Chlamydia trachomatis: From Urogenital Infections to the Pathway of Infertility
Abstract
1. Introduction
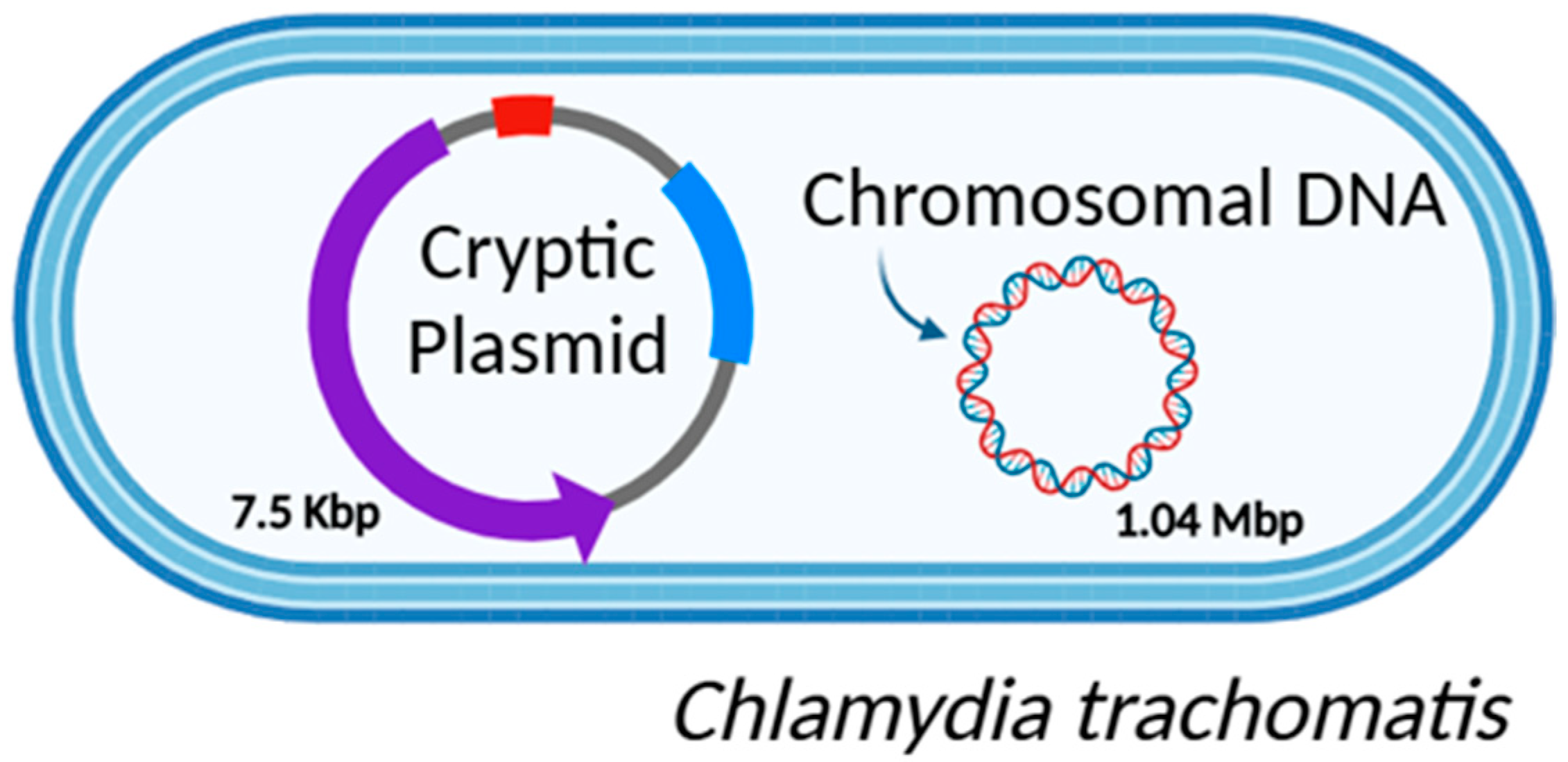
2. C. trachomatis Genome
2.1. CT Genomic DNA
2.2. CT Plasmid Diversity
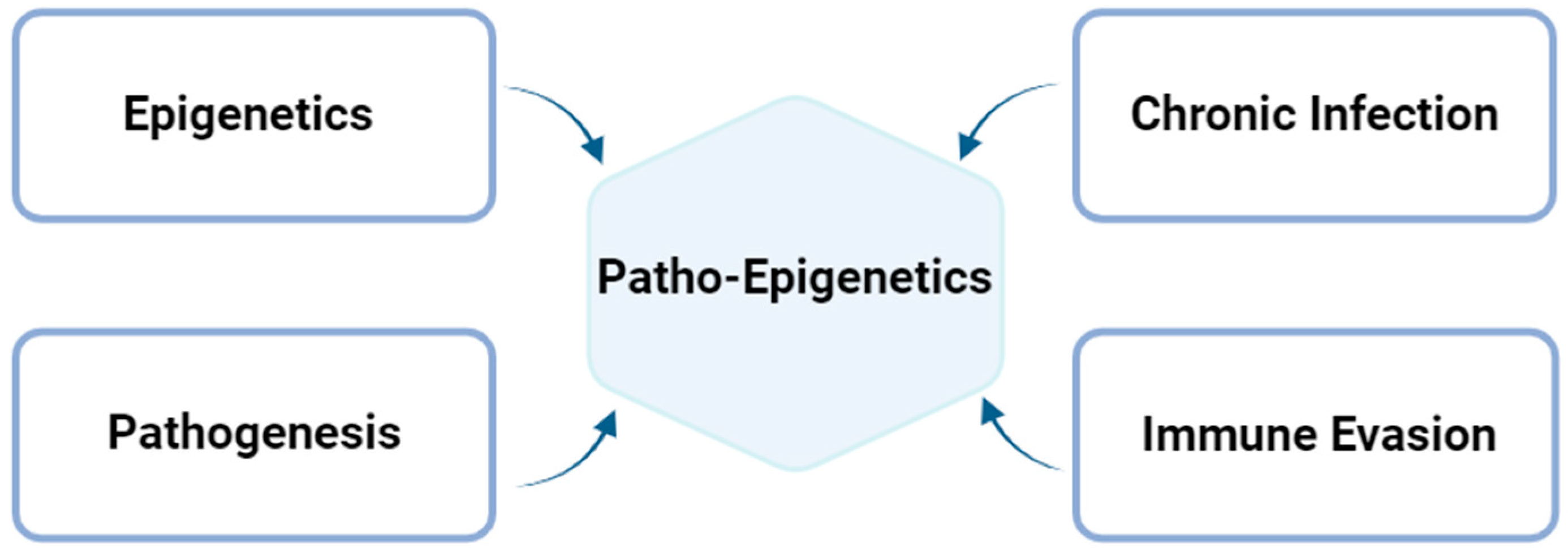
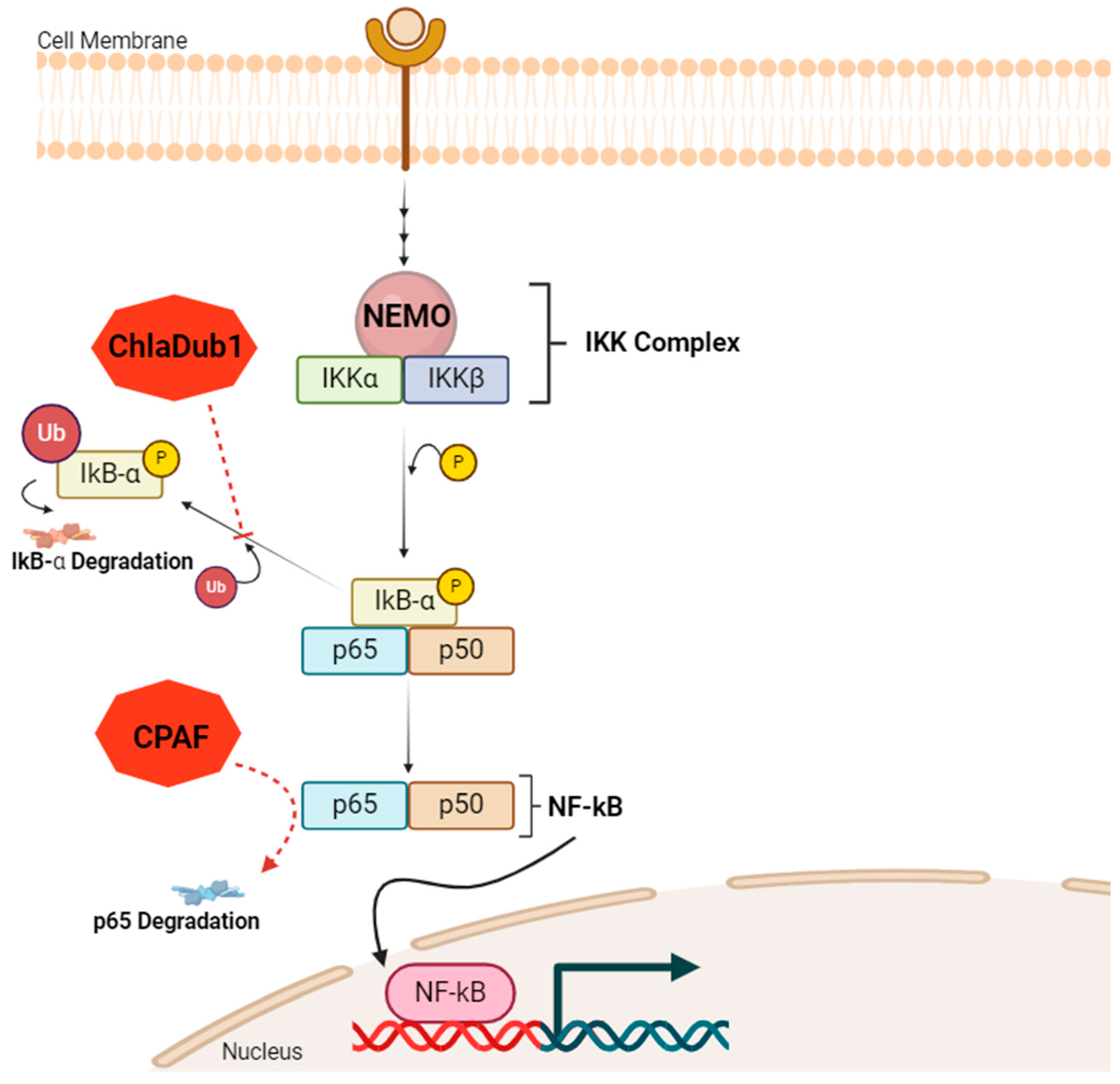
2.3. Patho-Epigenetics in Chlamydial Infection
