Identification of the SbDUF966 Gene Family in Sorghum and Investigation of It Role in Response to Abiotic Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Sorghum SbDUF966 Gene Family and Analysis of Chromosomal Localization
2.2. Analysis of SbDUF966 Chromosome Localization and Physicochemical Properties
2.3. Analysis of SbDUF966 Gene Structure, Conserved Motifs and Conserved Domains
2.4. Evolutionary Relationship Analysis of SbDUF966
2.5. Predictive Analysis of Cis-Elements of the SbDUF966 Promoter
2.6. Syntenic Analysis of the SbDUF966 Family Genes
2.7. Prediction of miRNAs Targeting SbDUF966
2.8. Analysis of SbDUF966 Gene Expression Pattern in Different Tissues
2.9. Plant Material and Abiotic Stresses
2.10. qRT-PCR Analysis
3. Results
3.1. Identification and Characterization of the Sorghum SbDUF966 Genes
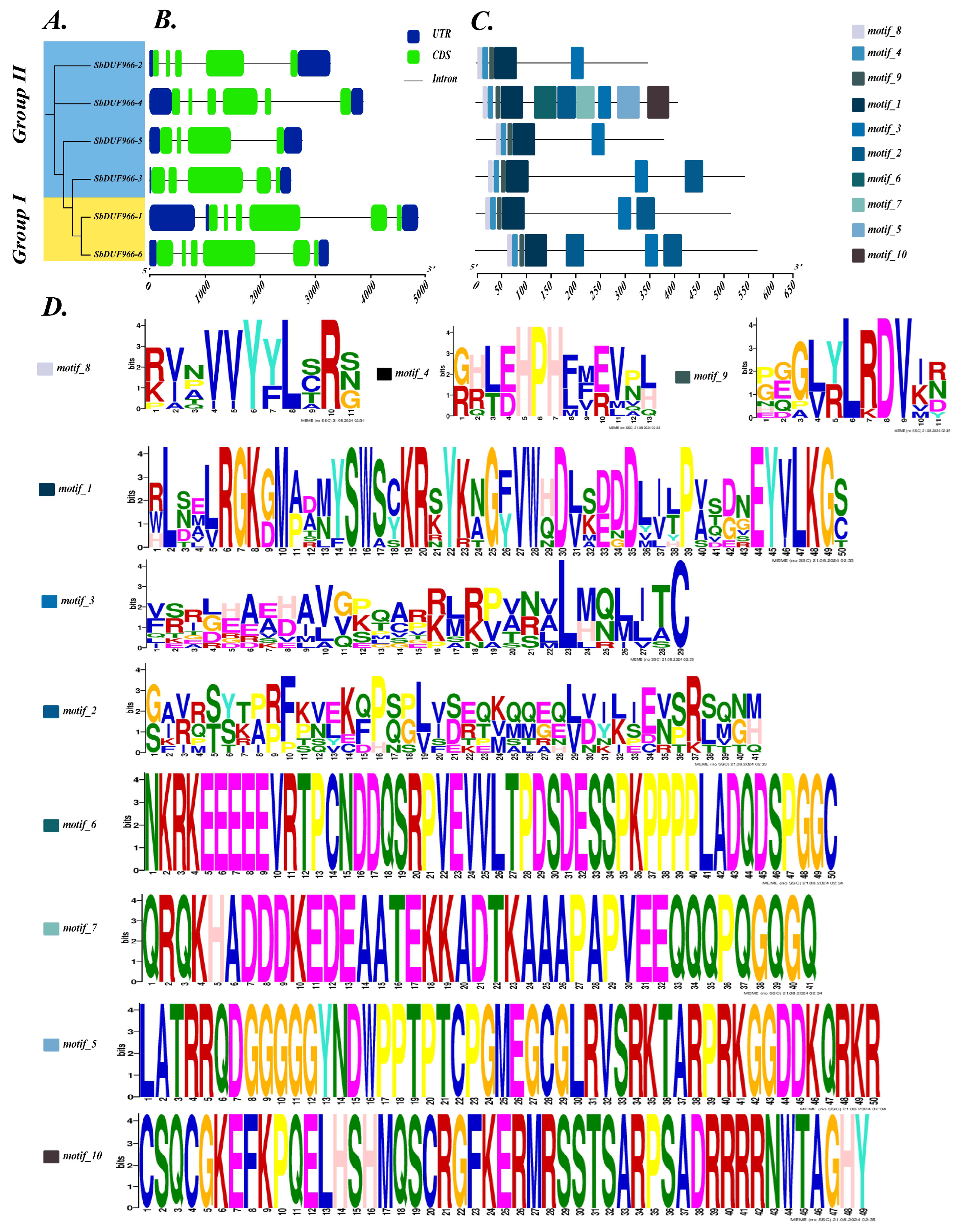
3.2. Phylogenetic Analysis of SbDUF966
3.3. Gene Structure and Motif Composition of SbDUF966
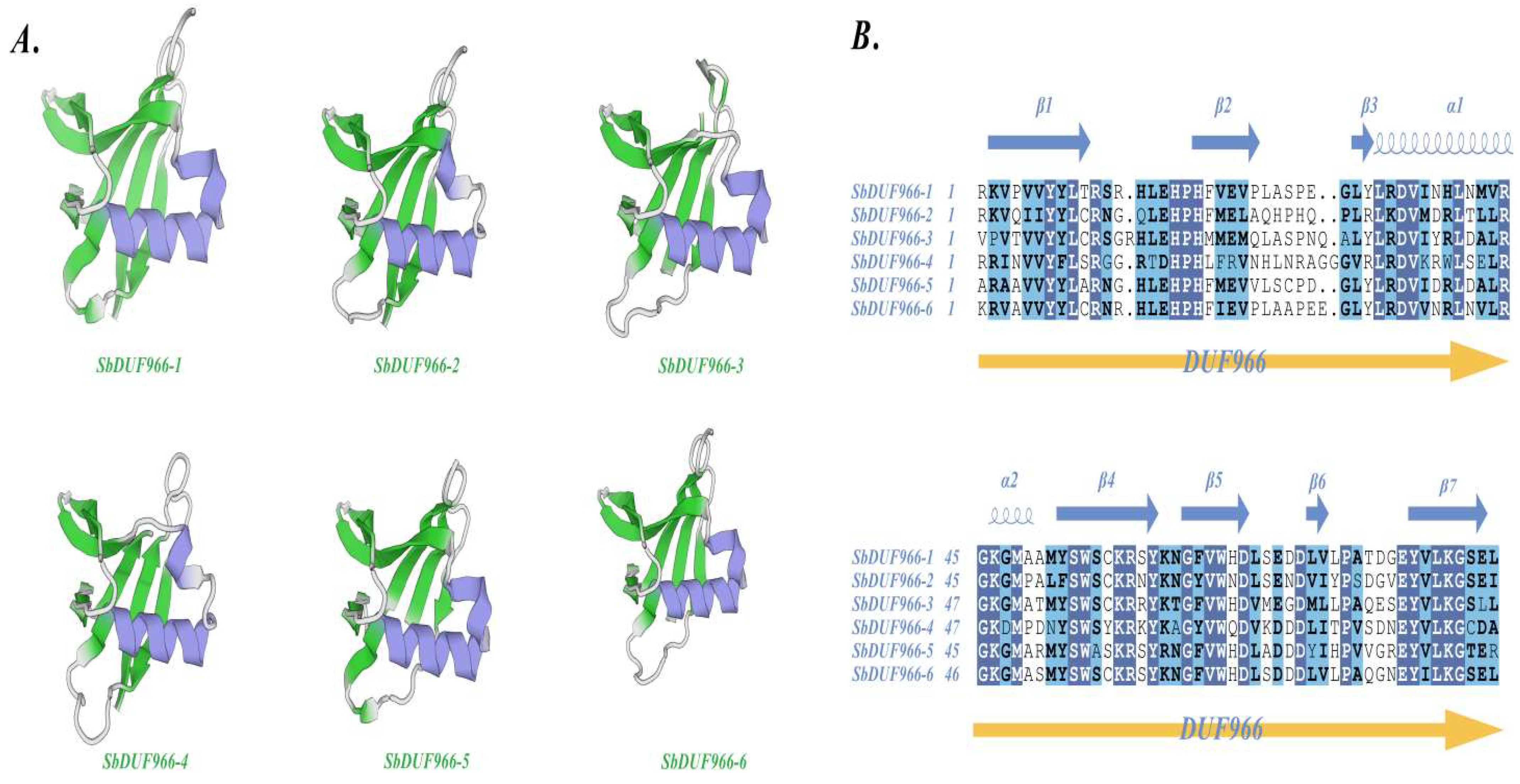
3.4. Tertiary Structure Prediction and Multiple Sequence Comparison of SbDUF966 Protein
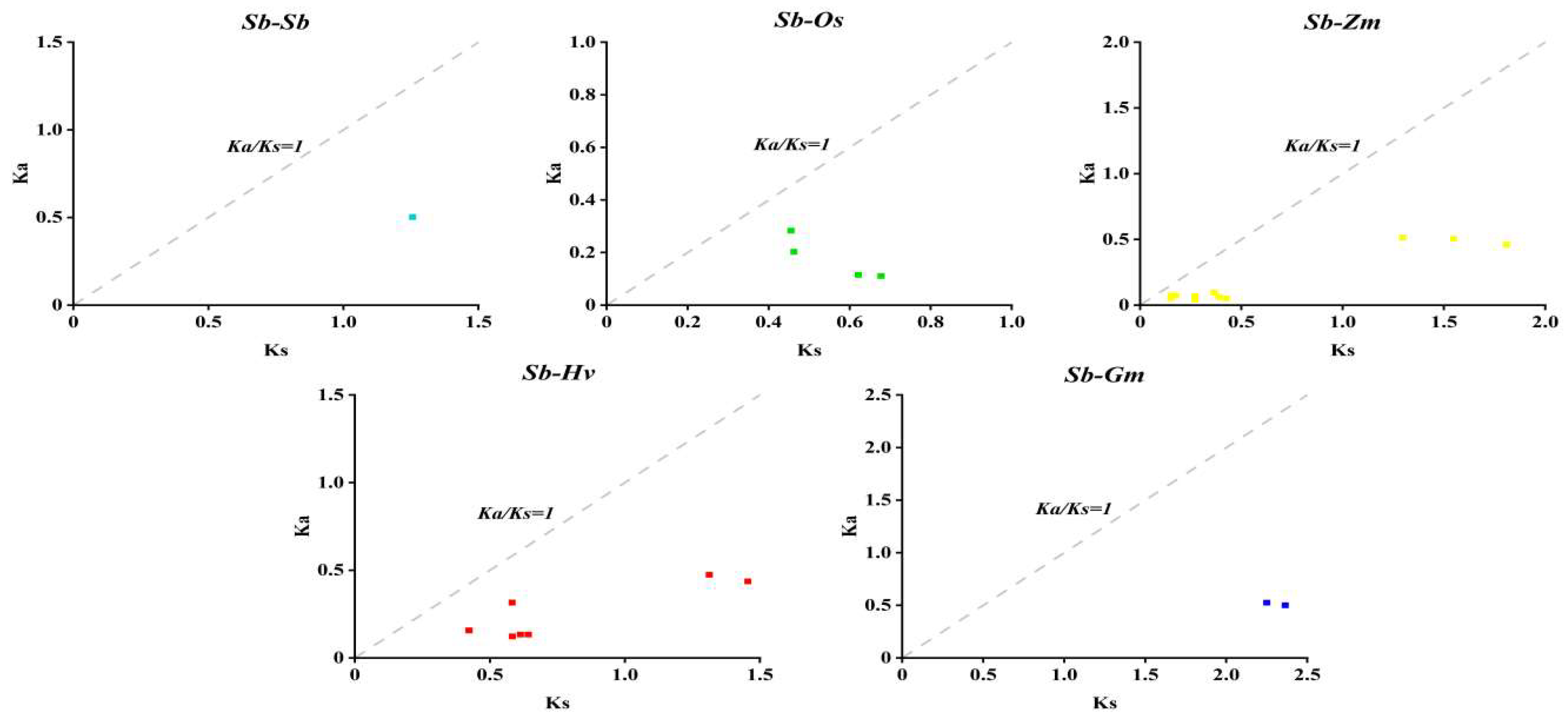
3.5. Analysis of Covariance Between SbDUF966 Gene Duplication Events and Other Species
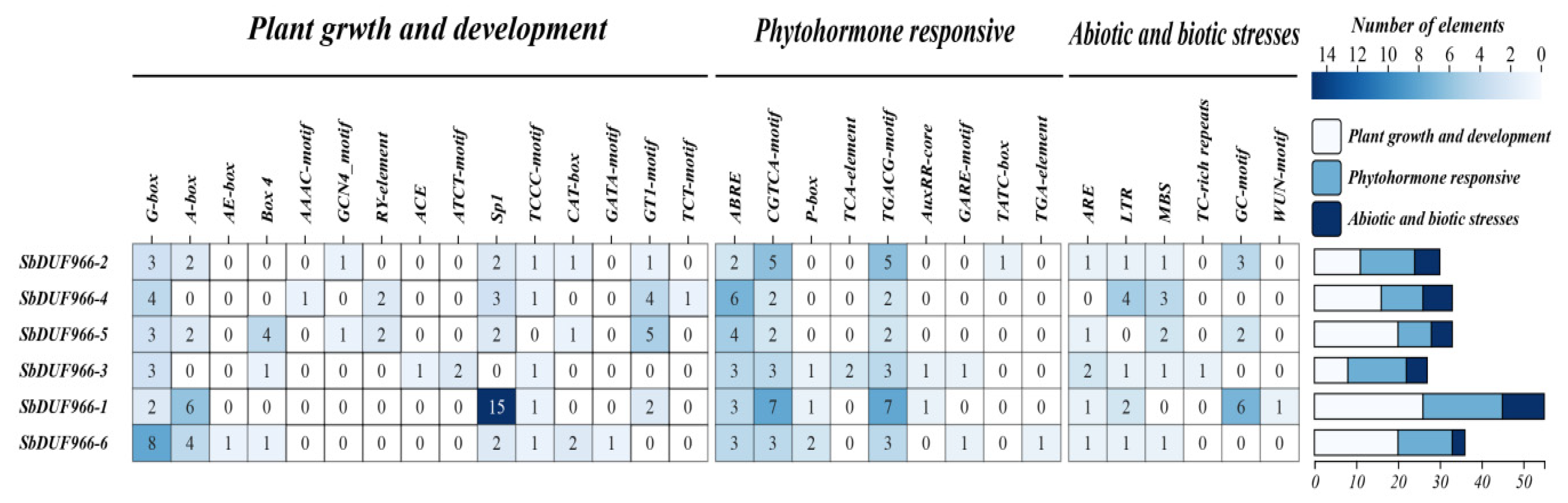
3.6. Analysis of Promoter Cis-Acting Elements of SbDUF966
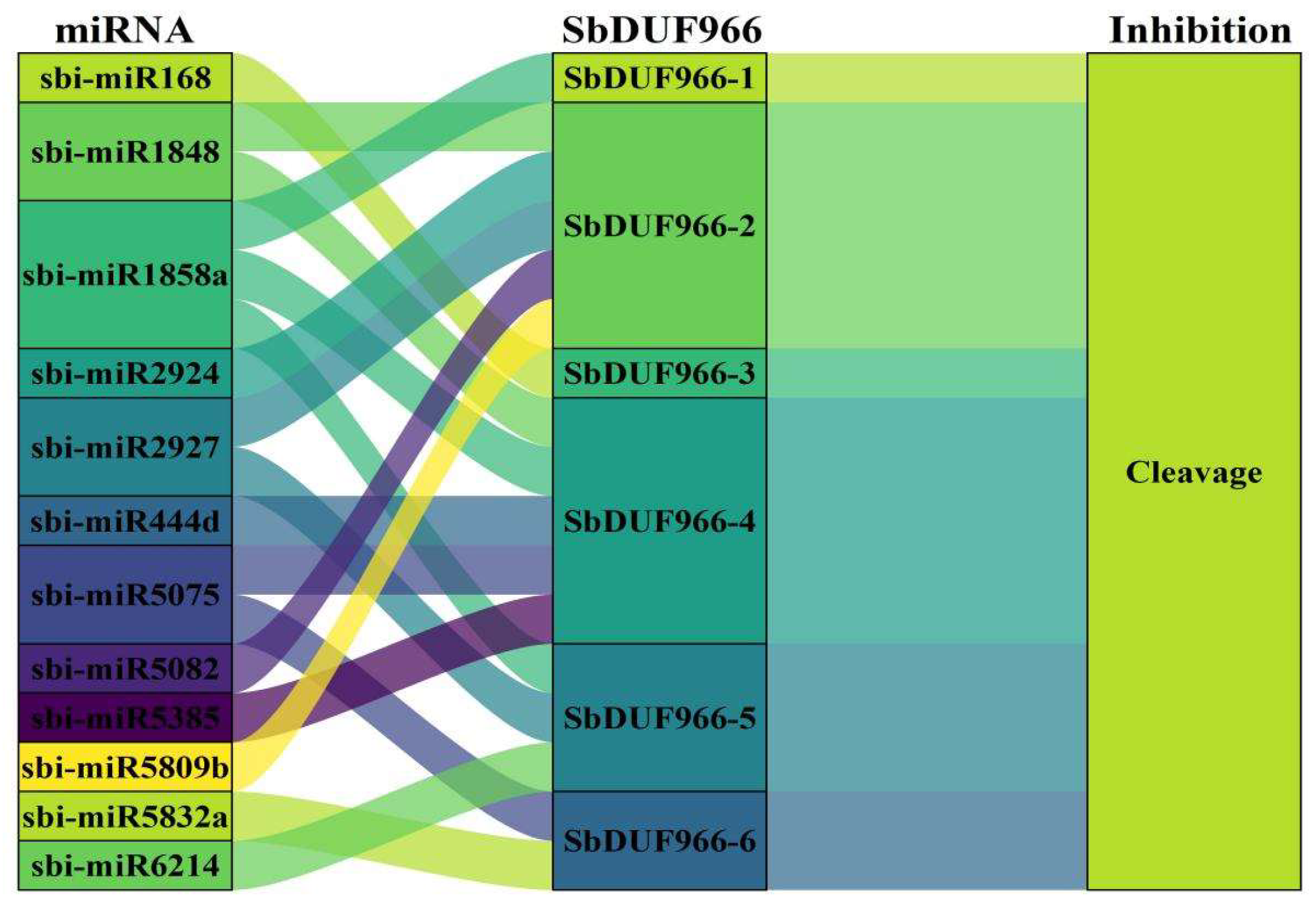
3.7. miRNA-Mediated Regulatory Mechanisms of SbDUF966 Expression
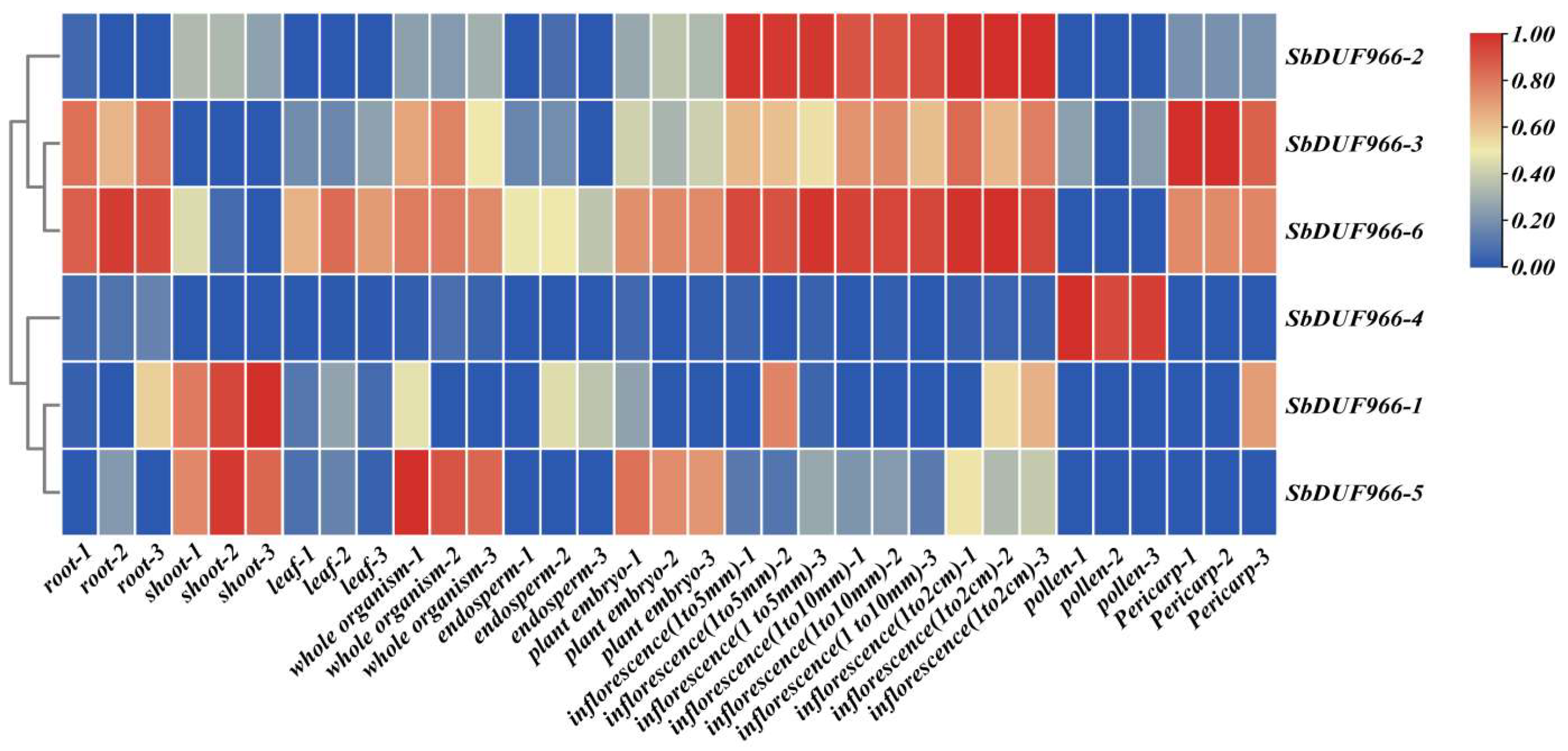
