Congenital Anomalies of the Kidney and Urinary Tract in Down Syndrome: Prevalence, Phenotypes, Genetics and Clinical Management
Abstract
1. Introduction
2. Autopsy and Small Studies
3. Prevalence of CAKUT in DS and Population-Based Studies
4. CAKUT Phenotypes in Down Syndrome
4.1. Kidney Anomalies
4.1.1. Renal Agenesis
4.1.2. Renal Hypoplasia
4.1.3. Renal Ectopia
4.1.4. Renal Fusion (Horseshoe Kidney)
4.1.5. Renal Cystic Disease
4.1.6. Hydronephrosis
4.1.7. Renal Pyelectasis
4.1.8. Acquired Glomerulopathies
4.1.9. Morphologic Changes of the Kidney
4.2. Ureter Anomalies
4.2.1. Ureteral Dilatation
4.2.2. Ureteral Stenosis
4.3. Bladder Anomalies
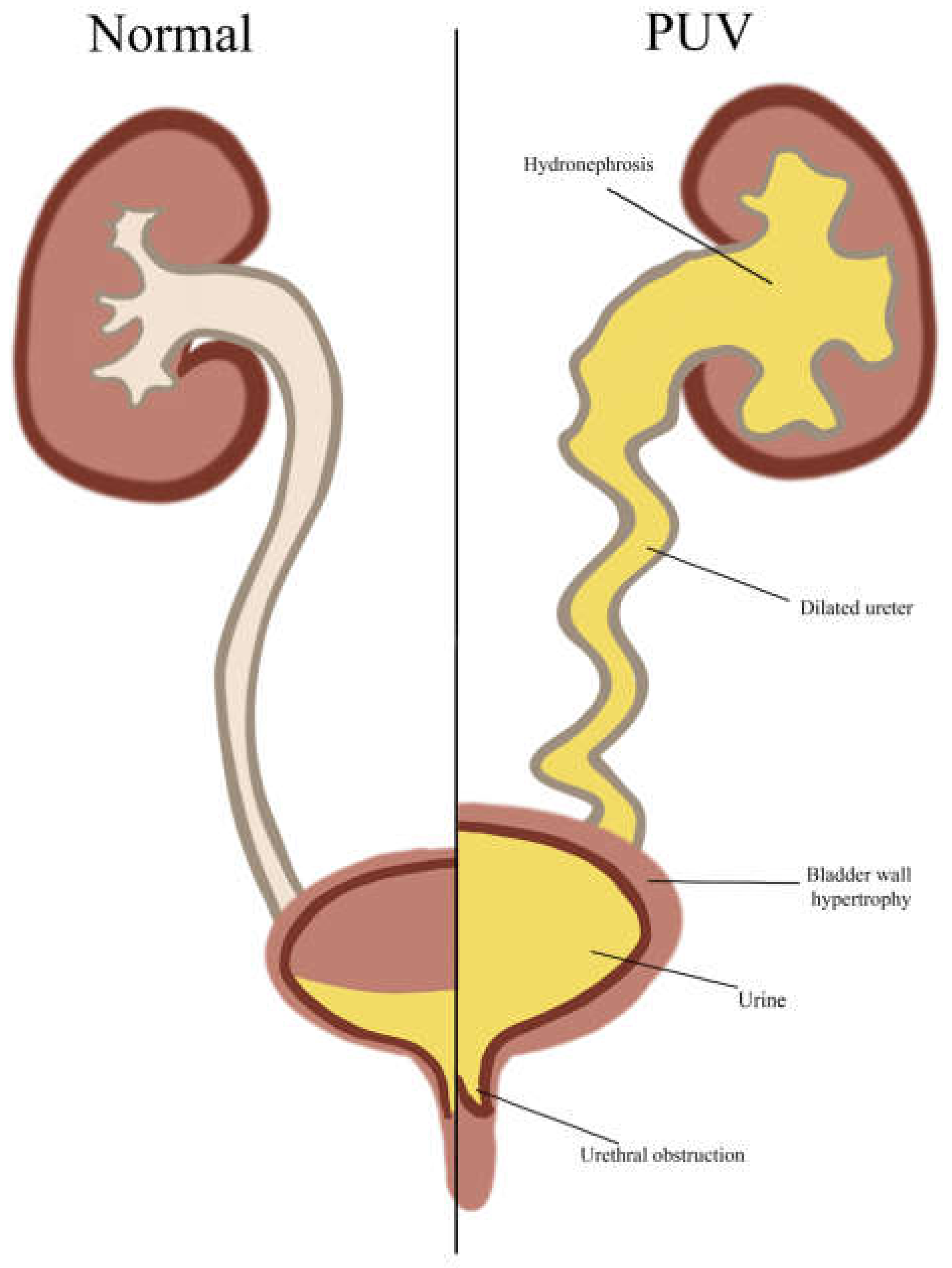
4.3.1. Posterior Urethral Valves (PUVs)
4.3.2. Lower Urinary Tract Dysfunction (LUTD)
4.4. Urethra Anomalies
Hypospadias and Epispadias
4.5. Other
Obstructive Defects
5. Kidney Function in Children with DS
6. Genetic Factors Contributing to CAKUT in Individuals with DS
7. Methods for Diagnosing CAKUT in Individuals with DS and the Importance of Early Detection and Screening Protocols
8. Medical and Surgical Management Options for CAKUT in Individuals with DS
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the number of people with Down syndrome in Europe. Eur. J. Hum. Genet. 2021, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Gruhn, J.R.; Zielinska, A.P.; Shukla, V.; Blanshard, R.; Capalbo, A.; Cimadomo, D.; Nikiforov, D.; Chan, A.C.H.; Newnham, L.J.; Vogel, I.; et al. Chromosome errors in human eggs shape natural fertility over reproductive lifespan. Science 2019, 365, 1466. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Postolache, L.; Parsa, A.; Simoni, P.; Boitsios, G.; Ismaili, K.; Schurmans, T.; Monier, A.; Casimir, G.; Albert, A.; Parsa, C.F. Widespread kidney anomalies in children with Down syndrome. Pediatr. Nephrol. 2022, 37, 2361–2368. [Google Scholar] [CrossRef]
- Chevalier, R.L. CAKUT: A Pediatric and Evolutionary Perspective on the Leading Cause of CKD in Childhood. Pediatr. Rep. 2023, 15, 143–153. [Google Scholar] [CrossRef]
- Down, J.L. Observations on an ethnic classification of idiots. Ment. Retard. 1995, 33, 54–56. [Google Scholar]
- Berg, J.M.; Crome, L.; France, N.E. Congenital cardiac malformations in mongolism. Br. Heart J. 1960, 22, 331–346. [Google Scholar] [CrossRef]
- Naeye, R.L. Prenatal organ and cellular growth with various chromosomal disorders. Biol. Neonatorum 1967, 11, 248–260. [Google Scholar] [CrossRef]
- Egli, F.; Stalder, G. Malformations of kidney and urinary tract in common chromosomal aberrations. I. Clinical studies. Humangenetik 1973, 18, 1–15. [Google Scholar] [CrossRef]
- Ariel, I.; Wells, T.R.; Landing, B.H.; Singer, D.B. The urinary system in Down syndrome: A study of 124 autopsy cases. Pediatr. Pathol. 1991, 11, 879–888. [Google Scholar] [CrossRef]
- Subrahmanyam, A.B.; Mehta, A.V. Renal anomalies in Down syndrome. Pediatr. Nephrol. 1995, 9, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Brown, H.G.; Fivush, B.A.; Neu, A.M.; Racusen, L.C. Renal disease in Down syndrome: Autopsy study with emphasis on glomerular lesions. Am. J. Kidney Dis. 1998, 31, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Málaga, S.; Pardo, R.; Málaga, I.; Orejas, G.; Fernández-Toral, J. Renal involvement in Down syndrome. Pediatr. Nephrol. 2005, 20, 614–617. [Google Scholar] [CrossRef]
- Hicks, J.A.; Carson, C.; Malone, P.S.J. Is there an association between functional bladder outlet obstruction and Down’s syndrome? J. Pediatr. Urol. 2007, 3, 369–374. [Google Scholar] [CrossRef]
- Ebert, A.K.; Brookman-Amissah, S.; Rösch, W.H. Urological manifestations of Down syndrome: Significance and long-term complications—Our own patient cohort with an overview. Urologe 2008, 47, 337–341. [Google Scholar] [CrossRef]
- Solomon, B.D.; Bous, S.M.; Bianconi, S.; Pineda-Alvarez, D. Consideration of VACTERL association in patients with trisomy 21. Clin. Dysmorphol. 2010, 19, 209. [Google Scholar] [CrossRef]
- Jain, M.; Singh, A.; Mantan, M.; Kapoor, S. Evaluation of structural anomalies of kidney and urinary tract in children with Down syndrome. Indian. J. Pediatr. 2014, 81, 734. [Google Scholar] [CrossRef]