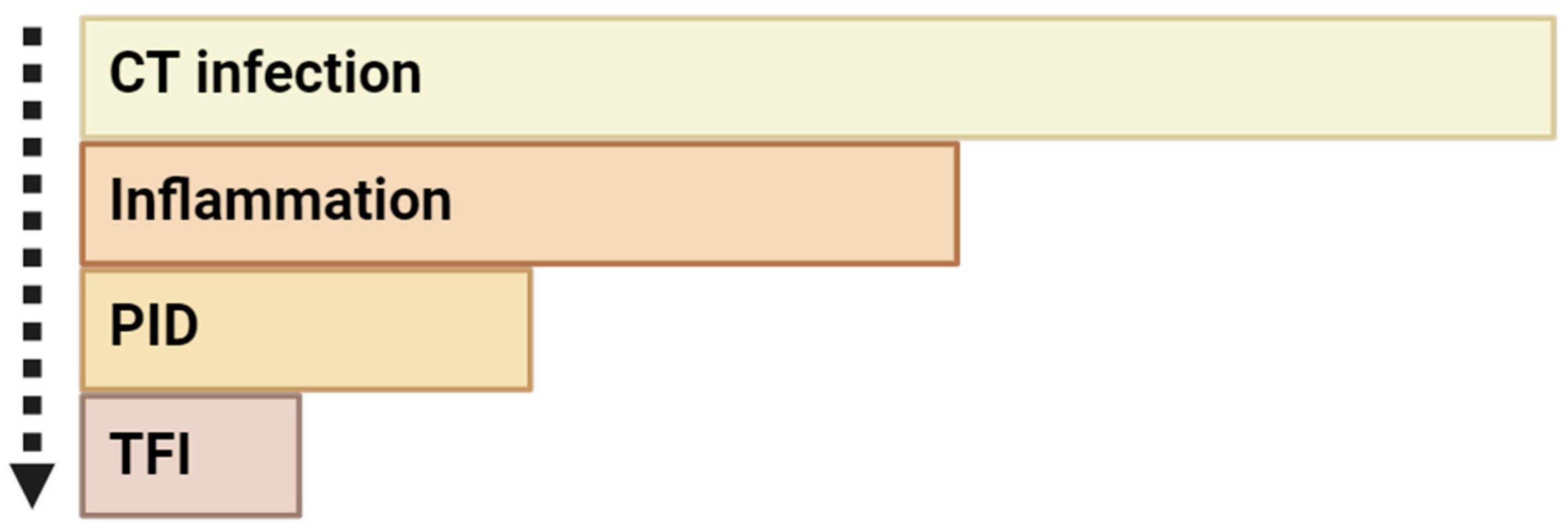
3. Long-Term Chlamydial Urogenital Infection and Infertility
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ECDC STI Cases on the Rise Across Europe. Available online: https://www.ecdc.europa.eu/en/news-events/sti-cases-rise-across-europe (accessed on 10 November 2024).
