Centenary Progress on Orchidaceae Research: A Bibliometric Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Bibliometric Analysis
2.3. Impact Factor
3. Results
3.1. Primary Performance of Selected Publications
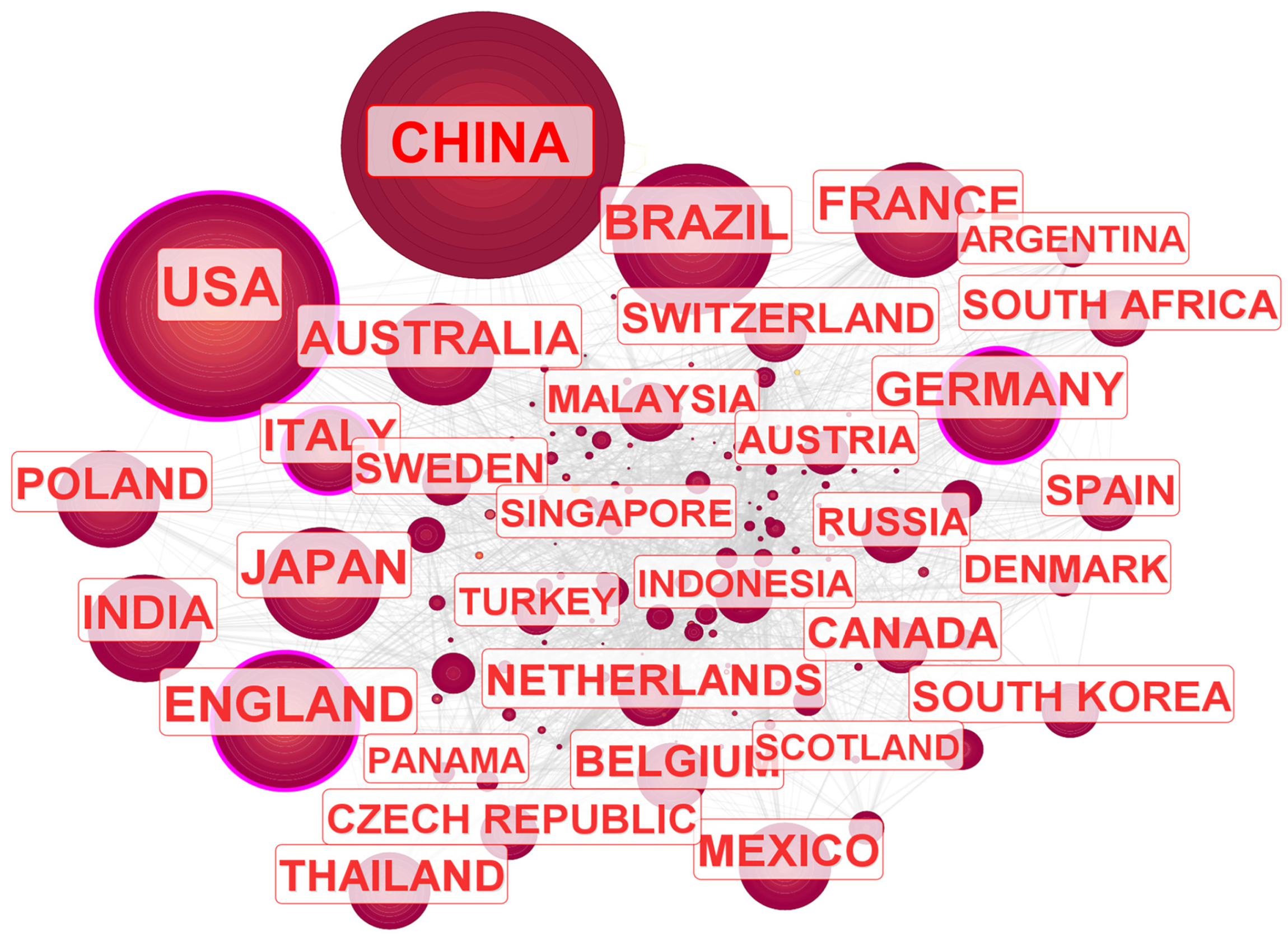
3.2. Author Collaboration and Subject Categories
3.3. Distribution of Journals, Research Institutions
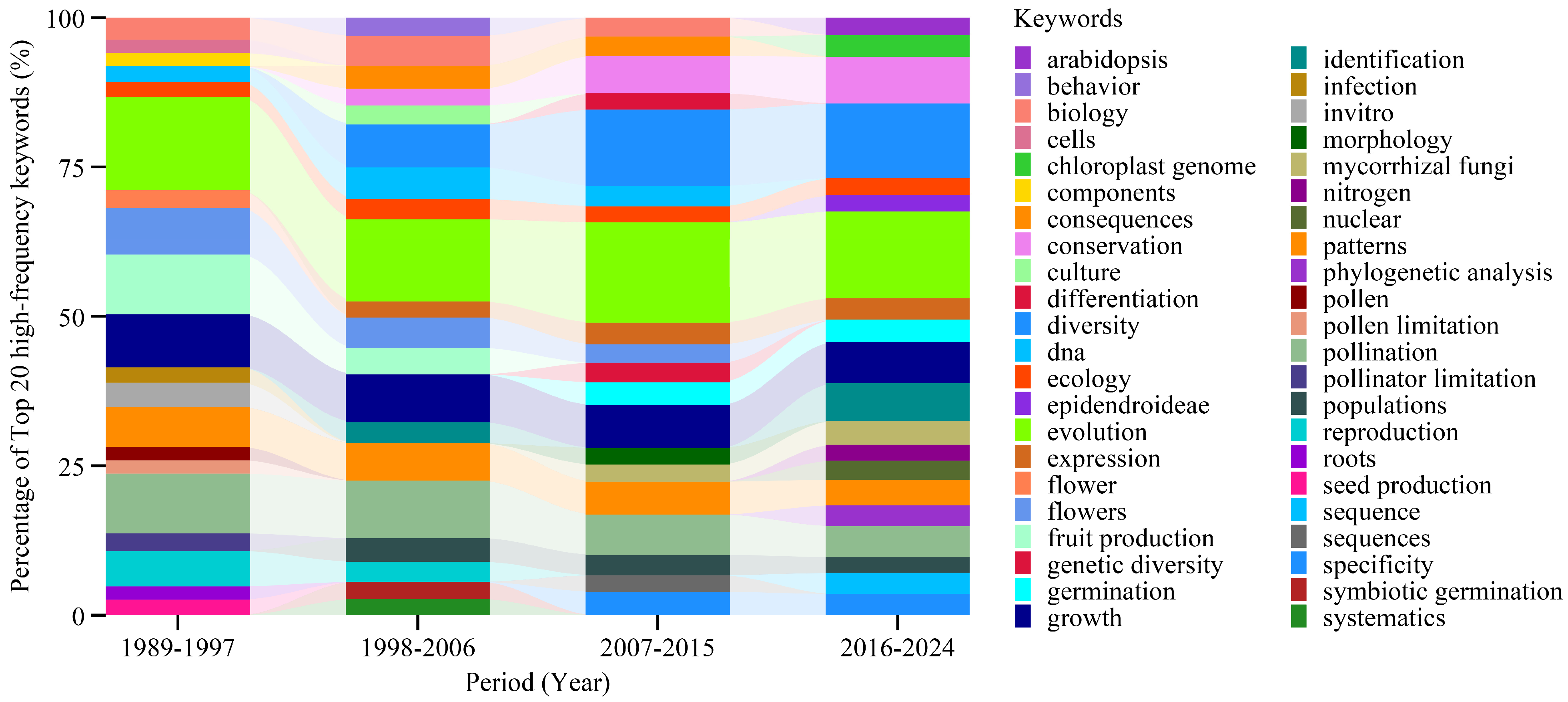
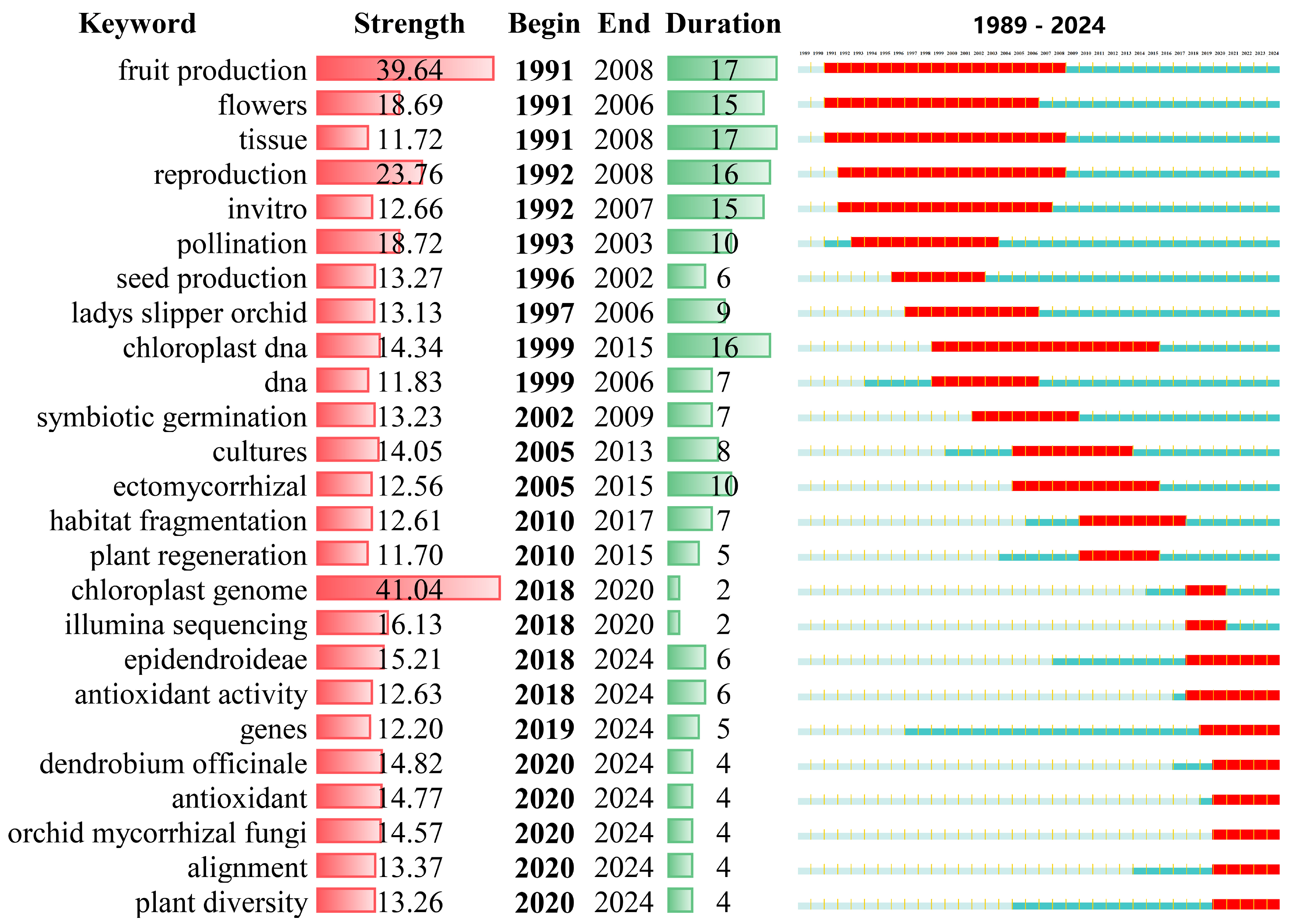
3.4. Time-Series Analysis of High-Frequency Keywords and Emerging Topics
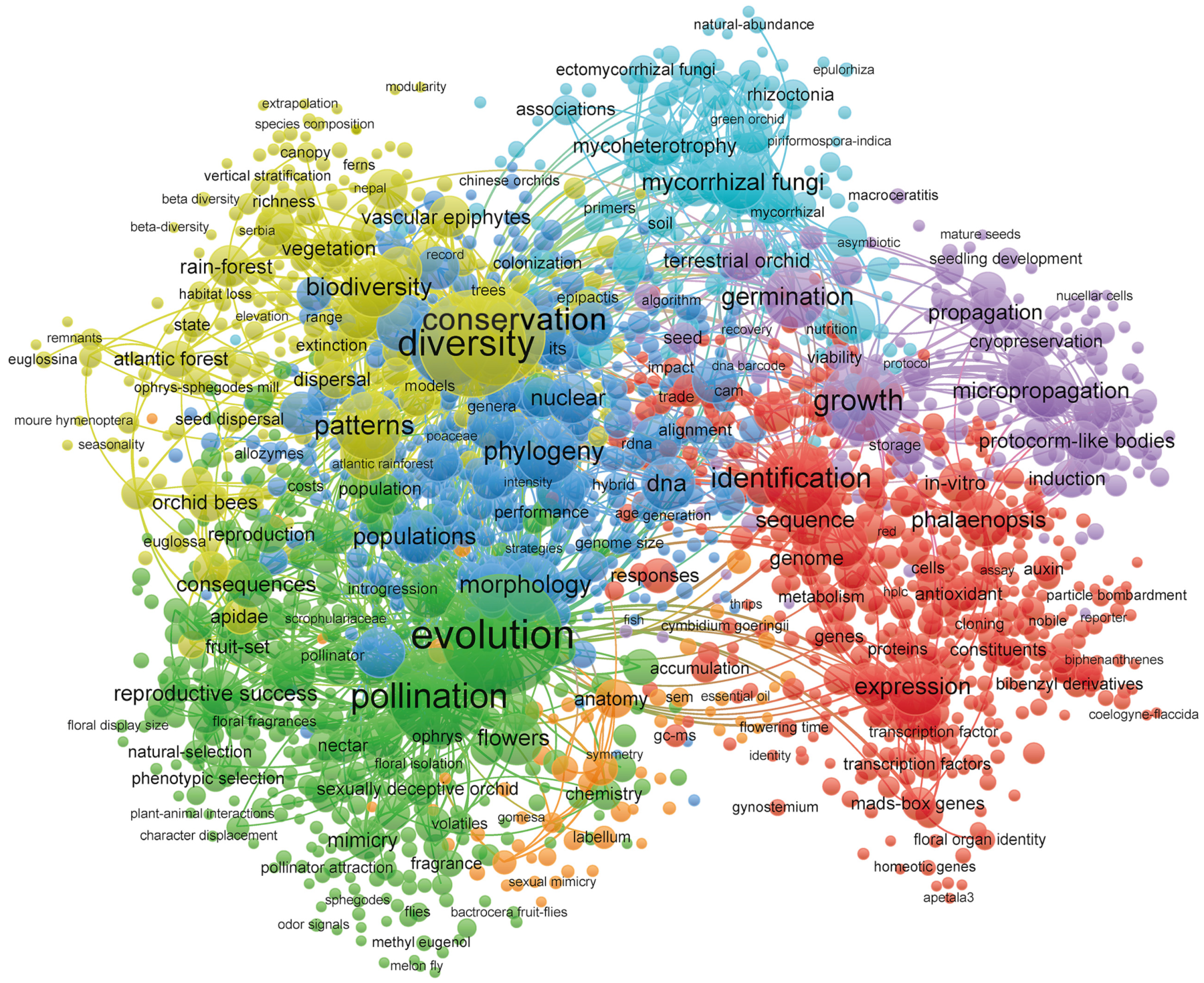
3.5. Evolution of Research Hotspots
3.6. Research Field Classification
3.6.1. The Impact of Evolutionary and Pollination Mechanisms in Orchidaceae Research
3.6.2. Orchid Conservation and Biodiversity Patterns in Changing Environments
3.6.3. In Vitro Propagation and Germination of Orchids
3.6.4. Molecular Mechanisms and Biotechnology in Orchid Research
3.6.5. Phylogenetics and Taxonomy in Orchid Research
3.6.6. Mycorrhizal Symbiosis and Nutrient Acquisition in Orchids
3.6.7. Morphological and Anatomical Features of Orchids
4. Discussion
4.1. Key Issues in the Orchid Field
4.2. Future Trend Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, F. Biological diversity, ecosystem stability and economic development. Ecol. Econ. 1996, 16, 191–203. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Martín-Forés, I.; Bywaters, S.L.; Sparrow, B.; Guerin, G.R. Simultaneous effect of habitat remnancy, exotic species, and anthropogenic disturbance on orchid diversity in South Australia. Conserv. Sci. Pract. 2022, 4, e12652. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Y.; Huang, M.Z.; Huang, W.C.; Liu, D.K.; Zhang, D.; Hu, H.; Downing, J.L.; Liu, Z.J.; Ma, H. Comprehensive phylogenetic analyses of Orchidaceae using nuclear genes and evolutionary insights into epiphytism. J. Integr. Plant Biol. 2023, 65, 1204–1225. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Perspectives on orchid conservation in botanic gardens. Trends Plant Sci. 2009, 14, 590–598. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. A continental scale analysis of threats to orchids. Biol. Conserv. 2019, 234, 7–17. [Google Scholar] [CrossRef]