- Lang, D.J.; Van Dyke, D.C.; Heide, F.; Lowe, P.L. Hypospadias and urethral abnormalities in Down syndrome. Clin. Pediatr. 1987, 26, 40–42. [Google Scholar] [CrossRef]
- Kupferman, J.C.; Druschel, C.M.; Kupchik, G.S. Increased prevalence of renal and urinary tract anomalies in children with Down syndrome. Pediatrics 2009, 124, e615–e621. [Google Scholar] [CrossRef]
- Sanna-Cherchi, S.; Westland, R.; Ghiggeri, G.M.; Gharavi, A.G. Genetic basis of human congenital anomalies of the kidney and urinary tract. J. Clin. Investig. 2018, 128, 4. [Google Scholar] [CrossRef]
- Sanna-Cherchi, S.; Kiryluk, K.; Burgess, K.E.; Bodria, M.; Sampson, M.G.; Hadley, D.; Nees, S.N.; Verbitsky, M.; Perry, B.J.; Sterken, R.; et al. Copy-Number Disorders Are a Common Cause of Congenital Kidney Malformations. Am. J. Hum. Genet. 2012, 91, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Plutecki, D.; Kozioł, T.; Bonczar, M.; Ostrowski, P.; Skorupa, A.; Matejuk, S.; Walocha, J.; Pękala, J.; Musiał, A.; Pasternak, A.; et al. Renal agenesis: A meta-analysis of its prevalence and clinical characteristics based on 15,641,184 patients. Nephrology 2023, 28, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, J.; Turpin, R.; Gautier, M. Chromosomic diagnosis of mongolism. Arch. Fr. Pediatr. 1959, 16, 962–963. [Google Scholar]
- Rossetti, C.M.; Simonetti, G.D.; Bianchetti, M.G.; Lava, S.A.G.; Treglia, G.; Agostoni, C.; Milani, G.P.; de Winter, J.P. Kidney and urogenital abnormalities in Down syndrome: A meta-analysis. Ital. J. Pediatr. 2024, 50, 79. [Google Scholar] [CrossRef]
- Savva, G.M.; Morris, J.K.; Mutton, D.E.; Alberman, E. Maternal Age-Specific Fetal Loss Rates in Down Syndrome Pregnancies. 2006. Available online: https://obgyn.onlinelibrary.wiley.com/doi/10.1002/pd.1443 (accessed on 24 June 2024).
- Yao, R.; Contag, S.A.; Goetzinger, K.R.; Crimmins, S.D.; Kopelman, J.N.; Turan, S.; Turan, O.M. The role of fetal growth restriction in the association between Down syndrome and perinatal mortality. J. Matern.-Fetal Neonatal Med. 2020, 33, 952–960. [Google Scholar] [CrossRef]
- Morris, J.K.; Garne, E.; Wellesley, D.; Addor, M.C.; Arriola, L.; Barisic, I.; Beres, J.; Bianchi, F.; Budd, J.; Dias, C.M.; et al. Major congenital anomalies in babies born with Down syndrome: A EUROCAT population-based registry study. Am. J. Med. Genet. Part A 2014, 164, 2979–2986. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 723. [Google Scholar] [CrossRef]
- Stoll, C.; Alembik, Y.; Dott, B.; Roth, M.P. Epidemiology of Down syndrome in 118,265 consecutive births. Am. J. Med. Genet. 1990, 7, 79–83. [Google Scholar] [CrossRef]
- Källén, B.; Mastroiacovo, P.; Robert, E. Major congenital malformations in Down syndrome. Am. J. Med. Genet. 1996, 65, 160–166. [Google Scholar] [CrossRef]
- Torfs, C.P.; Christianson, R.E. Anomalies in Down syndrome individuals in a large population-based registry. Am. J. Med. Genet. 1998, 77, 431–438. [Google Scholar] [CrossRef]
- Cleves, M.A.; Hobbs, C.A.; Cleves, P.A.; Tilford, J.M.; Bird, T.M.; Robbins, J.M. Congenital defects among liveborn infants with Down syndrome. Birth Defects Res. Part A—Clin. Mol. Teratol. 2007, 79, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.; Tennant, P.W.G.; Bythell, M.; Pearce, M.S. Predictors of survival in children born with Down syndrome: A registry-based study. Pediatrics 2012, 129, e1373–e1381. [Google Scholar] [CrossRef]
- Stoll, C.; Dott, B.; Alembik, Y.; Roth, M.P. Associated congenital anomalies among cases with Down syndrome. Eur. J. Med. Genet. 2015, 58, 674–680. [Google Scholar] [CrossRef]
- Safdar, O.; Albloushy, R.; Sait, S.; Almadani, S.; Ismail, A. Incidence and outcome of renal anomalies in children with down syndrome. Australas. Med. J. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Congenital Malformations Worldwide: A Report from the International Clearinghouse for Birth Defects Monitoring Systems | GHDx. Available online: https://ghdx.healthdata.org/record/congenital-malformations-worldwide-report-international-clearinghouse-birth-defects (accessed on 11 November 2024).