- Becker, Y. Chlamydia. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Rodrigues, R.; Sousa, C.; Vale, N. Chlamydia trachomatis as a Current Health Problem: Challenges and Opportunities. Diagnostics 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Sousa, C.; Vale, N. Deciphering the Puzzle: Literature Insights on Chlamydia trachomatis-Mediated Tumorigenesis, Paving the Way for Future Research. Microorganisms 2024, 12, 1126. [Google Scholar] [CrossRef]
- Stelzner, K.; Vollmuth, N.; Rudel, T. Intracellular lifestyle of Chlamydia trachomatis and host–pathogen interactions. Nat. Rev. Microbiol. 2023, 21, 448–462. [Google Scholar] [CrossRef]
- Witkin Steven, S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares Iara, M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, e00203-17. [Google Scholar] [CrossRef]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Bugalhão, J.N.; Mota, L.J. The multiple functions of the numerous Chlamydia trachomatis secreted proteins: The tip of the iceberg. Microb. Cell 2019, 6, 414–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Stephens, R.S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 1992, 69, 861–869. [Google Scholar] [CrossRef]
- Rodrigues, R.; Vieira-Baptista, P.; Catalão, C.; Borrego, M.J.; Sousa, C.; Vale, N. Chlamydial and Gonococcal Genital Infections: A Narrative Review. J. Pers. Med. 2023, 13, 1170. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Romano, S.; Sessa, R. Chlamydia trachomatis and Chlamydia pneumoniae Interaction with the Host: Latest Advances and Future Prospective. Microorganisms 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Marques, L.; Vieira-Baptista, P.; Sousa, C.; Vale, N. Therapeutic Options for Chlamydia trachomatis Infection: Present and Future. Antibiotics 2022, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Verma, R.; Dixit, S.; Igietseme, J.U.; Black, C.M.; Duncan, S.; Singh, S.R.; Dennis, V.A. Future of human Chlamydia vaccine: Potential of self-adjuvanting biodegradable nanoparticles as safe vaccine delivery vehicles. Expert Rev. Vaccines 2018, 17, 217–227. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, J.; Yu, J.; Sun, R.; Tuo, Y.; Bai, H. Insights into Chlamydia Development and Host Cells Response. Microorganisms 2024, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, M.R.; Kohlhoff, S.A. Treatment of chlamydial infections. Expert Opin. Pharmacother. 2012, 13, 545–552. [Google Scholar] [CrossRef]
- Riska Paul, F.; Kutlin, A.; Ajiboye, P.; Cua, A.; Roblin Patricia, M.; Hammerschlag Margaret, R. Genetic and Culture-Based Approaches for Detecting Macrolide Resistance in Chlamydia pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 3586–3590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Jury, B.; Fleming, C.; Huston, W.M.; Luu, L.D.W. Molecular pathogenesis of Chlamydia trachomatis. Front. Cell. Infect. Microbiol. 2023, 13, 1281823. [Google Scholar] [CrossRef] [PubMed]
- Ambildhuke, K.; Pajai, S.; Chimegave, A.; Mundhada, R.; Kabra, P. A Review of Tubal Factors Affecting Fertility and its Management. Cureus 2022, 14, 30990. [Google Scholar] [CrossRef]
- den Heijer, C.D.J.; Hoebe, C.; Driessen, J.H.M.; Wolffs, P.; van den Broek, I.V.F.; Hoenderboom, B.M.; Williams, R.; de Vries, F.; Dukers-Muijrers, N. Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease, Ectopic Pregnancy, and Female Infertility: A Retrospective Cohort Study Among Primary Care Patients. Clin. Infect. Dis. 2019, 69, 1517–1525. [Google Scholar] [CrossRef]
- Garcia, M.R.; Leslie, S.W.; Wray, A.A. Sexually Transmitted Infections; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hunt, S.; Vollenhoven, B. Pelvic inflammatory disease and infertility. Aust. J. Gen. Pract. 2023, 52, 215–218. [Google Scholar] [CrossRef]
- Jennings, L.K.; Krywko, D.M. Pelvic Inflammatory Disease; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mitchell, C.M.; Anyalechi, G.E.; Cohen, C.R.; Haggerty, C.L.; Manhart, L.E.; Hillier, S.L. Etiology and Diagnosis of Pelvic Inflammatory Disease: Looking Beyond Gonorrhea and Chlamydia. J. Infect. Dis. 2021, 224 (Suppl. 2), 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Donovan, B. Screening for genital Chlamydia trachomatis infection: Are men the forgotten reservoir? Med. J. Aust. 2003, 179, 124–125. [Google Scholar] [CrossRef]
- Mohseni, M.; Sung, S.; Takov, V. Chlamydia; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gaydos, C.A.; Ferrero, D.V.; Papp, J. Laboratory aspects of screening men for Chlamydia trachomatis in the new millennium. Sex. Transm. Dis. 2008, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.A.; Beagley, K.W. Male genital tract chlamydial infection: Implications for pathology and infertility. Biol. Reprod. 2008, 79, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Joki-Korpela, P.; Sahrakorpi, N.; Halttunen, M.; Surcel, H.M.; Paavonen, J.; Tiitinen, A. The role of Chlamydia trachomatis infection in male infertility. Fertil. Steril. 2009, 91, 1448–1450. [Google Scholar] [CrossRef]
- Rodrigues, R.; Silva, A.R.; Sousa, C.; Vale, N. Addressing Challenges in Chlamydia trachomatis Detection: A Comparative Review of Diagnostic Methods. Medicina 2024, 60, 1236. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.D.W.; Kasimov, V.; Phillips, S.; Myers, G.S.A.; Jelocnik, M. Genome organization and genomics in Chlamydia: Whole genome sequencing increases understanding of chlamydial virulence, evolution, and phylogeny. Front. Cell. Infect. Microbiol. 2023, 13, 1178736. [Google Scholar] [CrossRef]
- Meyer, T. Diagnostic Procedures to Detect Chlamydia trachomatis Infections. Microorganisms 2016, 4, 25. [Google Scholar] [CrossRef]