3.8. Expression Profile of SbDUF966 in Different Tissues
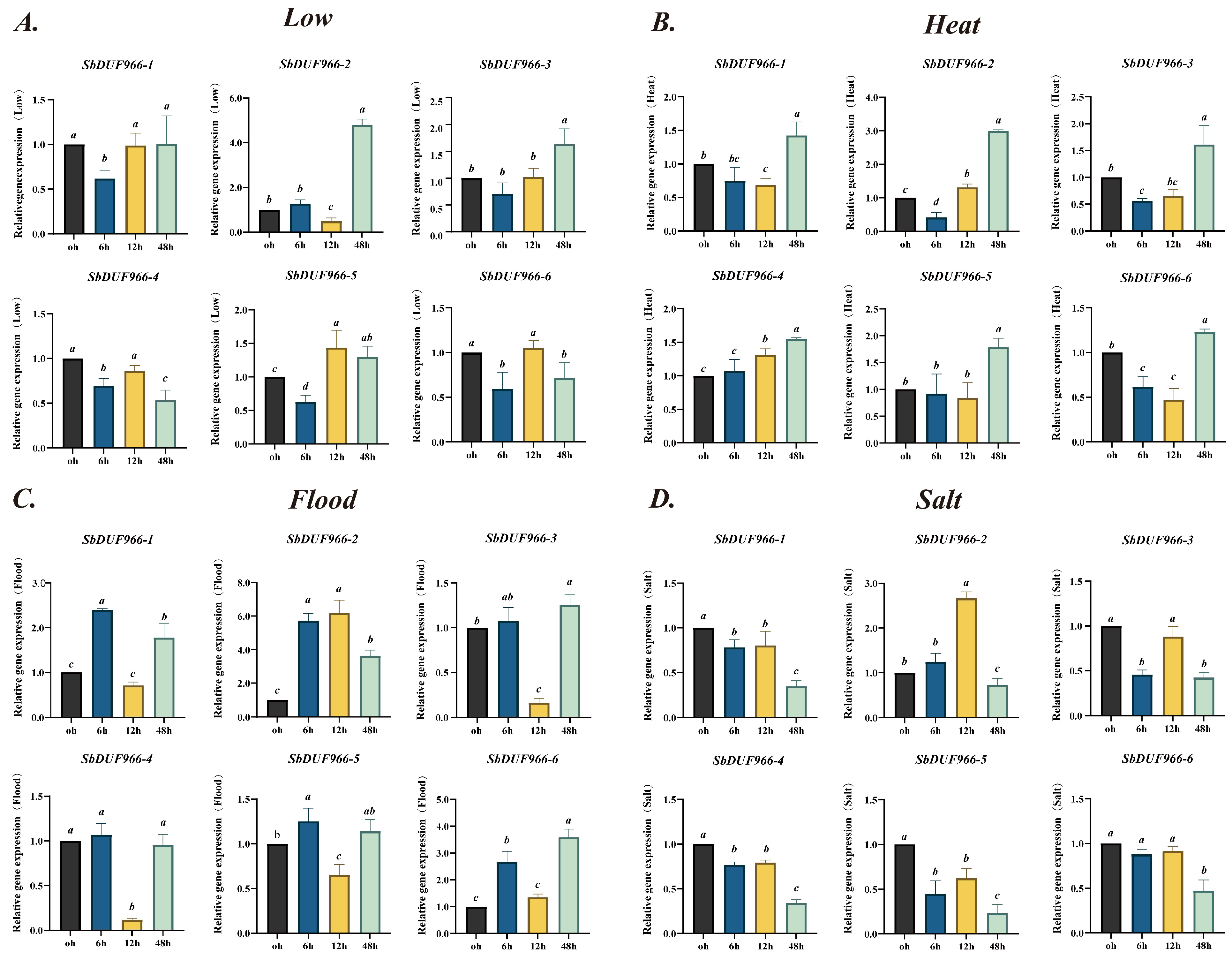
3.9. Analysis of SbDUF966 Gene Expression Under Abiotic Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bateman, A.; Coggill, P.; Finn, R.D. DUFs: Families in search of function. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1148–1152. [Google Scholar] [CrossRef]
- Goodacre, N.F.; Gerloff, D.L.; Uetz, P. Protein domains of unknown function are essential in bacteria. MBio 2014, 5, e00744-13. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Akhtar, M.; Min, W.; Bai, X.; Ma, T.; Liu, C. Domain of unknown function (DUF) proteins in plants: Function and perspective. Protoplasma 2024, 261, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Wan, J.; Zhang, C.; Hina, A.; Al Amin, G.; Begum, N.; Zhao, T. Unraveling the diverse roles of neglected genes containing domains of unknown function (DUFs): Progress and perspective. Int. J. Mol. Sci. 2023, 24, 4187. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Yoshikawa, M.; Kamada, A.; Ohtsuki, T.; Uchida, A.; Nakayama, K.; Satoh, H. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J. Plant Physiol. 2013, 170, 1549–1552. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Tan, L.; Liu, F.; Zhu, Z.; Fu, Y.; Sun, X.; Sun, X.; Xie, D.; Sun, C. TH1, a DUF640 domain-like gene controls lemma and palea development in rice. Plant Mol. Biol. 2012, 78, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Valderrama, J.E.; Gómez-Maqueo, X.; Salazar-Iribe, A.; Zúñiga-Sánchez, E.; Hernández-Barrera, A.; Quezada-Rodríguez, E.; Gamboa-deBuen, A. Overview of the role of cell wall DUF642 proteins in plant development. Int. J. Mol. Sci. 2019, 20, 3333. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Huang, X. Characterization of an Arabidopsis thaliana DUF761-containing protein with a potential role in development and defense responses. Theor. Exp. Plant Physiol. 2019, 31, 303–316. [Google Scholar] [CrossRef]
- Sakata, M.; Takano-Kai, N.; Miyazaki, Y.; Kanamori, H.; Wu, J.; Matsumoto, T.; Doi, K.; Yasui, H.; Yoshimura, A.; Yamagata, Y. Domain unknown function DUF1668-containing genes in multiple lineages are responsible for F1 pollen sterility in rice. Front. Plant Sci. 2021, 11, 632420. [Google Scholar] [CrossRef]
- Stonebloom, S.; Ebert, B.; Xiong, G.; Pattathil, S.; Birdseye, D.; Lao, J.; Pauly, M.; Hahn, M.G.; Heazlewood, J.L.; Scheller, H.V. A DUF-246 family glycosyltransferase-like gene affects male fertility and the biosynthesis of pectic arabinogalactans. BMC Plant Biol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, C.; Zhang, W.; Zheng, C.; Zhang, S.; Jia, H.; Yang, Y. Characterization of the DUF868 gene family in Nicotiana and functional analysis of NtDUF868-E5 involved in pigment metabolism. Plant Physiol. Biochem. 2024, 208, 108493. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.B.S.; Tayade, R.; Imran, Q.M.; Hussain, A.; Shahid, M.; Yun, B.