Toxoplasma Gondii Replication During Belatacept Treatment in Kidney Transplantation: A Case Report and a Review of the Literature
Abstract
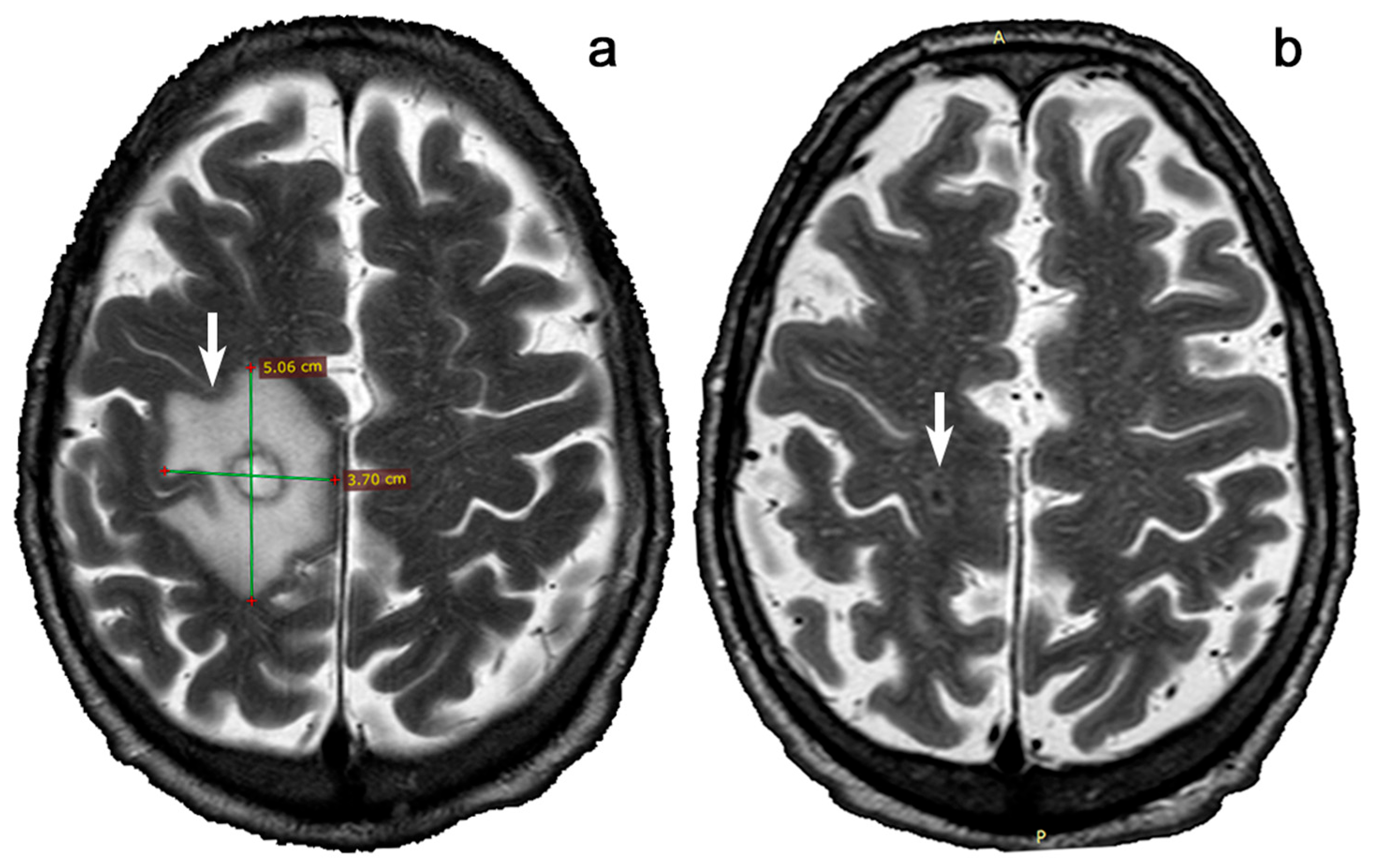
:1. Introduction
2. Belatacept Pharmacodynamics and Genetic Factors
3. Belatacept Pharmacokinetics
4. Adverse Events Related to Belatacept Treatment
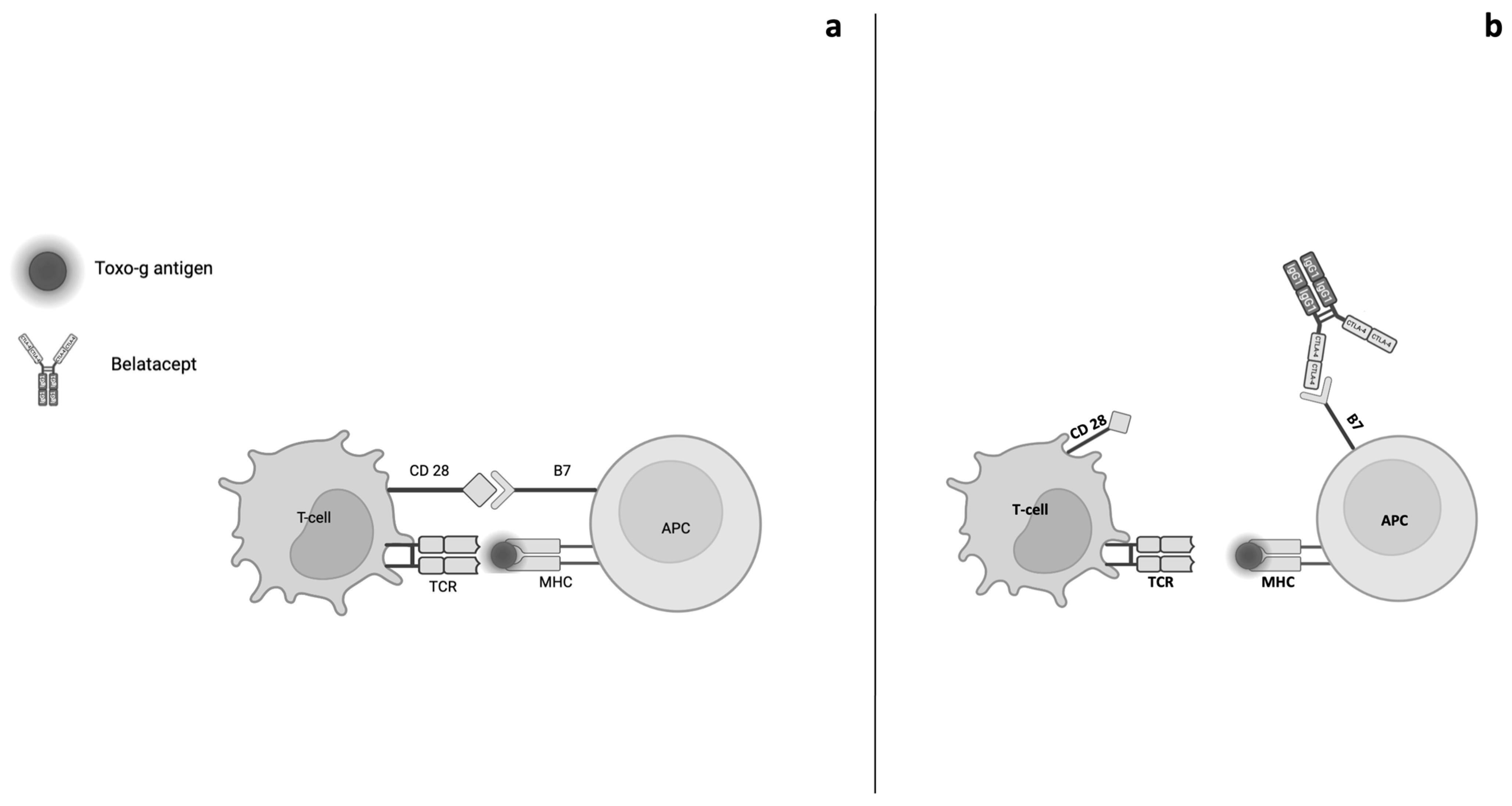
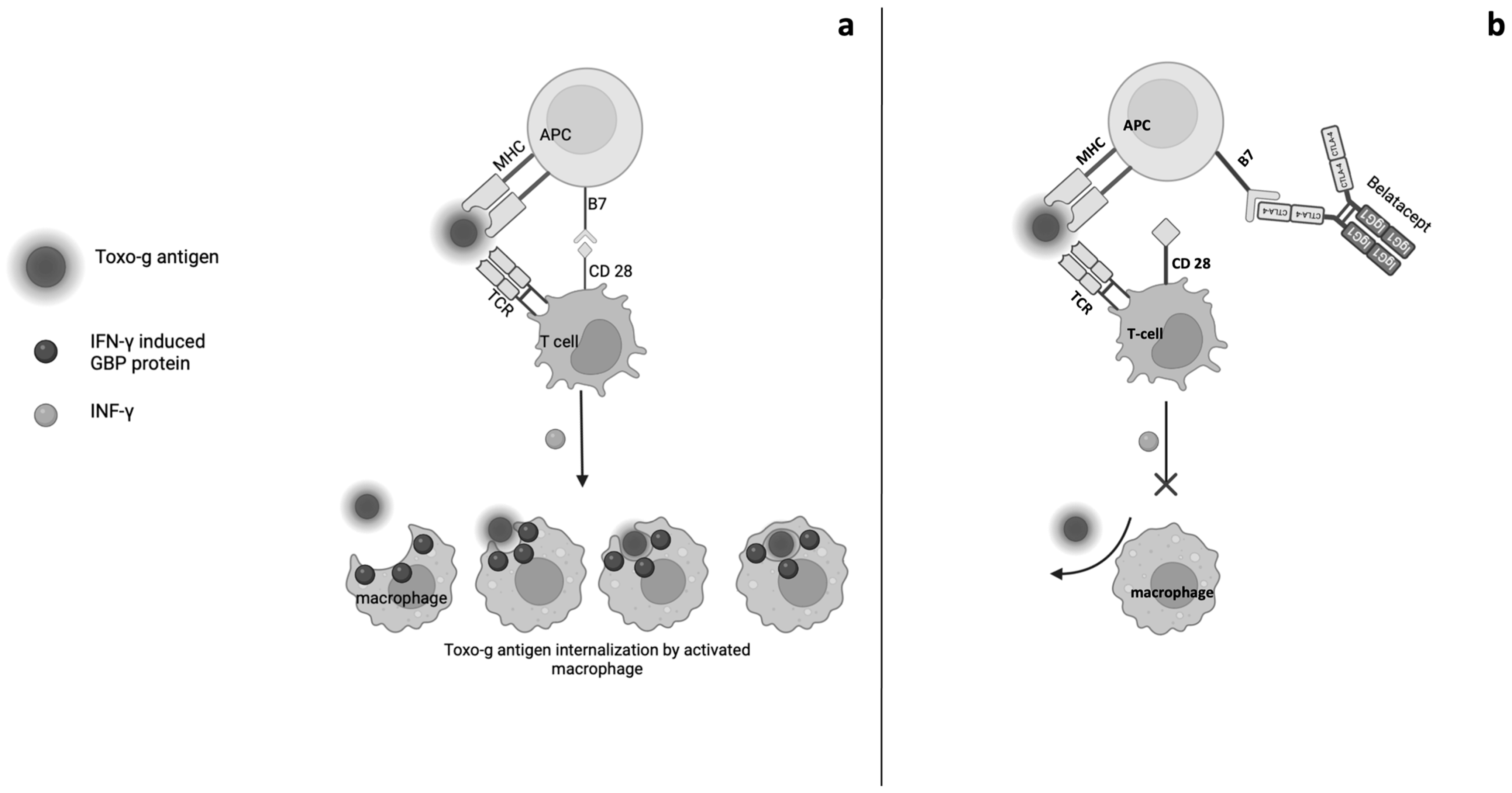
5. Opportunistic Infections and Belatacept
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Onofrio, G.; Simeoni, M.; Rizza, P.; Caroleo, M.; Capria, M.; Mazzitello, G.; Sacco, T.; Mazzuca, E.; Panzino, M.T.; Cerantonio, A.; et al. Quality of life, clinical outcome, personality and coping in chronic hemodialysis patients. Ren. Fail. 2017, 39, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Capasso, A.; Viggiano, D.; Lee, M.W.; Palladino, G.; Bilancio, G.; Simeoni, M.; Capolongo, G.; Secondulfo, C.; Ronchi, A.; Caputo, A.; et al. Kidney Transplant Modifies the Architecture and Microenvironment of Basal Cell Carcinomas. Kidney Blood Press. Res. 2020, 45, 368–377. [Google Scholar] [CrossRef]
- Leonardi, G.; Simeoni, M.; Bozzo, M.; Caglioti, A.; Fuiano, G. Diagnosis of Biliary Hamartomatosis in Kidney Transplant Recipient Affected by ADPKD. G. Ital. Nefrol. 2019, 36, 2019-vol1. (In Italian) [Google Scholar]
- Mella, A.; Mariano, F.; Dolla, C.; Gallo, E.; Manzione, A.M.; Di Vico, M.C.; Cavallo, R.; De Rosa, F.G.; Costa, C.; Biancone, L. Bacterial and Viral Infection and Sepsis in Kidney Transplanted Patients. Biomedicines 2022, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Khurana, A.; Mwangi, R.; Fama, A.; Ristow, K.M.; Maurer, M.J.; Macon, W.R.; Ansell, S.M.; Bennani, N.N.; Kudva, Y.C.; et al. Clinicopathologic Characteristics, Treatment, and Outcomes of Post-transplant Lymphoproliferative Disorders: A Single-Institution Experience Using 2017 WHO Diagnostic Criteria. Hemasphere 2021, 5, e640. [Google Scholar] [CrossRef]