- Stephens, R.S.; Kalman, S.; Lammel, C.; Fan, J.; Marathe, R.; Aravind, L.; Mitchell, W.; Olinger, L.; Tatusov, R.L.; Zhao, Q.; et al. Genome Sequence of an Obligate Intracellular Pathogen of Humans: Chlamydia trachomatis. Science 1998, 282, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Seth-Smith, H.M.; Harris, S.R.; Skilton, R.J.; Radebe, F.M.; Golparian, D.; Shipitsyna, E.; Duy, P.T.; Scott, P.; Cutcliffe, L.T.; O’Neill, C.; et al. Whole-genome sequences of Chlamydia trachomatis directly from clinical samples without culture. Genome Res. 2013, 23, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Ouellette Scot, P.; Lee, J.; Cox John, V. Division without Binary Fission: Cell Division in the FtsZ-Less Chlamydia. J. Bacteriol. 2020, 202, e00252-20. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B.; Folga, B.A. Chlamydia trachomatis—An Emerging Old Entity? Microorganisms 2023, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Ceovic, R.; Gulin, S.J. Lymphogranuloma venereum: Diagnostic and treatment challenges. Infect. Drug Resist. 2015, 8, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ausar, S.F.; Larson, N.R.; Wei, Y.; Jain, A.; Middaugh, C.R. Subunit-based vaccines: Challenges in developing protein-based vaccines. Pract. Asp. Vaccine Dev. 2022, 4, 79–135. [Google Scholar]
- Roe, S.K.; Zhu, T.; Slepenkin, A.; Berges, A.; Fairman, J.; de la Maza, L.M.; Massari, P. Structural Assessment of Chlamydia trachomatis Major Outer Membrane Protein (MOMP)-Derived Vaccine Antigens and Immunological Profiling in Mice with Different Genetic Backgrounds. Vaccines 2024, 12, 789. [Google Scholar] [CrossRef]
- Thomson, N.R.; Clarke, I.N. Chlamydia trachomatis: Small genome, big challenges. Future Microbiol. 2010, 5, 555–561. [Google Scholar] [CrossRef]
- Holló, P.; Jókai, H.; Herszényi, K.; Kárpáti, S. Genitourethral infections caused by D-K serotypes of Chlamydia trachomatis. Orvosi Hetil. 2015, 156, 19–23. [Google Scholar] [CrossRef]
- Dunlop, E.M.; Harris, R.J.; Darougar, S.; Treharne, J.D.; Al-Egaily, S.S. Subclinical pneumonia due to serotypes D-K of Chlamydia trachomatis: Case reports of two infants. Br. J. Vener. Dis. 1980, 56, 337–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nunes, A.; Borrego, M.J.; Gomes, J.P. Genomic features beyond Chlamydia trachomatis phenotypes: What do we think we know? Infect. Genet. Evol. 2013, 16, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.I. Chlamydia trachomatis strains and virulence: Rethinking links to infection prevalence and disease severity. J. Infect. Dis. 2010, 201, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, N.L.; Polkinghorne, A.; Timms, P. Chlamydia genomics: Providing novel insights into chlamydial biology. Trends Microbiol. 2014, 22, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Dimond, Z.E.; Suchland, R.J.; Baid, S.; LaBrie, S.D.; Soules, K.R.; Stanley, J.; Carrell, S.; Kwong, F.; Wang, Y.; Rockey, D.D.; et al. Inter-species lateral gene transfer focused on the Chlamydia plasticity zone identifies loci associated with immediate cytotoxicity and inclusion stability. Mol. Microbiol. 2021, 116, 1433–1448. [Google Scholar] [CrossRef]
- da Cunha, M.; Pais, S.V.; Bugalhão, J.N.; Mota, L.J. The Chlamydia trachomatis type III secretion substrates CT142, CT143, and CT144 are secreted into the lumen of the inclusion. PLoS ONE 2017, 12, 178856. [Google Scholar] [CrossRef] [PubMed]
- Borges, V.; Nunes, A.; Ferreira, R.; Borrego, M.J.; Gomes, J.P. Directional evolution of Chlamydia trachomatis towards niche-specific adaptation. J. Bacteriol. 2012, 194, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Borges, V.; Ferreira, R.; Borrego, M.J.; Gomes, J.P.; Mota, L.J. Polymorphisms in inc proteins and differential expression of inc genes among Chlamydia trachomatis strains correlate with invasiveness and tropism of lymphogranuloma venereum isolates. J. Bacteriol. 2012, 194, 6574–6585. [Google Scholar] [CrossRef]
- Nunes, A.; Gomes, J.P.; Karunakaran, K.P.; Brunham, R.C. Bioinformatic Analysis of Chlamydia trachomatis Polymorphic Membrane Proteins PmpE, PmpF, PmpG and PmpH as Potential Vaccine Antigens. PLoS ONE 2015, 10, 131695. [Google Scholar] [CrossRef] [PubMed]
- Debrine Abigail, M.; Karplus, P.A.; Rockey Daniel, D. A structural foundation for studying chlamydial polymorphic membrane proteins. Microbiol. Spectr. 2023, 11, e03242-23. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.; Stojanov, M.; Greub, G.; Baud, D. Chlamydial polymorphic membrane proteins: Regulation, function and potential vaccine candidates. Virulence 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shui, J.; Luo, L.; Ao, C.; Lin, H.; Liang, Y.; Wang, L.; Wang, H.; Chen, H.; Tang, S. Identification and characterization of mixed infections of Chlamydia trachomatis via high-throughput sequencing. Front. Microbiol. 2022, 13, 1041789. [Google Scholar] [CrossRef]
- Millman, K.; Black, C.M.; Stamm, W.E.; Jones, R.B.; Hook, E.W.; Martin, D.H.; Bolan, G.; Tavaré, S.; Dean, D. Population-based genetic epidemiologic analysis of Chlamydia trachomatis serotypes and lack of association between ompA polymorphisms and clinical phenotypes. Microbes Infect. 2006, 8, 604–611. [Google Scholar] [CrossRef]
- Pawlikowska-Warych, M.; Śliwa-Dominiak, J.; Deptuła, W. Chlamydial plasmids and bacteriophages. Acta Biochim. Pol. 2015, 62, 1–6. [Google Scholar] [CrossRef]
- Versteeg, B.; Bruisten, S.M.; Pannekoek, Y.; Jolley, K.A.; Maiden, M.C.J.; van der Ende, A.; Harrison, O.B. Genomic analyses of the Chlamydia trachomatis core genome show an association between chromosomal genome, plasmid type and disease. BMC Genom. 2018, 19, 130. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Y.; Yu, Y.; OuYang, X.; Zhou, D.; Song, Y.; Jiao, J. Autophagy: The Misty Lands of Chlamydia trachomatis Infection. Front. Cell. Infect. Microbiol. 2024, 14, 1442995. [Google Scholar] [CrossRef]