- Insunza, A.; González, F.; Guzmán, E.; Nielsen, E.; Gómez, C.; Castillo, S.; Valdés, A. Potter syndrome caused by bilateral renal agenesis and duodenal atresia. Rev. Chil. Obstet. Ginecol. 1993, 58, 477–480. [Google Scholar]
- Gupta, S.K.; Venkataseshan, V.S.; Churg, J. Mesangiocapillary glomerulonephritis in Down’s syndrome. Am. J. Nephrol. 1991, 11, 112–117. [Google Scholar] [CrossRef]
- Kute, V.B.; Vanikar, A.V.; Shah, P.R.; Gumber, M.R.; Patel, H.V.; Engineer, D.P.; Thakkar, U.G.; Trivedi, H.L. Down syndrome with end-stage renal disease. Indian. J. Clin. Biochem. 2013, 28, 429–432. [Google Scholar] [CrossRef][Green Version]
- Staicu, A.; Farcasanu, A.S.; Caracostea, G.; Turcu, R.V.F.; Simon, S.; Stamatian, F. Contribution of post-mortem MRI to the evaluation of subtle renal anomalies in a first trimester foetus with Down syndrome. J. Obstet. Gynaecol. 2016, 36, 359–360. [Google Scholar] [CrossRef]
- Boronat, F.; Pérez Bustamante, I.; Mayayo, T.; Gallego, N.; Romero, C. Thoracic ectopic right kidney associated with Down syndrome and a serious heart abnormality. Actas Urol. Esp. 1982, 6, 33–36. [Google Scholar]
- Stein, J.P.; Kurzrock, E.A.; Freeman, J.A.; Esrig, D.; Ginsberg, D.A.; Grossfeld, G.D.; Hardy, B.E. Right intrathoracic renal ectopia: A case report and review of the literature. Tech. Urol. 1999, 5, 166–168. [Google Scholar]
- Navarro, A.; Jiménez, J.; Ríos, T.; Mestanza, F.; Aguirre, I.; Urquizo, R. Unusual cause of lung and renal disease in a baby with trisomy 21. Pediatr. Pulmonol. 2005, 40, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Manjomi, F.M.; Al Mane, K.; Al-Nasser, A. A three-year old girl with Down’s syndrome and an abnormal finding in the chest. Ann. Saudi Med. 2005, 25, 349, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Kosmadakis, G.; Smirloglou, D.; Gobou, A.; Draganis, T.; Michail, S. Hemodialysis treatment on an adult patient with Down syndrome associated with ectopic right kidney chronic obstructive nephropathy and secondary amyloidosis. Saudi J. Kidney Dis. Transpl. 2013, 24, 322–325. [Google Scholar] [CrossRef]
- Anriquez, D.A.; Pussetto, M.B.; Seia, F. Kidney trasplantation in a female infant with Down syndrome, Pseudo Prune Belly Syndrome, and a post-transplant lymphoproliferative disorder. First case report in Argentina. Rev. Nefrol. Dial. Traspl. 2023, 43, 46–51. [Google Scholar]
- Webb, N.; Hébert, D.; Arbus, G. Renal replacement therapy in Down’s syndrome. Pediatr. Nephrol. 1993, 7, 771. [Google Scholar] [CrossRef]
- Narasimhan, K.L.; Kaur, B.; Marwaha, R.K. Posterior urethral valves in patients with Down syndrome. Indian. J. Pediatr. 2005, 72, 802. [Google Scholar] [CrossRef]
- Weijerman, M.E.; van Furth, A.M.; Vonk Noordegraaf, A.; van Wouwe, J.P.; Broers, C.J.M.; Gemke, R.J.B.J. Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: A national study. J. Pediatr. 2008, 152, 15–19. [Google Scholar] [CrossRef]
- Kim, G.E.; Sin, D.S.; Kim, S.S.; Lee, C.-H.; Cho, N.-J.; Lee, E.Y. End-stage renal disease in a Down syndrome patient caused by delayed diagnosis of nonneurogenic bladder: A case report. Medicine 2019, 98, e15145. [Google Scholar] [CrossRef]
- Kupferman, J.C.; Stewart, C.L.; Kaskel, F.J.; Katz, S.P.; Fine, R.N. Chronic peritoneal dialysis in a child with Down syndrome. Pediatr. Nephrol. 1994, 8, 644–645. [Google Scholar] [CrossRef]
- Hausmann, M.J.; Landau, D. A Down syndrome patient treated by peritoneal dialysis. Nephron 2002, 92, 484–486. [Google Scholar] [CrossRef]
- Lazarus, J.; Theron, A.; Smit, S. Posterior urethral valves and Down syndrome. Afr. J. Urol. 2015, 21, 4–5. [Google Scholar] [CrossRef]
- Xiang, A.; Weaver, J.; Nadeem, I.; D’Souza, N.; Rickard, M.; Weiss, D.; Milford, K.; Woo, L.; Hannick, J.; Lorenzo, A.; et al. Posterior urethral valves in patients with trisomy 21: Similar renal outcomes and rates of volitional voiding. J. Pediatr. Urol. 2023, 19, 637.e1–637.e5. [Google Scholar] [CrossRef] [PubMed]
- Yavascan, O.; Kara, O.D.; Anil, M.; Bal, A.; Pehlivan, O.; Aksu, N. Chronic peritoneal dialysis treatment in a pediatric patient with Down syndrome. Perit. Dial. Int. 2008, 28, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Husmann, D.A.; Vandersteen, D.R. Anatomy of Cloacal Exstrophy. In The Exstrophy—Epispadias Complex: Research Concepts and Clinical Applications; Gearhart, J.P., Mathews, R., Eds.; Springer: Boston, MA, USA, 1999; pp. 199–206. ISBN 978-1-4757-3056-2. [Google Scholar] [CrossRef]
- Reutter, H.; Betz, R.C.; Ludwig, M.; Boemers, T.M. MTHFR 677 TT genotype in a mother and her child with Down syndrome, atrioventricular canal and exstrophy of the bladder: Implications of a mutual genetic risk factor? Eur. J. Pediatr. 2006, 165, 566–568. [Google Scholar] [CrossRef]
- Potter, E.L. Normal and Abnormal Development of the Kidney; Year Book Medical Publishers: Chicago, IL, USA, 1972; ISBN 0-8151-6763-6. [Google Scholar]
- El-Reshaid, K.; El-Reshaid, W.; Al-Bader, D.; Varro, J.; Madda, J.; Sallam, H.T. Biopsy of small kidneys: A safe and a useful guide to potentially treatable kidney disease. Saudi J. Kidney Dis. Transpl. 2017, 28, 298–306. [Google Scholar] [CrossRef]
- Matsell, D.G.; Cojocaru, D.; Matsell, E.W.; Eddy, A.A. The impact of small kidneys. Pediatr. Nephrol. 2015, 30, 1501–1509. [Google Scholar] [CrossRef]
- Paueksakon, P.; Fogo, A.B. Autopsy Renal Pathology. Surg. Pathol. Clin. 2014, 7, 321–355. [Google Scholar] [CrossRef]
- Rowe, C.K.; Merguerian, P.A. Developmental Abnormalities of the Genitourinary System. In Avery’s Diseases of the Newborn; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1111–1124.e5. [Google Scholar] [CrossRef]
- Rosenblum, N.D. Kidney Development. In National Kidney Foundation’s Primer on Kidney Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–25. [Google Scholar] [CrossRef]
- Devlieger, R.; Hindryckx, A. 33—Kidney and Urinary Tract Disorders. In Fetal Medicine, 3rd ed.; Pandya, P.P., Oepkes, D., Sebire, N.J., Wapner, R.J., Eds.; Elsevier: London, UK, 2020; pp. 351–372.e2. ISBN 978-0-7020-6956-7. Available online: https://www.sciencedirect.com/science/article/pii/B9780702069567000336 (accessed on 6 November 2024).
- Je, B.-K.; Kim, H.K.; Horn, P.S. Incidence and Spectrum of Renal Complications and Extrarenal Diseases and Syndromes in 380 Children and Young Adults With Horseshoe Kidney. AJR Am. J. Roentgenol. 2015, 205, 1306–1314. [Google Scholar] [CrossRef]
- Goksu, S.Y.; Leslie, S.W.; Khattar, D. Renal Cystic Disease; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554504/ (accessed on 6 November 2024).