- Jersáková, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006, 81, 219–235. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Schlüter, P.M. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 2009, 54, 425–446. [Google Scholar] [CrossRef]
- Yu, H.; Goh, C.J. Molecular genetics of reproductive biology in orchids. Plant Physiol. 2001, 127, 1390–1393. [Google Scholar] [CrossRef]
- Roberts, D.L.; Dixon, K.W. Orchids. Curr. Biol. 2008, 18, R325–R329. [Google Scholar] [CrossRef]
- Hossain, M.M.; Kant, R.; Van, P.T.; Winarto, B.; Zeng, S.; Teixeira da Silva, J.A. The application of biotechnology to orchids. Crit. Rev. Plant Sci. 2013, 32, 69–139. [Google Scholar] [CrossRef]
- Pfeifer, M.; Wiegand, K.; Heinrich, W.; Jetschke, G. Long-term demographic fluctuations in an orchid species driven by weather: Implications for conservation planning. J. Appl. Ecol. 2006, 43, 313–324. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Whigham, D.F.; Burnett, R.K., Jr. Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. J. Ecol. 2016, 104, 744–754. [Google Scholar] [CrossRef]
- Wilcock, C.; Neiland, R. Pollination failure in plants: Why it happens and when it matters. Trends Plant Sci. 2002, 7, 270–277. [Google Scholar] [CrossRef]
- Fay, M.F. Orchid conservation: How can we meet the challenges in the twenty-first century? Bot. Stud. 2018, 59, 1–6. [Google Scholar] [CrossRef]
- Morales, C.L.; Sáez, A.; Garibaldi, L.A.; Aizen, M.A. Disruption of pollination services by invasive pollinator species. Impact Biol. Invasions Ecosyst. Serv. 2017, 12, 203–220. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Ye, C.; Wang, Z.; Ma, C.; Lin, D.; Jin, X. Progress in systematics and biogeography of Orchidaceae. Plant Divers. 2024, 46, 425–434. [Google Scholar] [CrossRef]