- Yang, C.; Kari, L.; Lei, L.; Carlson, J.H.; Ma, L.; Couch, C.E.; Whitmire, W.M.; Bock, K.; Moore, I.; Bonner, C.; et al. Chlamydia trachomatis Plasmid Gene Protein 3 Is Essential for the Establishment of Persistent Infection and Associated Immunopathology. Bio 2020, 11, e01902-20. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Carlson, J.H.; Whitmire, W.M.; Kari, L.; Virtaneva, K.; Sturdevant, D.E.; Watkins, H.; Zhou, B.; Sturdevant, G.L.; Porcella, S.F.; et al. Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect. Immun. 2013, 81, 636–644. [Google Scholar] [CrossRef]
- Turman, B.J.; Alzhanov, D.; Nagarajan, U.M.; Darville, T.; O’Connell, C.M. Virulence Protein Pgp3 Is Insufficient to Mediate Plasmid-Dependent Infectivity of Chlamydia trachomatis. Infect. Immun. 2023, 91, 39222. [Google Scholar] [CrossRef]
- Jones, C.A.; Hadfield, J.; Thomson, N.R.; Cleary, D.W.; Marsh, P.; Clarke, I.N.; O’Neill, C.E. The Nature and Extent of Plasmid Variation in Chlamydia trachomatis. Microorganisms 2020, 8, 373. [Google Scholar] [CrossRef]
- Seth-Smith, H.M.; Harris, S.R.; Persson, K.; Marsh, P.; Barron, A.; Bignell, A.; Bjartling, C.; Clark, L.; Cutcliffe, L.T.; Lambden, P.R.; et al. Co-evolution of genomes and plasmids within Chlamydia trachomatis and the emergence in Sweden of a new variant strain. BMC Genom. 2009, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Guerra, M.R.; Katoku-Herrera, M.; Lopez-Hurtado, M.; Villagrana-Zesati, J.R.; de Haro-Cruz, M.J.; Guerra-Infante, F.M. Identification of a new variant of Chlamydia trachomatis in Mexico. Enfermedades Infecc. Microbiol. Clin. 2019, 37, 93–99. [Google Scholar] [CrossRef]
- Sigar, I.M.; Schripsema, J.H.; Wang, Y.; Clarke, I.N.; Cutcliffe, L.T.; Seth-Smith, H.M.; Thomson, N.R.; Bjartling, C.; Unemo, M.; Persson, K.; et al. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog. Dis. 2014, 70, 61–69. [Google Scholar] [CrossRef]
- Aboud, N.M.A.; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, 160–167. [Google Scholar] [CrossRef]
- Niller, H.H.; Minarovits, J. Patho-epigenetics of Infectious Diseases Caused by Intracellular Bacteria. In Patho-Epigenetics of Infectious Disease. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 879, pp. 107–130. [Google Scholar]
- Darville, T.; Hiltke, T.J. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J. Infect. Dis. 2010, 201, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N. Infection-induced epigenetic changes and their impact on the pathogenesis of diseases. Semin. Immunopathol. 2020, 42, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H.; Hamon, M.; Cossart, P. Epigenetics and bacterial infections. Cold Spring Harb. Perspect. Med. 2012, 2, 10272. [Google Scholar] [CrossRef]
- Hayward, R.J.; Marsh, J.W.; Humphrys, M.S.; Huston, W.M.; Myers, G.S.A. Chromatin accessibility dynamics of Chlamydia-infected epithelial cells. Epigenet. Chromatin 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A.; Thompson, L.M. Epigenetic changes induced by pathogenic Chlamydia spp. Pathog. Dis. 2023, 81, ftad034. [Google Scholar] [CrossRef]
- Raisch, J.; Darfeuille-Michaud, A.; Nguyen, H.T. Role of microRNAs in the immune system, inflammation and cancer. World J. Gastroenterol. 2013, 19, 2985–2996. [Google Scholar] [CrossRef]
- Howard, S.; Richardson, S.; Benyeogor, I.; Omosun, Y.; Dye, K.; Medhavi, F.; Lundy, S.; Adebayo, O.; Igietseme, J.U.; Eko, F.O. Differential miRNA Profiles Correlate with Disparate Immunity Outcomes Associated with Vaccine Immunization and Chlamydial Infection. Front. Immunol. 2021, 12, 625318. [Google Scholar] [CrossRef] [PubMed]
- Eicher, S.C.; Dehio, C. Systems-level analysis of host–pathogen interaction using RNA interference. New Biotechnol. 2013, 30, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jiang, X.; Huang, L. RNA Interference Technology. Compr. Biotechnol. 2019, 560–575. [Google Scholar] [CrossRef]
- Theotoki, E.I.; Pantazopoulou, V.I.; Georgiou, S.; Kakoulidis, P.; Filippa, V.; Stravopodis, D.J.; Anastasiadou, E. Dicing the Disease with Dicer: The Implications of Dicer Ribonuclease in Human Pathologies. Int. J. Mol. Sci. 2020, 21, 7223. [Google Scholar] [CrossRef]
- Igietseme, J.U.; Omosun, Y.; Partin, J.; Goldstein, J.; He, Q.; Joseph, K.; Ellerson, D.; Ansari, U.; Eko, F.O.; Bandea, C.; et al. Prevention of Chlamydia-induced infertility by inhibition of local caspase activity. J. Infect. Dis. 2013, 207, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Pouncey, D.L.; Eledge, M.R.; Bhattacharya, S.; Luo, C.; Weatherford, E.W.; Ojcius, D.M.; Rank, R.G. MicroRNAs Modulate Pathogenesis Resulting from Chlamydial Infection in Mice. Infect. Immun. 2017, 85, e00768-16. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Luense, L.J.; McGinnis, L.K.; Nothnick, W.B.; Christenson, L.K. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 2008, 149, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Azevedo, J.; Lisboa, C.; Fernandes, C.; Borrego, M.J.; Borges-Costa, J.; Reis, J.; Santiago, F.; Santos, A.; Alves, J. Guidelines for the Diagnosis and Treatment of Uncomplicated Non-Lymphogranuloma Venereum Chlamydia trachomatis Infection in Portugal. Acta Médica Port. 2024, 37, 475–482. [Google Scholar]
- Lanjouw, E.; Ouburg, S.; de Vries, H.J.; Stary, A.; Radcliffe, K.; Unemo, M. European guideline on the management of Chlamydia trachomatis infections. Int. J. STD AIDS 2016, 27, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.H.; Mirsalehian, A.; Bahador, A. Association of Chlamydia trachomatis with infertility and clinical manifestations: A systematic review and meta-analysis of case-control studies. Infect. Dis. 2016, 48, 517–523. [Google Scholar] [CrossRef]
- Starrs, A.M.; Ezeh, A.C.; Barker, G.; Basu, A.; Bertrand, J.T.; Blum, R.; Coll-Seck, A.M.; Grover, A.; Laski, L.; Roa, M.; et al. Accelerate progress-sexual and reproductive health and rights for all: Report of the Guttmacher. Lancet 2018, 391, 2642–2692. [Google Scholar] [CrossRef]
- WHO: Infertility. Available online: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility (accessed on 8 September 2024).