-W. Functional insight of nitric-oxide induced DUF genes in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Guo, C.; Luo, C.; Guo, L.; Li, M.; Guo, X.; Zhang, Y.; Wang, L.; Chen, L. OsSIDP366, a DUF1644 gene, positively regulates responses to drought and salt stresses in rice. J. Integr. Plant Biol. 2016, 58, 492–502. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, M.; Zhou, H.; Li, M.; Huang, L.; Yin, X.; Zhao, G.; Lin, F.; Xia, X.; Xu, G. OsSGL, a novel DUF1645 domain-containing protein, confers enhanced drought tolerance in transgenic rice and Arabidopsis. Front. Plant Sci. 2016, 7, 2001. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lv, M.; Li, J.; Ye, T.; Li, X.; He, X.; Rong, S.; Dong, Y.; Guan, Y.; Zhao, L. Molecular characterisation and function analysis of the rice OsDUF872 family. Biotechnol. Biotechnol. Equip. 2018, 32, 881–887. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Y.; Li, X. Overexpression of OsDUF6 increases salt stress tolerance in rice. BMC Plant Biol. 2024, 24, 216. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Hu, Y.; Zhong, Q.; Yin, J.; Zhu, Y. Mining the roles of cucumber DUF966 genes in fruit development and stress response. Plants 2022, 11, 2497. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhong, T.; Wang, L.; Zhang, Q.; Jin, H.; Xu, M.; Ye, J. Characterization the role of a UFC homolog, AtAuxRP3, in the regulation of Arabidopsis seedling growth and stress response. J. Plant Physiol. 2019, 240, 152990. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, X.; Shao, W.; Song, J.; Jiang, W.; He, Y.; Yin, J.; Ma, D.; Qiao, Y. Genome-wide mining of wheat DUF966 gene family provides new insights into salt stress responses. Front. Plant Sci. 2020, 11, 569838. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Guo, C.; Wang, W.; Wang, L.; Chen, L. Overexpression of a new stress-repressive gene OsDSR2 encoding a protein with a DUF966 domain increases salt and simulated drought stress sensitivities and reduces ABA sensitivity in rice. Plant Cell Rep. 2014, 33, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, Y.; Akhtar, M.; Liu, C.; Ma, T.; Min, W.; Bai, X.; She, Y.; Chen, L.; Tian, L. A DUF966 gene family member OsDSR3 positively regulates alkali stress tolerance in rice. Plant Sci. 2024, 343, 112072. [Google Scholar] [CrossRef]
- Huang, R.-D. Research progress on plant tolerance to soil salinity and alkalinity in sorghum. J. Integr. Agric. 2018, 17, 739–746. [Google Scholar] [CrossRef]
- Tu, M.; Du, C.; Yu, B.; Wang, G.; Deng, Y.; Wang, Y.; Chen, M.; Chang, J.; Yang, G.; He, G. Current advances in the molecular regulation of abiotic stress tolerance in sorghum via transcriptomic, proteomic, and metabolomic approaches. Front. Plant Sci. 2023, 14, 1147328. [Google Scholar] [CrossRef] [PubMed]