- Swarts, N.D.; Sinclair, E.A.; Francis, A.; Dixon, K.W. Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid. Mol. Ecol. 2010, 19, 3226–3242. [Google Scholar] [CrossRef]
- Zhong-Jian, L.; Jian-Yong, Z.; Zheng-Zhong, R.; Si-Peng, L.; Li-Jun, C. Conservation biology of Paphiopedilum purpuratum (Orchidaceae). Biodivers. Sci. 2004, 12, 509. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Garfield, E. From the science of science to Scientometrics visualizing the history of science with HistCite software. J. Informetr. 2009, 3, 173–179. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Chellappandi, P.; Vijayakumar, C.S. Bibliometrics, Scientometrics, Webometrics/Cybermetrics, Informetrics and Altmetrics—An Emerging Field in Library and Information Science Research. Shanlax Int. J. Educ. 2018, 7, 5–8. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, P.; Li, T.; Wang, Z. Research Progress on endangered plants: A bibliometric analysis. Biodivers. Conserv. 2022, 31, 1125–1147. [Google Scholar] [CrossRef]
- Guo, Y.-M.; Huang, Z.-L.; Guo, J.; Guo, X.-R.; Li, H.; Liu, M.-Y.; Ezzeddine, S.; Nkeli, M.J. A bibliometric analysis and visualization of blockchain. Future Gener. Comput. Syst. 2021, 116, 316–332. [Google Scholar] [CrossRef]
- Garfield, E. The history and meaning of the journal impact factor. JAMA 2006, 295, 90–93. [Google Scholar] [CrossRef]
- Moussa, S. A bibliometric investigation of the journals that were repeatedly suppressed from Clarivate’s Journal Citation Reports. Account. Res. 2023, 30, 592–612. [Google Scholar] [CrossRef]
- Hanson, M.A.; Barreiro, P.G.; Crosetto, P.; Brockington, D. The strain on scientific publishing. Quant. Sci. Stud. 2024, 5, 823–843. [Google Scholar] [CrossRef]
- Normile, D. A ’home bias’ in citations boosts China’s global science ranking. Science 2024, 386, 11–12. [Google Scholar] [CrossRef]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S1), 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A.; Edwards, J.L. The global biodiversity information facility (GBIF). Syst. Assoc. Spec. Vol. 2007, 73, 1. [Google Scholar] [CrossRef]