- Desogus, M.; Crobe, A.; Fraschini, M.; Ottonello, G.; Melania, P.; Faa, G.; Fanos, V. Morphological changes in the kidney of fetuses with Down syndrome. J. Pediatr. Neonatal Individ. Med. 2016, 5, e050125. [Google Scholar] [CrossRef]
- Nagata, M.; Shibata, S.; Shu, Y. Pathogenesis of dysplastic kidney associated with urinary tract obstruction in utero. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 9), 37–38. [Google Scholar] [CrossRef]
- Patel, K.; Batura, D. An overview of hydronephrosis in adults. Br. J. Hosp. Med. 2020, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dagklis, T.; Plasencia, W.; Maiz, N.; Duarte, L.; Nicolaides, K.H. Choroid plexus cyst, intracardiac echogenic focus, hyperechogenic bowel and hydronephrosis in screening for trisomy 21 at 11 + 0 to 13 + 6 weeks. Ultrasound Obstet. Gynecol. 2008, 31, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Platt, L.D. Ultrasound screening: Status of markers and efficacy of screening for structural abnormalities. Semin. Perinatol. 2016, 40, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Mickelson, J.J.; Helfand, B.T.; Maizels, M.; Kaplan, W.E.; Yerkes, E.B. Fetal pyelectasis as predictor of decreased differential renal function. J. Urol. 2009, 182, 1849–1853. [Google Scholar] [CrossRef]
- Coco, C.; Jeanty, P. Isolated fetal pyelectasis and chromosomal abnormalities. Am. J. Obstet. Gynecol. 2005, 193, 732–738. [Google Scholar] [CrossRef]
- Benacerraf, B.R.; Mandell, J.; Estroff, J.A.; Harlow, B.L.; Frigoletto, F.D. Fetal pyelectasis: A possible association with Down syndrome. Obstet. Gynecol. 1990, 76, 58–60. [Google Scholar]
- Chudleigh, P.M.; Chitty, L.S.; Pembrey, M.; Campbell, S. The association of aneuploidy and mild fetal pyelectasis in an unselected population: The results of a multicenter study. Ultrasound Obstet. Gynecol. 2001, 17, 197–202. [Google Scholar] [CrossRef]
- Orzechowski, K.M.; Berghella, V. Isolated fetal pyelectasis and the risk of Down syndrome: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 42, 615–621. [Google Scholar] [CrossRef]
- Birk, P.E.; Burke, B.A.; Vernier, R.L. Glomerulonephritis in children with Down syndrome. Pediatr. Nephrol. 1996, 10, 549. [Google Scholar]
- Said, S.M.; Cornell, L.D.; Sethi, S.; Fidler, M.E.; Al Masri, O.; Marple, J.; Nasr, S.H. Acquired glomerular lesions in patients with Down syndrome. Hum. Pathol. 2012, 43, 81–88. [Google Scholar] [CrossRef]
- Alsultan, M.K.; Bdeir, Z.N.; Obeid, A.; Alsamarrai, O.; Nabil Al Houri, H.; Hassan, Q. Primary membranoproliferative glomerulonephritis in a child with down syndrome complicated with CVA: A case report. Ann. Med. Surg. 2022, 81, 104441. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, O.; Paksu, M.S.; Bek, K.; Yildiz, L.; Fişgin, T.; Gürmen, N.; Karagöz, F. Renal amyloidosis due to pulmonary tuberculosis in a patient with Down syndrome. Eur. J. Pediatr. 2006, 165, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Takemura, T.; Yoshioka, K.; Akano, N.; Michihata, I.; Okada, M.; Maki, S.; Shigematsu, H. Immunotactoid glomerulopathy in a child with Down syndrome. Pediatr. Nephrol. 1993, 7, 86–88. [Google Scholar] [CrossRef]
- Robson, W.L.; Leung, A.K.; Woodman, R.C.; Trevenen, C.L. Anti-neutrophil cytoplasmic antibody associated glomerulonephritis in a patient with Down’s syndrome. Pediatr. Nephrol. 1995, 9, 204–205. [Google Scholar] [CrossRef]
- Haseyama, T.; Imai, H.; Komatsuda, A.; Hamai, K.; Ohtani, H.; Kibira, S.; Miura, A.B. Proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) positive crescentic glomerulonephritis in a patient with Down’s syndrome and infectious endocarditis. Nephrol. Dial. Transplant. 1998, 13, 2142–2146. [Google Scholar] [CrossRef]
- Schwab, M.; Böswald, M.; Ludwig, K.; Wittekind, C.; Waldherr, R.; Ruder, H. A patient with Down’s syndrome and anti-neutrophilic cytoplasmic antibody-positive vasculitis. Pediatr. Nephrol. 1996, 10, 249–250. [Google Scholar] [CrossRef]
- Baqi, N.; Tejani, A.; Sullivan, E.K. Renal transplantation in Down syndrome: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr. Transplant. 1998, 2, 211–215. [Google Scholar]
- Assadi, F.K. IgG-associated mesangial glomerulonephritis in a patient with Down syndrome. Med. Sci. Monit. 2004, 10, CS54–CS56. [Google Scholar]
- Cherif, M.; Hedri, H.; Ounissi, M.; Gergah, T.; Goucha, R.; Barbouch, S.; Abderrahim, E.; Maiz, H.B.; Kheder, A. Pauci-immune crescentic glomerulonephritis in the Down’s syndrome. Saudi J. Kidney Dis. Transpl. 2013, 24, 1223–1227. [Google Scholar] [CrossRef]
- Ferrari, M.; Stagi, S. Autoimmunity and Genetic Syndromes: A Focus on Down Syndrome. Genes 2021, 12, 268. [Google Scholar] [CrossRef]
- Al-Aubodah, T.-A.; Piccirillo, C.A.; Trachtman, H.; Takano, T. The autoimmune architecture of childhood idiopathic nephrotic syndrome. Kidney Int. 2025, 107, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E. Down syndrome and the complexity of genome dosage imbalance. Nat. Rev. Genet. 2017, 18, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Fusch, C.; Olbertz, D.; Hartmann, K.; Rochow, N.; Renken, C.; Schneider, K. Analysis of the neonatal collective in the Federal Republic of Germany 12th report: Presentation of detailed percentiles for the body measurement of newborns. Geburtshilfe Frauenheilkd. 2006, 66, 956–970. [Google Scholar] [CrossRef]
- Wada, N.; Miyazaki, K.; Enya, T.; Okada, M.; Sugimoto, K. Renal impairment associated with oligonephronia in a patient with Down syndrome. Pediatr. Int. 2020, 62, 640–641. [Google Scholar] [CrossRef]
- Jain, V.; Beneck, D. Renal tubular dysgenesis in an hydropic fetus with trisomy 21: A case report with literature review. Pediatr. Dev. Pathol. 2003, 6, 568–572. [Google Scholar] [CrossRef]
- Thotakura, R.; Anjum, F. Hydronephrosis and Hydroureter; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK563217/ (accessed on 6 November 2024).
- Osmundson, S.S. Hydroureter. Am. J. Obstet. Gynecol. 2021, 225, B16–B17. [Google Scholar] [CrossRef]
- Vemulakonda, V.M. Ureteropelvic junction obstruction: Diagnosis and management. Curr. Opin. Pediatr. 2021, 33, 227–234. [Google Scholar] [CrossRef]
- Brownlee, E.; Wragg, R.; Robb, A.; Chandran, H.; Knight, M.; McCarthy, L. Current epidemiology and antenatal presentation of posterior urethral valves: Outcome of BAPS CASS National Audit. J. Pediatr. Surg. 2019, 54, 318–321. [Google Scholar] [CrossRef]