- Bertrand, D.; Chavarot, N.; Olagne, J.; Greze, C.; Gatault, P.; Danthu, C.; Colosio, C.; Jaureguy, M.; Duveau, A.; Bouvier, N.; et al. Biopsy-Proven T-Cell Mediated Rejection After Belatacept Rescue Conversion: A Multicenter Retrospective Study. Transpl. Int. 2024, 37, 13544. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Prashar, R.; Haller, H.; Rial, M.C.; Kamar, N.; Agarwal, A.; de Fijter, J.W.; Rostaing, L.; Berger, S.P.; Djamali, A.; et al. Conversion From Calcineurin Inhibitor to Belatacept-Based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial. J. Am. Soc. Nephrol. 2021, 32, 3252–3264. [Google Scholar] [CrossRef]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A Phase III Study of Belatacept-Based Immunosuppression Regimens versus Cyclosporine in Renal Transplant Recipients (BENEFIT Study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef]
- Bertrand, D.; Chavarot, N.; Gatault, P.; Garrouste, C.; Bouvier, N.; Grall-Jezequel, A.; Jaureguy, M.; Caillard, S.; Lemoine, M.; Colosio, C.; et al. Opportunistic Infections after Conversion to Belatacept in Kidney Transplantation. Nephrol. Dial. Transplant. 2020, 35, 336–345. [Google Scholar] [CrossRef]
- Ensor, C.R.; Goehring, K.C.; Iasella, C.J.; Moore, C.A.; Lendermon, E.A.; McDyer, J.F.; Morrell, M.R.; Sciortino, C.M.; Venkataramanan, R.; Wiland, A.M. Belatacept for Maintenance Immunosuppression in Cardiothoracic Transplantation: The Potential Frontier. Clin. Transplant. 2018, 32, e13363. [Google Scholar] [CrossRef]
- Larsen, C.P.; Pearson, T.C.; Adams, A.B.; Tso, P.; Shirasugi, N.; Strobert, E.; Anderson, D.; Cowan, S.; Price, K.; Naemura, J.; et al. Rational Development of LEA29Y (Belatacept), a High-Affinity Variant of CTLA4-Ig with Potent Immunosuppressive Properties. Am. J. Transplant. 2005, 5, 443–453. [Google Scholar] [CrossRef] [PubMed]