- Boyle, F. The Culture of Greenhouse Orchids: Old System and New; Chapman & Hall: London, UK, 1902. [Google Scholar]
- Catling, P.M.; Catling, V.R. Two new species of Lepanthes (Orchidaceae) from Mexico. Can. J. Bot. 1988, 66, 2130–2133. [Google Scholar] [CrossRef]
- Goh, C.J.; Kavaljian, L.G. Orchid industry of Singapore. Econ. Bot. 1989, 43, 241–254. [Google Scholar] [CrossRef]
- Cameron, K.M.; Chase, M.W.; Whitten, W.M.; Kores, P.J.; Jarrell, D.C.; Albert, V.A.; Yukawa, T.; Hills, H.G.; Goldman, D.H. A phylogenetic analysis of the Orchidaceae: Evidence from rbcL nucleotide sequences. Am. J. Bot. 1999, 86, 208–224. [Google Scholar] [CrossRef]
- Tremblay, R.L. Trends in the pollination ecology of the Orchidaceae: Evolution and systematics. Can. J. Bot. 1992, 70, 642–650. [Google Scholar] [CrossRef]
- Cozzolino, S.; Widmer, A. Orchid diversity: An evolutionary consequence of deception? Trends Ecol. Evol. 2005, 20, 487–494. [Google Scholar] [CrossRef]
- Dearnaley, J.D. Further advances in orchid mycorrhizal research. Mycorrhiza 2007, 17, 475–486. [Google Scholar] [CrossRef]
- Díaz, S.; Tilman, D.; Fargione, J.; Chapin, F.S., III; Dirzo, R.; Ktzberber, T. Biodiversity regulation of ecosystem services. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R., Ash, N., Eds.; Island Press: Washington, DC, USA, 2005; pp. 279–329. Available online: https://www.researchgate.net/publication/285678219_Biodiversity_regulation_of_ecosystem_services/citations (accessed on 11 March 2025).
- Pierce, S. The Conservation of Terrestrial Orchids; Centro Flora Autoctona della Regione Lombardia: Via Bertarelli, Italy, 2011. [Google Scholar]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Ari, Ş.; Arikan, M. Next-generation sequencing: Advantages, disadvantages, and future. In Plant Omics: Trends and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 109–135. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Wang, N.; Xu, Y.; Tang, G.; Xu, L.; Feng, Y. Bioactivities and mechanism of actions of Dendrobium officinale: A comprehensive review. Oxid. Med. Cell. Longev. 2022, 2022, 6293355. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Lei, Y.T.; Su, Z.X.; Zhao, M.; Zhang, J.X.; Shen, G.J.; Wang, L.; Li, J.; Qi, J.F.; Wu, J.Q. A chromosome-scale Gastrodia elata genome and large-scale comparative genomic analysis indicate convergent evolution by gene loss in mycoheterotrophic and parasitic plants. Plant J. 2021, 108, 1609–1623. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, M.; Wang, H.; Song, H.; Zhang, L.; Huang, Q.; Chen, R.; Song, C.; Li, G.; Cao, Y. Haplotype-resolved genome assembly of Bletilla striata (Thunb.) Reichb.f. to elucidate medicinal value. Plant J. 2022, 111, 1340–1353. [Google Scholar] [CrossRef]
- Piet, Q.; Droc, G.; Marande, W.; Sarah, G.; Bocs, S.; Klopp, C.; Bourge, M.; Siljak-Yakovlev, S.; Bouchez, O.; Lopez-Roques, C.; et al. A chromosome-level, haplotype-phased Vanilla planifolia genome highlights the challenge of partial endoreplication for accurate whole-genome assembly. Plant Commun. 2022, 3, 100330. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Li, Y.; Ke, S.; Yin, W.; Lan, S.; Liu, Z. Advances and prospects of orchid research and industrialization. Hortic. Res. 2022, 9, uhac220. [Google Scholar] [CrossRef]
- Feng, S.G.; Zheng, K.X.; Gao, Y.D.; Zhang, Z.H.; Jin, Y.Y.; Lin, W.T.; Ma, R.Y.; Hou, K.L.; Zhang, H.S.; Liang, X.S.; et al. Molecular mechanism of cadmium stress response in a traditional herbal medicine Anoectochilus roxburghii. Ind. Crop. Prod. 2023, 205, 11. [Google Scholar] [CrossRef]
- Fan, W.; He, Z.-S.; Zhe, M.; Feng, J.-Q.; Zhang, L.; Huang, Y.; Liu, F.; Huang, J.-L.; Ya, J.-D.; Zhang, S.-B.; et al. High-quality Cymbidium mannii genome and multifaceted regulation of crassulacean acid metabolism in epiphytes. Plant Commun. 2023, 4, 100564. [Google Scholar] [CrossRef]