- Thakkar, D.; Deshpande, A.V.; Kennedy, S.E. Epidemiology and demography of recently diagnosed cases of posterior urethral valves. Pediatr. Res. 2014, 76, 560–563. [Google Scholar] [CrossRef]
- Tambo, F.F.M.; Tolefac, P.N.; Ngowe, M.N.; Minkande, J.Z.; Mbouche, L.; Guemkam, G.; Telelen, N.A.; Angwafo, F.F.; Sosso, A.M. Posterior urethral valves: 10 years audit of epidemiologic, diagnostic and therapeutic aspects in Yaoundé gynaeco-obstetric and paediatric hospital. BMC Urol. 2018, 18, 46. [Google Scholar] [CrossRef]
- Meneghesso, D.; Partigiani, N.B.; Spagnol, R.; Brazzale, A.R.; Morlacco, A.; Vidal, E. Nadir creatinine as a predictor of renal outcomes in PUVs: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1085143. [Google Scholar] [CrossRef] [PubMed]
- Julie, K.; Chrystelle, L.; Cécile, C.; Justyna, S.; Petra, Z.; Mohammed, D.; Françoise, M.; Benjamin, B.; Angelique, S.; William, M.; et al. Fetal Urinary Peptides to Predict Postnatal Outcome of Renal Disease in Fetuses with Posterior Urethral Valves (PUV). Sci. Transl. Med. 2013, 5, 198ra106. [Google Scholar] [CrossRef]
- Bielek, J.; Carvajal-Busslinger, M.I.; Bianchetti, M.G. Posterior urethral valves in trisomy 21. Pediatr. Nephrol. 1996, 10, 678. [Google Scholar] [CrossRef]
- Mondal, K.; Maheshwari, A.; Aneja, S.; Seth, A. A case of Down syndrome with a posterior urethral valve. Indian. J. Nephrol. 2012, 22, 403–405. [Google Scholar] [CrossRef]
- Danacıoğlu, Y.O.; Karaman, M.İ.; Çaşkurlu, T.; Sılay, M.S. Congenital megalourethra and posterior urethral valve in a patient with Down syndrome. Turk. J. Urol. 2019, 45, S181–S184. [Google Scholar] [CrossRef]
- Garriboli, M.; Ibrahim, S.; Clothier, J. Spontaneous bladder rupture secondary to posterior urethral valves in a boy with Down syndrome. BMJ Case Rep. 2021, 14, e240857. [Google Scholar] [CrossRef]
- Ahmed, S. Vesico-ureteric reflux in Down’s syndrome: Poor prognosis. Aust. N. Z. J. Surg. 1990, 60, 113–116. [Google Scholar] [CrossRef]
- Frimberger, D.; Mercado-Deane, M.G.; McKenna, P.H.; Austin, J.C.; Austin, P.F.; Cooper, C.S.; Greenfield, S.P.; Herndon, C.D.; Kolon, T.F.; MacNeily, A.E.; et al. Establishing a Standard Protocol for the Voiding Cystourethrography. Pediatrics 2016, 138, e20162590. [Google Scholar] [CrossRef]
- Powers, M.K.; Brown, E.T.; Hogan, R.M.; Martin, A.D.; Ortenberg, J.; Roth, C.C. Trends in Toilet Training and Voiding Habits among Children with Down Syndrome. J. Urol. 2015, 194, 783–787. [Google Scholar] [CrossRef]
- Mrad, F.C.d.C.; de Figueiredo, A.A.; de Bessa, J.; Bastos Netto, J.M. Prolonged toilet training in children with Down syndrome: A case–control study. J. Pediatr. 2018, 94, 286–292. [Google Scholar] [CrossRef]
- Kızılay, F.; İrer, B.; Özalp Kızılay, D.; Şimşir, A.; Kalemci, S.; Şen, V.; Altay, B.; Çoğulu, Ö. Evaluation of lower urinary tract symptoms in children with Down syndrome: A prospective, case-controlled cohort study. Neurourol. Urodyn. 2020, 39, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ranchin, B.; Bidault, V.; Zekre, F.; DeMul, A.; Sanlaville, D.; Bacchetta, J. Kidney and urological involvement in Down syndrome: Frequent, underestimated, but associated with impaired quality of life and risk of kidney failure. Pediatr. Nephrol. 2024, 39, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Handel, L.N.; Barqawi, A.; Checa, G.; Furness, P.D.; Koyle, M.A. Males with Down’s syndrome and nonneurogenic neurogenic bladder. J. Urol. 2003, 169, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Groutz, A.; Blaivas, J.G.; Pies, C.; Sassone, A.M. Learned voiding dysfunction (non-neurogenic, neurogenic bladder) among adults. Neurourol. Urodyn. 2001, 20, 259–268. [Google Scholar] [CrossRef]
- Kai, N.; Seki, N.; Hirata, A.; Nakamuta, S.; Naito, S. A female case with Down syndrome and non-neurogenic neurogenic bladder. Int. J. Urol. 2007, 14, 867–868. [Google Scholar] [CrossRef]
- Kitamura, A.; Kondoh, T.; Noguchi, M.; Hatada, T.; Tohbu, S.; Mori, K.-I.; Matsuo, M.; Kunitsugu, I.; Kanetake, H.; Moriuchi, H. Assessment of lower urinary tract function in children with Down syndrome. Pediatr. Int. 2014, 56, 902–908. [Google Scholar] [CrossRef]
- Nijman, R.J. Neurogenic and non-neurogenic bladder dysfunction. Curr. Opin. Urol. 2001, 11, 577–583. [Google Scholar] [CrossRef]
- van der Horst, H.J.R.; de Wall, L.L. Hypospadias, all there is to know. Eur. J. Pediatr. 2017, 176, 435–441. [Google Scholar] [CrossRef]
- Baskin, L.S.; Ebbers, M.B. Hypospadias: Anatomy, etiology, and technique. J. Pediatr. Surg. 2006, 41, 463–472. [Google Scholar] [CrossRef]
- Pautonnier, J.; Goutte, S.; Dubourg, L.D.; Bacchetta, J.; Ranchin, B.; Rabilloud, M.; Sanlaville, D. Creatinine levels in French children with Down syndrome up to ten years old. Eur. J. Pediatr. 2024, 183, 1953–1957. [Google Scholar] [CrossRef]
- Nishino, T.; Endo, S.; Miyano, H.; Takemasa, Y.; Saito, M.; Umeda, C.; Tomii, Y.; Watanabe, Y.; Nakagawa, M.; Kakegawa, D.; et al. Reference serum creatinine levels according to sex, age, and height in children with Down syndrome. Eur. J. Pediatr. 2021, 180, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Mariño-Ramírez, L.; Sharma, S.; Rishishwar, L.; Conley, A.B.; Nagar, S.D.; Jordan, I.K. Effects of genetic ancestry and socioeconomic deprivation on ethnic differences in serum creatinine. Gene 2022, 837, 146709. [Google Scholar] [CrossRef]
- Chuang, G.-T.; Tsai, I.-J.; Tsau, Y.-K. Serum Creatinine Reference Limits in Pediatric Population-A Single Center Electronic Health Record-Based Database in Taiwan. Front. Pediatr. 2021, 9, 793446. [Google Scholar] [CrossRef]
- Yamakawa, S.; Nagai, T.; Uemura, O. Down syndrome and mild kidney dysfunction. Pediatr. Int. 2018, 60, 391–393. [Google Scholar] [CrossRef]
- Nishino, T.; Endo, S.; Miyano, H.; Umeda, C.; Tomii, Y.; Watanabe, Y.; Nakagawa, M.; Kakegawa, D.; Fujinaga, S. Is the estimated glomerular filtration rate formula useful for evaluating the renal function of Down syndrome? Pediatr. Int. 2021, 63, 944–950. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Haycock, G.B.; Spitzer, A. Plasma creatinine and urea concentration in children: Normal values for age and sex. J. Pediatr. 1976, 88, 828–830. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Uemura, O.; Honda, M.; Matsuyama, T.; Ishikura, K.; Hataya, H.; Yata, N.; Nagai, T.; Ikezumi, Y.; Fujita, N.; Ito, S.; et al. Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: A multicenter study. Clin. Exp. Nephrol. 2011, 15, 694–699. [Google Scholar] [CrossRef]