- El Hennawy, H.; Safar, O.; Al Faifi, A.S.; El Nazer, W.; Kamal, A.; Mahedy, A.; Zaitoun, M.; Fahmy, A.E. Belatacept rescue therapy of CNI-induced nephrotoxicity, meta-analysis. Transpl. Rev. 2021, 35, 100653. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Kemmner, S.; Renders, L.; Heemann, U. Should belatacept be the centrepiece of renal transplantation? Nephrol. Dial. Transplant. 2016, 31, 1995–2002. [Google Scholar] [CrossRef]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Gardner, D.; Jeffery, L.E.; Sansom, D.M. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am. J. Transplant. 2014, 14, 1985–1991. [Google Scholar] [CrossRef]
- Chavarot, N.; Morel, A.; Leruez-Ville, M.; Vilain, E.; Divard, G.; Burger, C.; Scemla, A. Weak Antibody Response to Three Doses of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Am. J. Transplant. 2021, 21, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Leibler, C.; Thiolat, A.; Hénique, C.; Samson, C.; Pilon, C.; Tamagne, M.; Grimbert, P. Control of Humoral Response in Renal Transplantation by Belatacept Depends on a Direct Effect on B Cells and Impaired T Follicular Helper-B Cell Crosstalk. J. Am. Soc. Nephrol. 2018, 29, 1049–1062. [Google Scholar] [CrossRef]
- Knechtle, S.; Kwun, J.; Song, S.; Jackson, A.; Williams, K.; Sanoff, S. Translation of Therapeutic Strategies to Modulate B Cell Responses from Non-Human Primate Models to Human Kidney Transplantation. Front. Transplant. 2023, 2, 1176796. [Google Scholar] [CrossRef]
- Lien, C.; Fang, C.; Huso, D.; Livák, F.; Lu, R.; Pitha, P. Critical Role of IRF-5 in Regulation of B-Cell Differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 4664–4668. [Google Scholar] [CrossRef]
- Hwang, S.; Cobb, D.; Bhadra, R.; Youngblood, B.; Khan, I. Blimp-1–Mediated CD4 T Cell Exhaustion Causes CD8 T Cell Dysfunction During Chronic Toxoplasmosis. J. Exp. Med. 2016, 213, 1799–1818. [Google Scholar] [CrossRef]
- Álvarez, E.; Cortés, A.; Alemán, G.; Alberú, J.; García, E.; Soldevila, M. Decreased Frequency and Impaired Function of Circulating Tregs from Renal Transplant Patients under Long-Term Belatacept Treatment. J. Immunol. 2016, 196, 141.1. [Google Scholar] [CrossRef]