- Tiwari, P.; Sharma, A.; Bose, S.K.; Park, K.-I. Advances in orchid biology: Biotechnological achievements, translational success, and commercial outcomes. Horticulturae 2024, 10, 152. [Google Scholar] [CrossRef]
- Russo, A.; Alessandrini, M.; El Baidouri, M.; Frei, D.; Galise, T.R.; Gaidusch, L.; Oertel, H.F.; Garcia Morales, S.E.; Potente, G.; Tian, Q. Genome of the early spider-orchid Ophrys sphegodes provides insights into sexual deception and pollinator adaptation. Nat. Commun. 2024, 15, 6308. [Google Scholar] [CrossRef]
- Harder, L.D.; Johnson, S.D. Darwin’s beautiful contrivances: Evolutionary and functional evidence for floral adaptation. New Phytol. 2009, 183, 530–545. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R. Lack of strong selection pressures maintains wide variation in floral traits in a food-deceptive orchid. Ann. Bot. 2020, 126, 445–453. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Johnson, S.D. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Brys, R.; Jongejans, E. Size-dependent flowering and costs of reproduction affect population dynamics in a tuberous perennial woodland orchid. J. Ecol. 2010, 98, 1204–1215. [Google Scholar] [CrossRef]
- Cozzolino, S.; Scopece, G. Specificity in pollination and consequences for postmating reproductive isolation in deceptive Mediterranean orchids. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3037–3046. [Google Scholar] [CrossRef]
- Kotilínek, M.; Těšitelová, T.; Košnar, J.; Fibich, P.; Hemrová, L.; Koutecký, P.; Münzbergová, Z.; Jersáková, J. Seed dispersal and realized gene flow of two forest orchids in a fragmented landscape. Plant Biol. 2020, 22, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Tang, Y.; Peng, X.-F.; Wang, J.; Zhang, S.-Q.; Feng, Y.; Peng, P.-H. Identifying priorities under highly heterogeneous environments through species distribution models to facilitate orchid conservation. Biodivers. Conserv. 2024, 33, 647–665. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.-T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef]