- CDC: Reproductive Health. Available online: https://www.cdc.gov/reproductive-health/about/index.html (accessed on 7 September 2024).
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Z. Association between serum Chlamydia trachomatis antibody levels and infertility among reproductive-aged women in the U.S. Front. Public Health 2023, 11, 1117245. [Google Scholar] [CrossRef]
- Russell, A.N.; Zheng, X.; O’Connell, C.M.; Taylor, B.D.; Wiesenfeld, H.C.; Hillier, S.L.; Zhong, W.; Darville, T. Analysis of Factors Driving Incident and Ascending Infection and the Role of Serum Antibody in Chlamydia trachomatis Genital Tract Infection. J. Infect. Dis. 2016, 213, 523–531. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.M.; Ferone, M.E. Chlamydia trachomatis Genital Infections. Microb. Cell 2016, 3, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.G.; Terraciano, P.; Wolf, N.; Oliveira, F.D.S.; Almeida, I.; Passos, E.P. The Correlation between Chlamydia trachomatis and Female Infertility: A Systematic Review. Rev. Bras. Ginecol. Obstet. 2022, 44, 614–620. [Google Scholar] [CrossRef]
- Keikha, M.; Hosseininasab-Nodoushan, S.A.; Sahebkar, A. Association between Chlamydia trachomatis Infection and Male Infertility: A Systematic Review and Meta-Analysis. Mini Rev. Med. Chem. 2023, 23, 746–755. [Google Scholar] [CrossRef] [PubMed]
- ECDC Factsheet About Chlamydia. Available online: https://www.ecdc.europa.eu/en/chlamydia/facts#:~:text=Chlamydia%20is%20responsible%20for%2050,than%20half%20of%20the%20cases (accessed on 4 December 2024).
- Mabonga, E.; Manabe, Y.C.; Elbireer, A.; Mbazira, J.K.; Nabaggala, M.S.; Kiragga, A.; Kisakye, J.; Gaydos, C.A.; Taylor, C.; Parkes-Ratanshi, R. Prevalence and predictors of asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae in a Ugandan population most at risk of HIV transmission. Int. J. STD AIDS 2021, 32, 510–516. [Google Scholar] [CrossRef]
- Reyes-Lacalle, A.; Carnicer-Pont, D.; Masvidal, M.G.; Montero-Pons, L.; Cabedo-Ferreiro, R.; Falguera-Puig, G. Prevalence and Characterization of Undiagnosed Youths at Risk of Chlamydia trachomatis Infection: A Cross-sectional Study. J. Low. Genit. Tract Dis. 2022, 26, 223–228. [Google Scholar] [CrossRef]
- Xiang, W.; Yu, N.; Lei, A.; Li, X.; Tan, S.; Huang, L.; Zhou, Z. Insights into Host Cell Cytokines in Chlamydia Infection. Front. Immunol. 2021, 12, 639834. [Google Scholar] [CrossRef]
- Buckner, L.R.; Lewis, M.E.; Greene, S.J.; Foster, T.P.; Quayle, A.J. Chlamydia trachomatis infection results in a modest pro-inflammatory cytokine response and a decrease in T cell chemokine secretion in human polarized endocervical epithelial cells. Cytokine 2013, 63, 151–165. [Google Scholar] [CrossRef]
- Darville, T. Pelvic Inflammatory Disease Due to Neisseria gonorrhoeae and Chlamydia trachomatis: Immune Evasion Mechanisms and Pathogenic Disease Pathways. J. Infect. Dis. 2021, 224, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, D.; Holzer, I.; Aschauer, J.; Selzer, C.; Parry, J.P.; Ott, J. Incidence and Causes of Tubal Occlusion in Infertility: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 3961. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Luo, L.; Dai, X.; Chen, H. Fallopian tubal infertility: The result of Chlamydia trachomatis-induced fallopian tubal fibrosis. Mol. Cell. Biochem. 2022, 477, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gradison, M. Pelvic inflammatory disease. Am. Fam. Physician 2012, 85, 791–796. [Google Scholar]
- Paavonen, J.; Turzanski Fortner, R.; Lehtinen, M.; Idahl, A. Chlamydia trachomatis, Pelvic Inflammatory Disease, and Epithelial Ovarian Cancer. J. Infect. Dis. 2021, 224, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hoenderboom, B.M.; van Benthem, B.H.B.; van Bergen, J.; Dukers-Muijrers, N.; Götz, H.M.; Hoebe, C.; Hogewoning, A.A.; Land, J.A.; van der Sande, M.A.B.; Morré, S.A.; et al. Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for chlamydia in a chlamydia screening trial. Sex. Transm. Infect. 2019, 95, 300–306. [Google Scholar]
- Mongane, J.; Hendwa, E.; Sengeyi, D.; Kajibwami, E.; Kampara, F.; Chentwali, S.; Kalegamire, C.; Barhishindi, I.; Kujirakwinja, Y.; Maningo, J.B.; et al. Association between bacterial vaginosis, Chlamydia trachomatis infection and tubal factor infertility in Bukavu, Democratic Republic of Congo. BMC Infect. Dis. 2024, 24, 480. [Google Scholar] [CrossRef]
- Vitale, S.G.; Mikuš, M.; Herman, M.; D’Alterio, M.N.; Goldštajn, M.Š.; Tesarik, J.; Angioni, S. Tubal factor infertility: Which is the possible role of tubal microbiota? A fresh look to a busy corner focusing on the potential role of hysteroscopy. Gynecol. Reprod. Endocrinol. Metab. 2023, 3, 88–93. [Google Scholar]
- In Brief: How Does the Immune System Work? Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany. 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279364/ (accessed on 26 November 2024).