- Santeusanio, A.; Bhansali, A.; Weinberg, A.; Shapiro, R.; Delaney, V.; Florman, S.; Boccardo, G. Conversion to Belatacept within 1-Year of Renal Transplantation in a Diverse Cohort Including Patients with Donor-Specific Antibodies. Clin. Transplant. 2020, 34, e13823. [Google Scholar] [CrossRef]
- Renton, A.; Pliner, H.; Provenzano, C.; Evoli, A.; Ricciardi, R.; Nalls, M.; Traynor, B. A Genome-Wide Association Study of Myasthenia Gravis. JAMA Neurol. 2015, 72, 396. [Google Scholar] [CrossRef]
- Khalaf, A.; Song, J.; Gao, T.; Yu, X.; Lei, T. CTLA-4 Gene Polymorphism and the Risk of Systemic Lupus Erythematosus in the Chinese Population. Biomed Res. Int. 2011, 2011, 167395. [Google Scholar] [CrossRef]
- Barreto, M.; Santos, E.; Ferreira, R.; Fesel, C.; Moraes-Fontes, M.; Pereira, C.; Vicente, A. Evidence for CTLA4 as a Susceptibility Gene for Systemic Lupus Erythematosus. Eur. J. Hum. Genet. 2004, 12, 620–626. [Google Scholar] [CrossRef]
- Jury, E.; Flores-Borja, F.; Kalsi, H.; Lazarus, M.; Isenberg, D.; Mauri, C.; Ehrenstein, M. Abnormal CTLA-4 Function in T Cells from Patients with Systemic Lupus Erythematosus. Eur. J. Immunol. 2010, 40, 569–578. [Google Scholar] [CrossRef]
- Stumpf, M.; Zhou, X.; Bluestone, J. The B7-Independent Isoform of CTLA-4 Functions to Regulate Autoimmune Diabetes. J. Immunol. 2013, 190, 961–969. [Google Scholar] [CrossRef]
- Hwang, K.; Sweatt, W.; Mashayekhi, M.; Palucki, D.; Sattar, H.; Chuang, E.; Alegre, M. Transgenic Expression of CTLA-4 Controls Lymphoproliferation in IL-2-Deficient Mice. J. Immunol. 2004, 173, 5415–5424. [Google Scholar] [CrossRef]
- Pavlova, A.; Diaz-Lacava, A.; Zeitler, H.; Satoguina, J.; Niemann, B.; Krause, M.; Scharrer, I.; Hoerauf, A.; Wienker, T.; Oldenburg, J. Increased Frequency of the CTLA-4 49 A/G Polymorphism in Patients with Acquired Haemophilia A Compared to Healthy Controls. Haemophilia 2008, 14, 355–360. [Google Scholar] [CrossRef]
- Wing, K.; Yamaguchi, T.; Sakaguchi, S. Cell-Autonomous and -Non-Autonomous Roles of CTLA-4 in Immune Regulation. Trends Immunol. 2011, 32, 428–433. [Google Scholar] [CrossRef]