- Popova, E.; Kim, H.H.; Saxena, P.K.; Engelmann, F.; Pritchard, H.W. Frozen beauty: The cryobiotechnology of orchid diversity. Biotechnol. Adv. 2016, 34, 380–403. [Google Scholar] [CrossRef]
- Novak, S.D.; Luna, L.J.; Gamage, R.N. Role of auxin in orchid development. Plant Signal. Behav. 2014, 9, e972277. [Google Scholar] [CrossRef]
- Sarmah, D.; Kolukunde, S.; Sutradhar, M.; Singh, B.K.; Mandal, T.; Mandal, N. A review on: In vitro cloning of orchids. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1909–1927. [Google Scholar] [CrossRef]
- Suetsugu, K.; Sugita, R.; Yoshihara, A.; Okada, H.; Akita, K.; Nagata, N.; Tanoi, K.; Kobayashi, K. Aerial roots of the leafless epiphytic orchid Taeniophyllum are specialized for performing crassulacean acid metabolism photosynthesis. New Phytol. 2023, 238, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chaudhary, V.; Sirohi, U.; Singh, J. Advances in biochemical and molecular marker techniques and their applications in genetic studies of orchid: A review. Int. J. Chem. Stud. 2018, 6, 806–822. [Google Scholar]
- Chen, H.-Y.; Zhang, Z.-R.; Yao, X.; Ya, J.-D.; Jin, X.-H.; Wang, L.; Lu, L.; Li, D.-Z.; Yang, J.-B.; Yu, W.-B. Plastid phylogenomics provides new insights into the systematics, diversification, and biogeography of Cymbidium (Orchidaceae). Plant Divers. 2024, 46, 448–461. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, K.W.; Li, Z.; Lohaus, R.; Hsiao, Y.Y.; Niu, S.C.; Wang, J.Y.; Lin, Y.C.; Xu, Q.; Chen, L.J.; et al. The Apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef]
- Wu, S.-S.; Jiang, M.-T.; Miao, J.-l.; Li, M.-H.; Wang, J.-Y.; Shen, L.-M.; Peng, D.-H.; Lan, S.-R.; Zhai, J.-W.; Liu, Z.-J. Origin and diversification of a Himalayan orchid genus Pleione. Mol. Phylogenet. Evol. 2023, 184, 107797. [Google Scholar] [CrossRef]
- Pinheiro, F.; De Barros, F.; Palma-Silva, C.; Meyer, D.; Fay, M.F.; Suzuki, R.M.; Lexer, C.; Cozzolino, S. Hybridization and introgression across different ploidy levels in the Neotropical orchids Epidendrum fulgens and E. puniceoluteum (Orchidaceae). Mol. Ecol. 2010, 19, 3981–3994. [Google Scholar] [CrossRef]