- Tian, J.; Liu, H.; Che, J.; Song, L. Editorial: Innate immunity against intracellular bacteria: Mechanisms and strategies. Front. Immunol. 2024, 15, 1396114. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, M.C.; Singer, M.; Ouburg, S. The two-sided role of the vaginal microbiome in Chlamydia trachomatis and Mycoplasma genitalium pathogenesis. J. Reprod. Immunol. 2018, 130, 11–17. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Fang, C.; Li, Z. Insights into innate immune cell evasion by Chlamydia trachomatis. Front. Immunol. 2024, 15, 1289644. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wen, Y.; Li, Z. Clear Victory for Chlamydia: The Subversion of Host Innate Immunity. Front. Microbiol. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.; Greub, G.; Nardelli-Haefliger, D.; Baud, D. Genital Chlamydia trachomatis: Understanding the roles of innate and adaptive immunity in vaccine research. Clin. Microbiol. Rev. 2014, 27, 346–370. [Google Scholar] [CrossRef] [PubMed]
- Buchacher, T.; Ohradanova-Repic, A.; Stockinger, H.; Fischer, M.B.; Weber, V. M2 Polarization of Human Macrophages Favors Survival of the Intracellular Pathogen Chlamydia pneumoniae. PLoS ONE 2015, 10, e0143593. [Google Scholar] [CrossRef] [PubMed]
- Lausen, M.; Christiansen, G.; Bouet Guldbæk Poulsen, T.; Birkelund, S. Immunobiology of monocytes and macrophages during Chlamydia trachomatis infection. Microbes Infect. 2019, 21, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; He, P.; Zhou, Q.; Chen, H. The dual role of cytokine responses to Chlamydia trachomatis infection in host pathogen crosstalk. Microb. Pathog. 2022, 173 Pt A, 105812. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; McKay, P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine 2021, 39, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Alfano, V.; Pelloni, M.; Splendiani, E.; Po, A.; Paoli, D.; Ferretti, E.; Sessa, R. Chlamydia trachomatis elicits TLR3 expression but disrupts the inflammatory signaling down-modulating NFκB and IRF3 transcription factors in human Sertoli cells. J. Biol. Regul. Homeost. Agents 2020, 34, 977–986. [Google Scholar]
- Russell, T.; Richardson, D. The good Samaritan glutathione-S-transferase P1: An evolving relationship in nitric oxide metabolism mediated by the direct interactions between multiple effector molecules. Redox Biol. 2022, 59, 102568. [Google Scholar] [CrossRef] [PubMed]
- Le Negrate, G.; Krieg, A.; Faustin, B.; Loeffler, M.; Godzik, A.; Krajewski, S.; Reed, J.C. ChlaDub1 of Chlamydia trachomatis suppresses NF-κB activation and inhibits IκBα ubiquitination and degradation. Cell. Microbiol. 2008, 10, 1879–1892. [Google Scholar] [CrossRef]
- Christian, J.; Vier, J.; Paschen, S.A.; Häcker, G. Cleavage of the NF-κB family protein p65/RelA by the chlamydial protease-like activity factor (CPAF) impairs proinflammatory signaling in cells infected with Chlamydiae. J. Biol. Chem. 2010, 285, 41320–41327. [Google Scholar] [CrossRef] [PubMed]
- Del Balzo, D.; Capmany, A.; Cebrian, I.; Damiani, M.T. Chlamydia trachomatis Infection Impairs MHC-I Intracellular Trafficking and Antigen Cross-Presentation by Dendritic Cells. Front. Immunol. 2021, 12, 662096. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Fan, T.; Liu, L. Chlamydia inhibits interferon γ-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J. Exp. Med. 1999, 189, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, R.; Matthiesen, S.; Zaeck, L.M.; Finke, S.; Knittler, M.R. Chlamydia trachomatis Cell-to-Cell Spread through Tunneling Nanotubes. Microbiol. Spectr. 2022, 10, e02817-22. [Google Scholar] [CrossRef] [PubMed]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually transmitted diseases and infertility. Am. J. Obs. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yount, K.S.; Darville, T. Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines. Vaccines 2024, 12, 863. [Google Scholar] [CrossRef]
- Borgogna, J.-L.C.; Shardell, M.D.; Yeoman, C.J.; Ghanem, K.G.; Kadriu, H.; Ulanov, A.V.; Gaydos, C.A.; Hardick, J.; Robinson, C.K.; Bavoil, P.M.; et al. The association of Chlamydia trachomatis and Mycoplasma genitalium infection with the vaginal metabolome. Sci. Rep. 2020, 10, 3420. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, L.; Rogua, H.; El Mansouri, N.; Kassidi, F.; Aksim, M.; El Farouqi, A.; Chouham, S.; Nejmeddine, M. The association of Chlamydia trachomatis and human papillomavirus co-infection with abnormal cervical cytology among women in south of Morocco. Microb. Pathog. 2023, 175, 105971. [Google Scholar] [CrossRef]
- Tran, V.C.; Nguyen, S.H.; Bui, H.T.; Pham, T.D.; Van Nguyen, A.T. Impact of Gender, Location, and Seasonality on the Prevalence of Gonorrhea and Chlamydia Co-infections in Northern Vietnam. Indian. J. Microbiol. 2024. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Weidner, W.; Naber, K.G. Chlamydial infections in urology. World J. Urol. 2006, 24, 4–12. [Google Scholar] [CrossRef]
- Badalyan, R.R.; Fanarjyan, S.V.; Aghajanyan, I.G. Chlamydial and ureaplasmal infections in patients with nonbacterial chronic prostatitis. Andrologia 2003, 35, 263–265. [Google Scholar] [CrossRef]
- Ouzounova-Raykova, V.; Ouzounova, I.; Mitov, I.G. May Chlamydia trachomatis be an aetiological agent of chronic prostatic infection? Andrologia 2010, 42, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Ostaszewska, I.; Zdrodowska-Stefanow, B.; Badyda, J.; Pucilo, K.; Trybula, J.; Bulhak, V. Chlamydia trachomatis: Probable cause of prostatitis. Int. J. STD AIDS 1998, 9, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.H.; Mirsalehian, A.; Sadighi Gilani, M.A.; Bahador, A.; Afraz, K. Association of asymptomatic Chlamydia trachomatis infection with male infertility and the effect of antibiotic therapy in improvement of semen quality in infected infertile men. Andrologia 2018, 50, e12944. [Google Scholar] [CrossRef]