- Xu, H.; Perez, S.; Cheeseman, J.; Mehta, A.; Kirk, A. The Allo- and Viral-Specific Immunosuppressive Effect of Belatacept, but Not Tacrolimus, Attenuates with Progressive T Cell Maturation. Am. J. Transplant. 2014, 14, 319–332. [Google Scholar] [CrossRef]
- Australian Public Assessment Report for Belatacept. Australian Department of Health and Ageing, Therapeutic Goods Administration: Canberra, Australia, 2012; pp. 1–198.
- Zhou, Z.; Shen, J.; Hong, Y.; Kaul, S.; Pfister, M.; Roy, A. Time-varying belatacept exposure and its relationship to efficacy/safety responses in kidney-transplant recipients. Clin. Pharmacol. Ther. 2012, 92, 251–257. [Google Scholar] [CrossRef]
- Latek, R.; Fleener, C.; Lamian, V.; Kulbokas, E., 3rd; Davis, P.M.; Suchard, S.J.; Curran, M.; Vincenti, F.; Townsend, R. Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation 2009, 87, 926–933. [Google Scholar] [CrossRef]
- Chopra, B.; Sureshkumar, K.K. Co-stimulatory blockade with belatacept in kidney transplantation. Expert Opin. Biol. Ther. 2014, 14, 563–567. [Google Scholar] [CrossRef]
- Durrbach, A.; Pestana, J.M.; Pearson, T.; Vincenti, F.; Garcia, V.D.; Campistol, J.; Rial, M.D.C.; Florman, S.; Block, A.; Di Russo, G.; et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am. J. Transplant. 2010, 10, 547–557. [Google Scholar] [CrossRef]
- Rostaing, L.; Massari, P.; Garcia, V.D.; Mancilla-Urrea, E.; Nainan, G.; del Carmen Rial, M.; Steinberg, S.; Vincenti, F.; Shi, R.; Di Russo, G.; et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: A randomized phase II study. Clin. J. Am. Soc. Nephrol. 2011, 6, 430–439. [Google Scholar] [CrossRef]
- Grinyó, J.; Alberu, J.; Contieri, F.L.; Manfro, R.C.; Mondragon, G.; Nainan, G.; Rial, M.D.C.; Steinberg, S.; Vincenti, F.; Dong, Y.; et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: 2-year results from the long-term extension of a phase II study. Transpl. Int. 2012, 25, 1059–1064. [Google Scholar] [CrossRef]