- Dilanthi, H.W.; Kularatnam, G.a.M.; Jayasena, S.; Jasinge, E.; Samaranayake, D.B.D.L.; Wickramasinghe, V.P. Validity of the use of Schwartz formula against creatinine clearance in the assessment of renal functions in children. Sri Lanka J. Child Health 2017, 46, 155–159. [Google Scholar] [CrossRef]
- Pottel, H.; Delanaye, P.; Cavalier, E. Exploring Renal Function Assessment: Creatinine, Cystatin C, and Estimated Glomerular Filtration Rate Focused on the European Kidney Function Consortium Equation. Ann. Lab. Med. 2024, 44, 135–143. [Google Scholar] [CrossRef]
- Huang, Y.-N.; Huang, J.-Y.; Wang, C.-H.; Su, P.-H. Long-Term Non-Congenital Cardiac and Renal Complications in Down Syndrome: A Study of 32,936 Patients. Children 2023, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Fujiyama, A.; Taylor, T.D.; Watanabe, H.; Yada, T.; Park, H.S.; Toyoda, A.; Ishii, K.; Totoki, Y.; Choi, D.K.; et al. The DNA sequence of human chromosome 21. Nature 2000, 405, 311–319. [Google Scholar] [CrossRef]
- Shapiro, B.L. Down syndrome—A disruption of homeostasis. Am. J. Med. Genet. 1983, 14, 241–269. [Google Scholar] [CrossRef]
- Pritchard, M.A.; Kola, I. The “gene dosage effect” hypothesis versus the “amplified developmental instability” hypothesis in Down syndrome. J. Neural Transm. Suppl. 1999, 57, 293–303. [Google Scholar]
- Duchon, A.; Herault, Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front. Behav. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Aoidi, R.; Llorian, M.; Gibbins, D.; Buechsenschuetz, C.; Bussi, C.; Flynn, H.; Gilmore, T.; Watson-Scales, S.; Hansen, M.H.; et al. Increased dosage of DYRK1A leads to congenital heart defects in a mouse model of Down syndrome. Sci. Transl. Med. 2024, 16, eadd6883. [Google Scholar] [CrossRef]
- Redhead, Y.; Gibbins, D.; Lana-Elola, E.; Watson-Scales, S.; Dobson, L.; Krause, M.; Liu, K.J.; Fisher, E.M.C.; Green, J.B.A.; Tybulewicz, V.L.J. Craniofacial dysmorphology in Down syndrome is caused by increased dosage of Dyrk1a and at least three other genes. Development 2023, 150, dev201077. [Google Scholar] [CrossRef]
- Laguna, A.; Barallobre, M.-J.; Marchena, M.-Á.; Mateus, C.; Ramírez, E.; Martínez-Cue, C.; Delabar, J.M.; Castelo-Branco, M.; de la Villa, P.; Arbonés, M.L. Triplication of DYRK1A causes retinal structural and functional alterations in Down syndrome. Hum. Mol. Genet. 2013, 22, 2775–2784. [Google Scholar] [CrossRef]
- Blackburn, A.T.M.; Bekheirnia, N.; Uma, V.C.; Corkins, M.E.; Xu, Y.; Rosenfeld, J.A.; Bainbridge, M.N.; Yang, Y.; Liu, P.; Madan-Khetarpal, S.; et al. DYRK1A-related intellectual disability: A syndrome associated with congenital anomalies of the kidney and urinary tract. Genet. Med. 2019, 21, 2755–2764. [Google Scholar] [CrossRef]
- Lozic, M.; Minarik, L.; Racetin, A.; Filipovic, N.; Saraga Babic, M.; Vukojevic, K. CRKL, AIFM3, AIF, BCL2, and UBASH3A during Human Kidney Development. Int. J. Mol. Sci. 2021, 22, 9183. [Google Scholar] [CrossRef] [PubMed]
- Tsygankov, A.Y. TULA Proteins in Men, Mice, Hens, and Lice: Welcome to the Family. Int. J. Mol. Sci. 2023, 24, 9126. [Google Scholar] [CrossRef] [PubMed]
- Tsygankov, A.Y. TULA proteins as signaling regulators. Cell Signal 2020, 65, 109424. [Google Scholar] [CrossRef] [PubMed]
- Tsygankov, A.Y. TULA-family proteins: Jacks of many trades and then some. J. Cell Physiol. 2018, 234, 274–288. [Google Scholar] [CrossRef]
- Yuen, R.K.; Neumann, S.M.; Fok, A.K.; Peñaherrera, M.S.; Mcfadden, D.E.; Robinson, W.P.; Kobor, M.S. Extensive epigenetic reprogramming in human somatic tissues between fetus and adult. Epigenetics Chromatin 2011, 4, 7. [Google Scholar] [CrossRef]
- Henriksen, K.J.; Chang, A.; Bayliss, G.P. Kidney Transplant Outcomes in 2 Adults With Down Syndrome. Kidney Int. Rep. 2018, 3, 979–984. [Google Scholar] [CrossRef]
- Ohki, Y.; Kawabe, M.; Yamamoto, I.; Kobayashi, A.; Kanzaki, G.; Koike, K.; Ueda, H.; Tanno, Y.; Urabe, F.; Miki, J.; et al. Early Recurrence of Immunoglobulin A Nephropathy after Kidney Transplantation in a Patient with Down Syndrome. Nephron 2023, 147 (Suppl. 1), 35–40. [Google Scholar] [CrossRef]
- Edvardsson, V.O.; Kaiser, B.A.; Polinsky, M.S.; Baluarte, H.J. Successful living-related renal transplantation in an adolescent with Down syndrome. Pediatr. Nephrol. 1995, 9, 398–399. [Google Scholar] [CrossRef]

| Authors, Reference no. | Year of Publication | Country | Study Design | No. of Subjects with DS | No. of Subjects with CAKUT and DS | % of CAKUT | Age Range |
|---|---|---|---|---|---|---|---|
| Berg et al. [7] | 1960 | UK | autopsy | 141 | 5 | 3.55 | 0–27 y, SB |
| Naeye [8] | 1967 | USA | autopsy | 21 | 3 | 14.29 | 36–40 gw |
| Egli and Stalder [9] | 1973 | Switzerland | autopsy | 103 | 7 | 6.80 | (−14) |
| Ariel et al. [10] | 1991 | USA | autopsy | 124 | NA | NA | 16 gw–25 y |
| Subrahmanyam and Mehta [11] | 1995 | USA | retrospective chart review | 54 | 1 | 1.85 | 2 m–24 y |
| Lo et al. [12] | 1998 | Hong Kong, USA | case study and retrospective autopsy review | 45 | 29 | 64.44 | NA |
| Malaga et al. [13] | 2004 | Spain | cross-sectional study | 69 | 2 | 2.90 | 1–24 y |
| Hicks et al. [14] | 2007 | UK | retrospective chart review and cross-sectional study | 7 | 7 | 100.00 | 0–9 y |
| Ebert et al. [15] | 2008 | Germany | retrospective chart review | 24 | 24 | NA | 3 m–27 y |
| Solomon et al. [16] | 2010 | USA | case study | 2 | 2 | NA | 6 w; 18 y |
| Jain et al. [17] | 2014 | India | cross-sectional study | 40 | NA | 0.00 | 0–18 y |
| Postolache et al. [4] | 2022 | Belgium | retrospective cohort study | 49 | 7 | 14.29 | 0–18 y |
| Lang et al. [18] | 1987 | USA | cross-sectional study | 91 | 5 | 5.49 | NA |
| Authors, Reference no. | Year of Publication | Country | Study Years | No. of Subjects with DS | No. of CAKUT | % of CAKUT | No. of Controls | Age Range |
|---|---|---|---|---|---|---|---|---|
| Stoll et al. [29] | 1990 | France | 1979–1987 | 139 | 4 | 2.88 | NA | 20 gw–9 d |
| Kallen et al. [30] | 1996 | France, Italy, Sweeden | 1976–1993 | 5581 | 12 | 0.22 | # | NA |