- Baqi, A.; Samiullah; Rehman, W.; Bibi, I.; Menaa, F.; Khan, Y.; Albalawi, D.A.; Sattar, A. Identification and validation of functional miRNAs and their main targets in Sorghum bicolor. Mol. Biotechnol. 2023, 67, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zheng, H.; Dang, Y.; Sui, N. Sorghum: A Multipurpose Crop. J. Agric. Food Chem. 2023, 71, 17570–17583. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon–intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Tang, C.; Zhu, X.; Qiao, X.; Gao, H.; Li, Q.; Wang, P.; Wu, J.; Zhang, S. Characterization of the pectin methyl-esterase gene family and its function in controlling pollen tube growth in pear (Pyrus bretschneideri). Genomics 2020, 112, 2467–2477. [Google Scholar] [CrossRef]
- Luo, C.; Guo, C.; Zhang, Y.; Chen, J.; Xiao, S.; Chen, L. Expression analysis of a stress repressed gene osDSR4 from DUF966 family and generation of OsDSR4-overexpressing transgenic rice (Oryza sativa L. ssp. japonica). J. Agric. Biotechnol. 2013, 21, 1287–1294. [Google Scholar]
- Han, X.-W.; Han, S.; Zhu, Y.-X.; Wang, F.; Gao, S.-H.; Liu, Y.-Q.; Yin, J.-L. Identification and expression pattern analysis of pepper DUF966 gene family. J. South. Agric. 2023, 54, 1341–1351. [Google Scholar]
- Rehman, M.; Saeed, M.S.; Fan, X.; Salam, A.; Munir, R.; Yasin, M.U.; Khan, A.R.; Muhammad, S.; Ali, B.; Ali, I. The multifaceted role of jasmonic acid in plant stress mitigation: An overview. Plants 2023, 12, 3982. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Ou, X.; Tang, H.; Wang, R.; Wu, P.; Jia, Y.; Wei, X.; Xu, X.; Kang, S.; Kim, S. Rice microRNA osa-miR1848 targets the obtusifoliol 14a-demethylase gene Os CYP 51G3 and mediates the biosynthesis of phytosterols and brassinosteroids during development and in response to stress. New Phytol. 2015, 208, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhang, H.; Guo, R.; Wang, Q.; Huang, X.; Liao, J.; Li, Y.; Huang, Y.; Wang, Z. Characterization and functional divergence of a novel DUF668 gene family in rice based on comprehensive expression patterns. Genes 2019, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Ying, S. Genome-wide identification and transcriptional analysis of Arabidopsis DUF506 gene family. Int. J. Mol. Sci. 2021, 22, 11442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Akher, S.A.; Xue, J.; Wang, J.; Guo, C.; Li, Z.; Guo, Y. Integrative Analysis of the DUF668 Gene Family in Nicotiana tabacum to Excavate Their Potential Roles in Abiotic Stress Responses. Agronomy 2024, 14, 445. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Li, C.; Li, X.; Fan, J.; Dong, Y.; Zhu, J.; Huang, Z.; Xu, Z.; Li, L. Molecular characterization and function analysis of the rice OsDUF1664 family. Biotechnol. Biotechnol. Equip. 2021, 35, 53–60. [Google Scholar] [CrossRef]




| Name | Gene ID | Number of Amino Acid (aa) | Molecular Weight (KD) | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| SbDUF966-1 | XM_002464013.2 | Chr01 | 556 | 62,625.16 | 8.73 | 71.71 | 63.65 | −0.851 |
| SbDUF966-2 | XM_002465667.2 | Chr01 | 374 | 40,953.67 | 7.16 | 61.77 | 60.32 | −0.805 |