- Grinyó, J.M.; Del Carmen Rial, M.; Alberu, J.; Steinberg, S.M.; Manfro, R.C.; Nainan, G.; Vincenti, F.; Jones-Burton, C.; Kamar, N. Safety and efficacy outcomes 3 years after switching to belatacept from a calcineurin inhibitor in kidney transplant recipients: Results from a phase 2 randomized trial. Am. J. Kidney Dis. 2017, 69, 587–594. [Google Scholar] [CrossRef]
- Holdaas, H.; Mjøen, G.; Jardine, A.G. Belatacept: Where the BENEFITS Outweigh the Risk. Am. J. Kidney Dis. 2017, 69, 561–563. [Google Scholar] [CrossRef]
- Jehn, U.; Siam, S.; Wiening, V.; Pavenstädt, H.; Reuter, S. Belatacept as a Treatment Option in Patients with Severe BK Polyomavirus Infection and High Immunological Risk—Walking a Tightrope between Viral Control and Prevention of Rejection. Viruses 2022, 14, 1005. [Google Scholar] [CrossRef]
- Liverman, R.; Chandran, M.; Crowther, B. Considerations and Controversies of Pharmacologic Management of the Pediatric Kidney Transplant Recipient. Pharmacotherapy 2021, 41, 77–102. [Google Scholar] [CrossRef]
- Huang, H.; Schechtman, K.; Askar, M.; Bernadt, C.; Doré, P.; Goodarzi, A.; Hachem, R. A Pilot Randomized Controlled Trial of De Novo Belatacept-Based Immunosuppression after Lung Transplantation. Transplantation 2024, 108, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Rengel, T.; Andrews, J.; Paulero, M.; Iotti, A.; Forastiero, A.; Agorio, I. Belatacept and Mediastinal Histoplasmosis in a Kidney Transplant Patient. J. Nephropathol. 2016, 5, 84–87. [Google Scholar]
- Blew, K.; Chua, A.; Foreman, J.; Gbadegesin, R.; Jackson, A.; Nagaraj, S.; Chambers, E. Tailored Use of Belatacept in Adolescent Kidney Transplantation. Am. J. Transplant. 2020, 20, 884–888. [Google Scholar] [CrossRef]
- Schlüter, D.; Deckert, M.; Hof, H.; Frei, K. Toxoplasma gondii infection of neurons induces neuronal cytokine and chemokine production, but gamma interferon- and tumor necrosis factor-stimulated neurons fail to inhibit the invasion and growth of T Gondii. Infect. Immun. 2001, 69, 7889–7893. [Google Scholar] [CrossRef]
