl. Sci. 2025, 210, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Rim, M.H.; Dean, C.; Aliaj, E.; Karas, B.L.; Barada, F.; Levitsky, A.M. Recent and anticipated novel drug approvals (3Q 2024 through 2Q 2025). Am. J. Health Syst. Pharm. 2024, 81, 1103–1108. [Google Scholar] [CrossRef]
- Mohan, R.R.; Martin, L.M.; Sinha, N.R. Novel insights into gene therapy in the cornea. Exp. Eye Res. 2021, 202, 108361. [Google Scholar] [CrossRef] [PubMed]
- Hudde, T.; Rayner, S.A.; De Alwis, M.; Thrasher, A.J.; Smith, J.; Coffin, R.S.; George, A.J.; Larkin, D.F. Adeno-associated and herpes simplex viruses as vectors for gene transfer to the corneal endothelium. Cornea 2000, 19, 369–373. [Google Scholar] [CrossRef]
- Klebe, S.; Sykes, P.J.; Coster, D.J.; Bloom, D.C.; Williams, K.A. Gene transfer to ovine corneal endothelium. Clin. Exp. Ophthalmol. 2001, 29, 316–322. [Google Scholar] [CrossRef]

| FECD | Gene | Mutation—Significance or ACMG Classification *: 5–1 | References |
|---|---|---|---|
| Early-onset | COL8A2 | Q455K-5 L450W-5 R155Q-1 R304Q-3 R434H-3 | [13,14,15,16] |
| Late-onset | TCF4 | CTG repeat within third intron—pathogenic above >40–50 repeats rs613872-3 rsl 7595731-3 rs9954153-3 rs2286812-3 rs784257-3 | [17,18,19,20] |
| DMPK | CTG repeat within the 3′ UTR—not common | [21,22] | |
| SLC4A11 | E399K-4 G709E-4 T754M-4 c.99–100delTC-4 E167D-3 R282P-3 Y526C-3 V575M-3 G583D-3 G742R-3 G834S-3 W240S-3 V507I-3 T434I-3 | [23,24,25] | |
| TCF8/ZEB1 (chr 10p11.22) | N78T-4 Q810P-4 Q840P-4 A905G-4 P649A-4 N696S-3 rs77516068-3 rsl49166539-3 IVS2+276-3 S234S-3 E733K-3 A818V-3 L947Stop-3 | [24,26,27] | |
| L0XHD1 | R547C-4 rs450997-3 | [20,28] | |
| AGBL1 | R1028Stop-3 c.2969G>C-3 | [29] | |
| KANK4 (chr 1q25.3) | rs79742895-3 | [20] | |
| LAMC1 | rs3768617-3 | [20,30] | |
| LINC00970/ATP1B1 | rsl022114-3 | [20] | |
| DNA Repair enzymes RAD51 FEN1 XRCC1 NEIL1 LIG3 | c.−61G>T/rs1801321)-3 c.−98G>C/rs1801320-3 rs4246215-4 c.H96A>G-4 g.46438521G>C-3rslO52536-3 rs3135967-3 | [31,32,33,34] | |
| Extracellular Matrix TGFBI CLU COL8A2 | one haplotype-3 rs 17466684-4 R304Q-3 R434H-3 | [34,35,36] | |
| Mitochondrial ND3 TSPOAP1 | A10398G-1 Variants-3,4 | [18,37] | |
| Other PITX2 PTPRG FASLG KCNJ13 | g.20913G>T-3 rs7640737-3 rs 10490775 c.−671A>G-3 R162W-4 | [19,36,38,39] |
| FECD | Locus on Chromosome | Reference |
|---|---|---|
| Early-onset | 1p34.3-p32 (FECD1) | [45] |
| Late-onset | 13pter-q12.13 (FECD2) 18q21.2-q21.3 (FECD3) 20p13-p12 (FECD4) 5q33.1-q35.2 (FECD5) 10p11.2 (FECD6) 9p24.1-p22.1 (FECD7) 15q25 (FECD8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stunf Pukl, S. Genetic Therapy of Fuchs Endothelial Corneal Dystrophy: Where Are We? A Review. Genes 2025, 16, 1222. https://doi.org/10.3390/genes16101222
Stunf Pukl S. Genetic Therapy of Fuchs Endothelial Corneal Dystrophy: Where Are We? A Review. Genes. 2025; 16(10):1222. https://doi.org/10.3390/genes16101222
Chicago/Turabian StyleStunf Pukl, Spela. 2025. "Genetic Therapy of Fuchs Endothelial Corneal Dystrophy: Where Are We? A Review" Genes 16, no. 10: 1222. https://doi.org/10.3390/genes16101222
APA StyleStunf Pukl, S. (2025). Genetic Therapy of Fuchs Endothelial Corneal Dystrophy: Where Are We? A Review. Genes, 16(10), 1222. https://doi.org/10.3390/genes16101222