| SbDUF966-3 | XM_021453260.1 | Chr02 | 587 | 63,305.47 | 8.6 | 65.47 | 69.45 | −0.53 |
| SbDUF966-4 | XM_002456393.2 | Chr03 | 441 | 48,779.1 | 9.33 | 66.88 | 48.28 | −1.138 |
| SbDUF966-5 | XM_002458689.2 | Chr03 | 410 | 44,266.75 | 9.44 | 53.53 | 70.8 | −0.548 |
| SbDUF966-6 | XM_021465023.1 | Chr07 | 615 | 66,240.58 | 7.61 | 69.24 | 61.72 | −0.692 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wang, M.; Jiao, W.; Huang, K.; Li, J.; Chen, H.; Zhang, R.; Cao, X. Identification of the SbDUF966 Gene Family in Sorghum and Investigation of It Role in Response to Abiotic Stresses. Genes 2025, 16, 206. https://doi.org/10.3390/genes16020206
Luo Y, Wang M, Jiao W, Huang K, Li J, Chen H, Zhang R, Cao X. Identification of the SbDUF966 Gene Family in Sorghum and Investigation of It Role in Response to Abiotic Stresses. Genes. 2025; 16(2):206. https://doi.org/10.3390/genes16020206
Chicago/Turabian StyleLuo, Yu, Minli Wang, Wenda Jiao, Kun Huang, Jiaqi Li, Haiyun Chen, Ruidong Zhang, and Xiong Cao. 2025. "Identification of the SbDUF966 Gene Family in Sorghum and Investigation of It Role in Response to Abiotic Stresses" Genes 16, no. 2: 206. https://doi.org/10.3390/genes16020206
APA StyleLuo, Y., Wang, M., Jiao, W., Huang, K., Li, J., Chen, H., Zhang, R., & Cao, X. (2025). Identification of the SbDUF966 Gene Family in Sorghum and Investigation of It Role in Response to Abiotic Stresses. Genes, 16(2), 206. https://doi.org/10.3390/genes16020206