- Fisch, D.; Clough, B.; Khan, R.; Healy, L.; Frickel, E.M. Toxoplasma-proximal and distal control by GBPs in human macrophages. Pathog. Dis. 2022, 79, ftab058. [Google Scholar] [CrossRef]
- Antinori, A.; Ammassari, A.; De Luca, A.; Cingolani, A.; Murri, R.; Scoppettuolo, G.; Fortini, M.; Tartaglione, T.; Larocca, L.M.; Zannoni, G.; et al. Diagnosis of AIDS-related focal brain lesions: A decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 1997, 48, 687–694. [Google Scholar] [CrossRef]
- Marra, C.M. Central nervous system infection with Toxoplasma gondii. Handb. Clin. Neurol. 2018, 152, 117–122. [Google Scholar] [CrossRef]
- Santos, J.; Brož, P. Sensing of Invading Pathogens by GBPs: At the Crossroads between Cell-Autonomous and Innate Immunity. J. Leukoc. Biol. 2018, 104, 729–735. [Google Scholar] [CrossRef]
- Tretina, K.; Park, E.; Mamińska, A.; MacMicking, J. Interferon-Induced Guanylate-Binding Proteins: Guardians of Host Defense in Health and Disease. J. Exp. Med. 2019, 216, 482–500. [Google Scholar] [CrossRef]
- Kutsch, M.; Coers, J. Human Guanylate Binding Proteins: Nanomachines Orchestrating Host Defense. FEBS J. 2021, 288, 5826–5849. [Google Scholar] [CrossRef]
- Meunier, É.; Wallet, P.; Dreier, R.; Costanzo, S.; Anton, L.; Rühl, S.; Brož, P. Guanylate-Binding Proteins Promote Activation of the AIM2 Inflammasome during Infection with Francisella novicida. Nat. Immunol. 2015, 16, 476–484. [Google Scholar] [CrossRef]
- Kravets, E.; Poschmann, G.; Hänsch, S.; Raba, V.; Weidtkamp-Peters, S.; Degrandi, D.; Stühler, K.; Pfeffer, K. mGBP2 Engages Galectin-9 for Immunity against Toxoplasma gondii. PLoS ONE 2025, 20, e0316209. [Google Scholar] [CrossRef]
- Steffens, N.; Beuter-Gunia, C.; Kravets, E.; Reich, A.; Legewie, L.; Pfeffer, K.; Degrandi, D. Essential Role of mGBP7 for Survival of Toxoplasma gondii Infection. mBio 2020, 11, e02993-19. [Google Scholar] [CrossRef]