| Torfs and Christianson [31] | 1998 | USA (California) | 1983–1993 | 2894 | 56 | 1.94 | 2,490,437 | 0–1 y |
| Cleves et al. [32] | 2007 | USA | 1993–2002 | 11372 | 991 | 8.71 | 7,884,209 | newborns |
| Kupferman et al. [19] | 2009 | USA (New York State) | 1992–2004 | 3832 | 123 | 3.21 | 3,411,833 | 0–2 y |
| Rankin et al. [33] | 2012 | UK | 1985–2008 | 1115 | 11 | 0.99 | NA | SB, miscarriages, TOPFAs, 0–12 y (1985–2001), 0–16 y (2001–2003) |
| Morris et al. [27] | 2014 | 18 EU countries | 2000–2010 | 7025 † | 159 | 2.26 | NA | 20 gw–1 y, SB |
| Stoll et al. [34] | 2015 | France | 1979–2008 | 728 | 28 | 3.85 | 401,804 | 0–2 y, SB, TOPFAs |
| Safdar et al. [35] | 2018 | Saudi Arabia | 2005–2016 | 241 | 51 | 21.16 | NA | 1 m–57 y |
| CAKUT | Authors and Year | Study Cases | Total |
|---|---|---|---|
| Reported | |||
| Kidney Anomalies | |||
| Renal agenesis | Berg et al., 1960 [7] | 2 of 141 DS autopsies | 33 * |
| Insunza et al., 1993 [37] | 1 DS autopsy case of Potter syndrome | ||
| Kallen et al., 1996 [30] | 2 agenesis/dysgenesis of 5581 DS infants | ||
| Torfs and Christianson, 1998 [31] | 1 of 2894 DS infants | ||
| Cleves et al., 2007 [32] | 14 agenesis/hypoplasia of 43,463 DS infants | ||
| Kupferman et al., 2009 [19] | 9 of 3832 DS infants | ||
| Morris et al., 2014 [27] | 4 of 7044 DS live births and fetal deaths | ||
| Renal hypoplasia | Berg et al., 1960 [7] | 2 of 141 DS autopsies | 42 * |
| Ariel et al., 1991 [10] | 18 of 124 DS autopsies | ||
| Gupta et al., 1991 [38] | 2 cases | ||
| Lo et al., 1998 [12] | 1 of 45 cases | ||
| Malaga et al., 2004 [13] | 1 of 69 DS cases | ||
| Cleves et al., 2007 [32] | 14 agenesis/hypoplasia of 11,372 DS newborns | ||
| Ebert et al., 2008 [15] | 2 out of 11 DS cases | ||
| Kute et al., 2013 [39] | 1 case | ||
| Jain, 2014 [17] | NA | ||
| Staicu et al., 2015 [40] | 1 case | ||
| Renal ectopia | Boronat et al., 1982 [41] | 1 case of intrathoracic kidney | 12 |
| Stein, 1999 [42] | 1 case of intrathoracic kidney | ||
| Malaga et al., 2004 [13] | 1 of 69 DS cases | ||
| Navarro et al., 2005 [43] | 1 case of intrathoracic kidney | ||
| Al-Manjomi et al., 2005 [44] | 1 case of intrathoracic kidney | ||
| Kupferman et al., 2009 [19] | 1 of 3832 DS infants | ||
| Kosmadakis et al., 2013 [45] | 1 case | ||
| Safdar et al., 2018 [35] | 5 of 241 DS cases | ||
| Renal fusion (Horseshoe kidney) | Berg et al., 1960 [7] | 1 of 141 DS autopsies | 5 |
| Naeye, 1967 [8] | 1 of 21 DS autopsies | ||
| Stoll et al., 1990 [29] | 1 of 139 DS fetuses/newborns | ||
| Torfs and Christianson, 1998 [31] | 2 of 2894 DS infants | ||
| Renal dysplasia | Morris et al., 2014 [27] | 11 of 7025 DS fetuses/infants/SB | 12 |
| Anriquez et al., 2023 [46] | 1 case | ||
| Renal cystic disease | |||
| Glomerulocystic disease | Ariel et al., 1991 [10] | 23 of 124 DS autopsies | 41 |
| Lo et al., 1998 [12] | 28 of 45 cases | ||
| Simple renal cysts | Ariel et al., 1991 [10] | 7 of 124 DS autopsies | 8 |
| Staicu et al., 2015 [40] | 1 case | ||
| Renal dysplasia with cysts | Ariel et al., 1991 [10] | 4 of 124 DS autopsies | 8 |
| Webb et al., 1993 [47] | 1 case | ||
| Kupferman et al., 2009 [19] | 3 of 3832 DS infants | ||
| Hydronephrosis | Naeye, 1967 [8] | 1 of 21 DS autopsies | ~203 |
| Egli and Stalder, 1973 [9] | 1 of 103 DS autopsies | ||
| Ariel et al., 1991 [10] | 3 of 124 DS autopsies | ||
| Kupferman et al., 1995 [19] | 2 cases | ||
| Narashiman et al., 2005 [48] | 4 out of 6 DS cases with PUV | ||
| Hicks et al., 2007 [14] | 7 of 7 DS children | ||
| Ebert et al., 2008 [15] | 7 of 24 DS cases | ||
| Weijerman et al., 2008 [49] | 2 of 176 DS infants | ||
| Kupferman et al., 2009 [19] | 69 of 3832 DS infants | ||
| Rankin et al., 2012 [33] | 12 of 1115 DS SBs, miscarriages, TOPFAs | ||
| Jain, 2014 [17] | 8 of 40 DS children | ||
| Morris et al., 2014 [27] | 66 of 7044 DS live births and fetal deaths | ||
| Staicu et al., 2015 [40] | 1 case | ||
| Safdar et al., 2018 [35] | 18 of 241 DS cases | ||
| Postolache et al., 2022 [4] | 2 of 49 DS children | ||
| Pyelectasis | Malaga et al., 2004 [13] | 1 of 69 DS cases | 4 |
| Postolache et al., 2022 [4] | 3 of 49 DS children | ||
| Ureter Anomalies | |||
| Ureteral dilatation (hydroureter, megaureter) | Egli and Stalder, 1973 [9] | 3 of 103 DS autopsies | 28 * |
| Stoll et al., 1990 [29] | 1 of 139 DS fetuses/newborns | ||
| Ariel et al., 1991 [10] | 6 of 124 DS autopsies | ||
| Hicks et al., 2007 [14] | 5 of 7 DS children | ||
| Ebert et al., 2008 [15] | 7 of 24 DS cases | ||
| Kupferman et al., 2009 [19] | 5 of 3832 DS infants | ||
| Jain, 2014 [17] | NA | ||
| Kim et al., 2019 [50] | 1 case | ||
| Uretocele | Ariel et al., 1991 [10] | 1 of 124 DS autopsies | |
| Ureteral stenosis | Egli and Stalder, 1973 [9] | 2 of 103 DS autopsies | 10 * |
| Ariel et al., 1991 [10] | 2 of 124 DS autopsies | ||
| Subrahmanyam and Mehta, 1995 [11] | 1 of 54 DS cases | ||
| Jain, 2014 [17] | NA | ||
| Ebert et al., 2008 [15] | 2 cases of UPJO of 24 DS cases | ||
| Kupferman et al., 2009 [19] | 1 case of UPJO of 3832 DS infants | ||
| Naeye, 1967 [8] | 2 cases of UPJO of 21 DS autopsies | ||
| Duplication of the ureter and renal pelvis | Ariel et al., 1991 [10] | 1 of 124 DS autopsies | |
| Bladder Anomalies | |||
| PUV | Berg et al., 1960 [7] | 1 of 141 DS autopsies | 41 * |
| Webb et al., 1993 [47] | 2 cases | ||
| Kupferman et al., 1995 [51] | 3 cases | ||
| Hausmann et al., 2002 [52] | 1 case | ||
| Narashiman et al., 2005 [48] | 6 cases | ||
| Ebert et al., 2008 [15] | 2 of 24 DS cases | ||
| Kupferman et al., 2009 [51] | 2 of 3832 DS infants | ||
| Morris et al., 2014 [27] | 4 cases of PUV and/or prune belly of 7044 DS live births and fetal deaths | ||
| Lazarus et al., 2014 [53] | 2 cases | ||
| Xiang et al., 2023 [54] | 18 cases | ||
| VUR | Stoll et al., 1990 [29] | 1 of 139 DS fetuses/newborns | 20 * |
| Webb et al., 1993 [47] | 2 cases | ||
| Kupferman et al., 1995 [51] | 1 case | ||
| Narashiman et al., 2005 [48] | 3 out of 6 DS cases with PUV | ||
| Hicks et al., 2007 [14] | 3 of 7 DS children | ||
| Yavascan et al., 2008 [55] | 1 case | ||
| Ebert et al., 2008 [15] | 4 of 24 DS cases | ||
| Solomon et al., 2010 [16] | 1 case | ||
| Jain, 2014 [17] | NA | ||
| Safdar et al., 2018 [35] | 4 of 241 DS cases | ||
| Bladder exstrophy | Stoll et al., 1990 [29] | 1 of 139 DS fetuses/newborns | 9 * |