- Ahmadi, K.; Moosavian, M.; Mardaneh, J.; Pouresmaeil, O.; Afzali, M. Prevalence of Chlamydia trachomatis, Ureaplasma parvum and Mycoplasma genitalium in Infertile Couples and the Effect on Semen Parameters. Ethiop. J. Health Sci. 2023, 33, 133–142. [Google Scholar]
- Samplaski, M.K.; Domes, T.; Jarvi, K.A. Chlamydial Infection and Its Role in Male Infertility. Adv. Androl. 2014, 2014, 307950. [Google Scholar] [CrossRef]
- Stojanov, M.; Baud, D.; Greub, G.; Vulliemoz, N. Male infertility: The intracellular bacterial hypothesis. New Microbes New Infect. 2018, 26, 37–41. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial Infections Affect Male Fertility: A Focus on the Oxidative Stress-Autophagy Axis. Front. Cell Dev. Biol. 2021, 9, 727812. [Google Scholar] [CrossRef]
- Moazenchi, M.; Totonchi, M.; Salman Yazdi, R.; Hratian, K.; Mohseni Meybodi, M.A.; Ahmadi Panah, M.; Chehrazi, M.; Mohseni Meybodi, A. The impact of Chlamydia trachomatis infection on sperm parameters and male fertility: A comprehensive study. Int. J. STD AIDS 2018, 29, 466–473. [Google Scholar] [CrossRef]
- Makarounis, K.; Leventopoulos, M.; Georgoulias, G.; Nikolopoulos, D.; Zeginiadou, T.; Xountasi, M.; Kotrotsos, P.; Nosi, E.; Gennimata, V.; Venieratos, D.; et al. Detection of Chlamydia trachomatis inside spermatozoa using flow cytometry: Effects of antibiotic treatment (before and after) on sperm count parameters. J. Microbiol. Methods 2022, 203, 106604. [Google Scholar] [CrossRef] [PubMed]
- Haratian, K.; Borjian Boroujeni, P.; Sabbaghian, M.; Maghareh Abed, E.; Moazenchi, M.; Mohseni Meybodi, A. DEFB126 2-nt Deletion (rs11467417) as a Potential Risk Factor for Chlamydia trachomatis Infection and Subsequent Infertility in Iranian Men. J. Reprod. Infertil. 2024, 25, 20–27. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Diaco, F.; Sessa, R. In Vitro Modelling of Chlamydia trachomatis Infection in the Etiopathogenesis of Male Infertility and Reactive Arthritis. Front. Cell. Infect. Microbiol. 2022, 12, 840802. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.J.; Patel, M. The economic impact of infertility on women in developing countries—A systematic review. Facts Views Vis. Obgyn 2012, 4, 102–109. [Google Scholar]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Adamson, G.D.; Boivin, J.; Chambers, G.M.; de Geyter, C.; Dyer, S.; Inhorn, M.C.; Schmidt, L.; Serour, G.I.; Tarlatzis, B.; et al. Declining global fertility rates and the implications for family planning and family building: An IFFS consensus document based on a narrative review of the literature. Hum. Reprod. Update 2024, 30, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, M.; Wright-Schnapp, T.J.; Chandra, A.; Johnson, R.; Satterwhite, C.L.; Pulver, A.; Berman, S.M.; Wang, R.Y.; Farr, S.L.; Pollack, L.A. A public health focus on infertility prevention, detection, and management. Fertil. Steril. 2010, 93, 161–1610. [Google Scholar] [CrossRef]
- Bowen, V.B.; Braxton, J.; Davis, D.W.; Flagg, E.W.; Grey, J.; Grier, L.; Harvey, A.; Kidd, S.; Kreisel, K.; Llata, E.; et al. Sexually Transmitted Disease Surveillance; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. [Google Scholar] [CrossRef]

| Biovar | Serovar |
|---|---|
| Trachoma | A |
| B | |
| Ba | |
| C | |
| Ano-urogenital | D |
| Da | |
| E | |
| F | |
| G | |
| Ga | |
| H | |
| I | |
| Ia | |
| J | |
| Ja | |
| K | |
| LGV | L1 |
| L2 | |
| L2a | |
| L2b | |
| L3 |
| Genomic Elements | Characteristics/Functions |
|---|---|
| Plasticity zone (PZ) | Contains more than 45 genes (Trp operons; tox). Highly variable among the distinct CT strains. |
| T3SS | Contains 20–25 genes encoding membrane-associated components and surface structures. Crucial role involved in the translocation of effector proteins into host cells. |
| TarP | T3SS effector. Involved in actin cytoskeleton remodeling, facilitating CT entry into host cells. Gene variations contribute to bacterial virulence and tissue tropism. |
| Incs | Associated with tissue tropisms and disease severity between LGV and trachoma biovars. Gene variations may influence host–pathogen interactions, tissue tropism, and immune recognition. |
| Pmps | Encodes for potential virulent factors and some membrane proteins. Potentially involved in CT attachment, invasion, and immune evasion processes. |
| ompA | Highly polymorphic. Encodes for the CT major outer membrane protein (MOMP), used for serovar classification. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.; Sousa, C.; Barros, A.; Vale, N. Chlamydia trachomatis: From Urogenital Infections to the Pathway of Infertility. Genes 2025, 16, 205. https://doi.org/10.3390/genes16020205
Rodrigues R, Sousa C, Barros A, Vale N. Chlamydia trachomatis: From Urogenital Infections to the Pathway of Infertility. Genes. 2025; 16(2):205. https://doi.org/10.3390/genes16020205
Chicago/Turabian StyleRodrigues, Rafaela, Carlos Sousa, Alberto Barros, and Nuno Vale. 2025. "Chlamydia trachomatis: From Urogenital Infections to the Pathway of Infertility" Genes 16, no. 2: 205. https://doi.org/10.3390/genes16020205
APA StyleRodrigues, R., Sousa, C., Barros, A., & Vale, N. (2025). Chlamydia trachomatis: From Urogenital Infections to the Pathway of Infertility. Genes, 16(2), 205. https://doi.org/10.3390/genes16020205