- Ólafsson, E.; Barragán, A. The Unicellular Eukaryotic Parasite Toxoplasma gondii Hijacks the Migration Machinery of Mononuclear Phagocytes to Promote Its Dissemination. Biol. Cell 2020, 112, 239–250. [Google Scholar] [CrossRef]
- Baird, J.; Fox, B.; Sanders, K.; Lizotte, P.; Cubillos-Ruiz, J.; Scarlett, U.; Bzik, D. Avirulent Toxoplasma gondii Generates Therapeutic Antitumor Immunity by Reversing Immunosuppression in the Ovarian Cancer Microenvironment. Cancer Res. 2013, 73, 3842–3851. [Google Scholar] [CrossRef]
- Catchpole, A.; Zabriskie, B.; Bassett, P.; Embley, B.; White, D.; Gale, S.; Dawson, W. Association between Toxoplasma gondii Infection and Type-1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 4436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigilante, R.; Izhar, R.; Paola, R.D.; De, A.; Pollastro, R.M.; Capolongo, G.; Viceconte, G.; Simeoni, M. Toxoplasma Gondii Replication During Belatacept Treatment in Kidney Transplantation: A Case Report and a Review of the Literature. Genes 2025, 16, 391. https://doi.org/10.3390/genes16040391
Vigilante R, Izhar R, Paola RD, De A, Pollastro RM, Capolongo G, Viceconte G, Simeoni M. Toxoplasma Gondii Replication During Belatacept Treatment in Kidney Transplantation: A Case Report and a Review of the Literature. Genes. 2025; 16(4):391. https://doi.org/10.3390/genes16040391
Chicago/Turabian StyleVigilante, Raffaella, Raafiah Izhar, Rossella Di Paola, Ananya De, Rosa Maria Pollastro, Giovanna Capolongo, Giulio Viceconte, and Mariadelina Simeoni. 2025. "Toxoplasma Gondii Replication During Belatacept Treatment in Kidney Transplantation: A Case Report and a Review of the Literature" Genes 16, no. 4: 391. https://doi.org/10.3390/genes16040391
APA StyleVigilante, R., Izhar, R., Paola, R. D., De, A., Pollastro, R. M., Capolongo, G., Viceconte, G., & Simeoni, M. (2025). Toxoplasma Gondii Replication During Belatacept Treatment in Kidney Transplantation: A Case Report and a Review of the Literature. Genes, 16(4), 391. https://doi.org/10.3390/genes16040391