| Hausmann and Vandersteen, 1999 [56] | 1 case | ||
| Reutter et al., 2006 [57] | 1 case | ||
| Ebert et al., 2008 [15] | 2 of 24 DS cases | ||
| Solomon et al., 2010 [16] | 1 case | ||
| Morris et al., 2014 [27] | 3 cases of bladder exstrophy and/or epispadias of 7044 DS live births and fetal deaths | ||
| Bladder hypertrophy | Egli and Stalder, 1973 [9] | 3 of 103 DS autopsies | 6 |
| Ariel et al., 1991 [10] | 1 of 124 DS autopsies | ||
| Malaga et al., 2004 [13] | 1 of 69 DS cases | ||
| Anriquez et al., 2023 [46] | 1 case of megabladder | ||
| Trabeculated bladder | Ariel et al., 1991 [10] | 3 of 124 DS autopsies | 10 |
| Kupferman et al., 1995 [51] | 2 cases | ||
| Hicks et al., 2007 [14] | 5 of 7 DS children | ||
| Bladder neck stenosis | Ariel et al., 1991 [10] | 3 of 124 DS autopsies | 3 |
| Urethra Anomalies | |||
| Urethral atresia | Solomon et al., 2010 [16] | 1 case | 1 |
| Hypospadias/epispadias | Lang et al., 1987 [18] | 5 of 77 DS males | 467 |
| Kallen et al., 1996 [30] | 10 of 5581 DS cases | ||
| Torfs and Christianson, 1998 [31] | 36 of 2894 DS infants | ||
| Cleves et al., 2007 [32] | 355 of 11,372 DS newborns | ||
| Ebert et al., 2008 [15] | 2 of 24 DS cases | ||
| Kupferman et al., 2009 [19] | 31 of 3832 DS infants | ||
| Morris et al., 2014 [27] | 24 of 7044 DS live births and fetal deaths | ||
| Stoll et al., 2015 [34] | 4 of 728 DS SBs/TOPFAs/infants | ||
| Other | |||
| Unspecified obstructive uropathy | Ariel et al., 1991 [10] | 8 of 124 DS autopsies | 665 |
| Cleves et al., 2007 [32] | 638 of 11,372 DS newborns | ||
| Stoll et al., 2015 [34] | 14 of 728 DS SBs/TOPFAs/infants | ||
| Safdar et al., 2018 [35] | 4 of 241 DS cases | ||
| Anterior urethral obstruction | Kupferman et al., 2009 [19] | 1 of 3832 DS infants |
| Acquired Glomerulopathies | Authors and Year | Study Cases | Total Reported |
|---|---|---|---|
| Membranoproliferative glomerulonephritis | Gupta et al., 1991 [38] Birk et al., 1996 [77] Said et al., 2012 [78] Alsultan et al., 2022 [79] | 4 cases 1 case 2 of 17 biopsies 1 case | 8 |
| Renal amyloidosis | Ozkaya et al., 2005 [80] | 1 case | 1 |
| Immunotactoid glomerulopathy | Takemura et al., 1993 [81] | 1 case | 1 |
| Anti-neutrophilic cytoplasmic antibody (ANCA)-associated glomerulonephritis | Robson et al., 1995 [82] Haseyama et al., 1998 [83] Schwab et al., 1996 [84] | 1 case 1 case 1 case | 3 |
| Focal segmental glomerulosclerosis (FSGS) | Lo et al., 1998 [12] Baqi et al., 1998 [85] Said et al., 2012 [78] | 2 cases 2 cases 4 of 17 biopsies | 8 |
| Minimal change disease (MCD) | Lo et al., 1998 [12] | 1 of 43 autopsies | 1 |
| IgA glomerulonephritis | Birk et al., 1996 [77] Said et al., 2012 [78] | 1 case 5 of 17 biopsies | 6 |
| IgG glomerulonephritis | Assadi, 2004 [86] | 1 case | 1 |
| Membranous glomerulonephritis | Lo et al., 1998 [12] Said et al., 2012 [78] | 1 of 43 autopsies 1 of 17 biopsies | 2 |
| Pauci-immune crescentic glomerulonephritis | Birk et al., 1996 [77] Cherif et al., 2013 [87] Said et al., 2012 [78] | 1 case 1 case 2 of 17 biopsies | 4 |
| Lupus nephritis | Said et al., 2012 [78] | 1 of 17 biopsies | 1 |
| Pautonnier et al. 2023 [119] | Nishino et al. 2021 [120] | Postolache et al. 2021 [4] | Nishino et al. 2020 [124] | Yamakawa et al. 2018 [123] | Malaga et al. 2005 ¥ [13] | |
|---|---|---|---|---|---|---|
| Retrospective 2004–2021 | Retrospective 2003–2020 | Retrospective cohort study 2017–2019 | Retrospective 2003–2018 | Retrospective 2002–2014 | Cross-sectional | |
| Number of children | 279 | 568 | 49 | 379 | 108 | 69 |
| Age, years Median | 0–10 | 0.3–18 | 0–18 8.0 ± 4.2 | 2–18 | 2–18 | 1–24 9.7 |
| Sex M F | 162 117 | 336 232 | 30 19 | 224 155 | 59 49 | 37 32 |
| Exclusion criteria | Yes £ | Yes © | Yes ™ | Yes ® | Yes ∞ | No |
| Country | France | Japan | Belgium | Japan | Japan | Spain |
| Number of creatinine measurements | 857 | 3765 | ns | 2421 | ns | ns |
| Method | enzymatic | enzymatic | Jaffe | enzymatic | enzymatic | ns |
| Serum creatinine (μmol/L) Median | 24.0–50.0 | * 0.3–9 y 21.2–38.9 9–17 y Boys: 41.5–76.0 Girls: 39.8–62.8 | * 44.2 | ns | * 33.6 | * 16.5 y M: 114.1 3.3 y M: 107.0 |
| Serum creatinine (mg/dl) All children Boys: Girls: | 0.27–0.57 | 0.3–9 y 0.24–0.44 9–17 y 0.47–0.86 0.45–0.71 | 0.5 ± 0.1 | ns | 0.38 | 16.5 y M:1.29 3.3 y M: 1.21 |
| eGFR mL/min/1.73 m2 All children BoysGirls | ns | ns | 94.3 ± 16.6 | 93.4 ± 17.14 90.86 ± 15.7 96.3 ± 18.21 | 90.0 | 16.5 y: 50.1 3.3 y: 56.3 |
| Formula | ns | ns | Bedside Schwartz formula | Uemura | Uemura | Schwartz formula |
| Limitations | 1. single-center design 2. relatively low number of children 3. not take sex into account 4. only French children were studied | 1. single-center design 2. only Japanese children were studied | 1. utilized a Jaffe creatinine assay, as opposed to an enzymatic assay, as used in the Bedside Schwartz eGFR formula 2. only Belgian children were studied | 1. single-center design 2. included premature births and low-birth-weight infants 3. only Japanese children were studied | 1. small number of subjects 2. the single-centerdesign 3. the short observation period 4. only Japanese children were studied | 1. single-center design 2. only Spanish children were studied |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leskur, M.; Leskur, D.; Marijan, S.; Minarik, L.; Lozić, B. Congenital Anomalies of the Kidney and Urinary Tract in Down Syndrome: Prevalence, Phenotypes, Genetics and Clinical Management. Genes 2025, 16, 245. https://doi.org/10.3390/genes16030245
Leskur M, Leskur D, Marijan S, Minarik L, Lozić B. Congenital Anomalies of the Kidney and Urinary Tract in Down Syndrome: Prevalence, Phenotypes, Genetics and Clinical Management. Genes. 2025; 16(3):245. https://doi.org/10.3390/genes16030245
Chicago/Turabian StyleLeskur, Mirela, Dario Leskur, Sandra Marijan, Luka Minarik, and Bernarda Lozić. 2025. "Congenital Anomalies of the Kidney and Urinary Tract in Down Syndrome: Prevalence, Phenotypes, Genetics and Clinical Management" Genes 16, no. 3: 245. https://doi.org/10.3390/genes16030245
APA StyleLeskur, M., Leskur, D., Marijan, S., Minarik, L., & Lozić, B. (2025). Congenital Anomalies of the Kidney and Urinary Tract in Down Syndrome: Prevalence, Phenotypes, Genetics and Clinical Management. Genes, 16(3), 245. https://doi.org/10.3390/genes16030245