- Chauhan, P.; Attri, L. Mycorrhizal Associations in Orchids: A Review. Asian J. Biol. Life Sci. 2024, 13, 278–286. [Google Scholar] [CrossRef]
- Sathiyadash, K.; Muthukumar, T.; Karthikeyan, V.; Rajendran, K. Orchid mycorrhizal fungi: Structure, function, and diversity. In Orchid Biology: Recent Trends & Challenges; Springer: Berlin/Heidelberg, Germany, 2020; pp. 239–280. [Google Scholar] [CrossRef]
- Li, M.H.; Liu, K.W.; Li, Z.; Lu, H.C.; Ye, Q.L.; Zhang, D.; Wang, J.Y.; Li, Y.F.; Zhong, Z.M.; Liu, X.; et al. Genomes of leafy and leafless Platanthera orchids illuminate the evolution of mycoheterotrophy. Nat. Plants 2022, 8, 373–388. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Weiss, M.; Kull, T.; Taylors, D.L. High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Mol. Ecol. 2005, 14, 613–626. [Google Scholar] [CrossRef]
- Zahn, F.E. Exploring Plant Nutritional Strategies in Orchid and Arbuscular Mycorrhizal Associations Using Stable Isotope Natural Abundance. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2024. [Google Scholar]
- Ercole, E.; Adamo, M.; Rodda, M.; Gebauer, G.; Girlanda, M.; Perotto, S. Temporal variation in mycorrhizal diversity and carbon and nitrogen stable isotope abundance in the wintergreen meadow orchid Anacamptis morio. New Phytol. 2015, 205, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Pabón-Mora, N.; González, F. The gynostemium: More than the sum of its parts with emerging floral complexities. Curr. Opin. Plant Biol. 2024, 81, 102609. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.A.; Davies, K.L.; Stpiczyńska, M.; Carlsward, B.S.; Ionta, G.M.; Gerlach, G. Floral elaiophores in Lockhartia Hook. (Orchidaceae: Oncidiinae): Their distribution, diversity and anatomy. Ann. Bot. 2013, 112, 1775–1791. [Google Scholar] [CrossRef] [PubMed]
- Baishnab, B.; Bora, D.; Banik, B.; Paul, S.; Sarma, D.; Majumdar, K.; Datta, B.K. Comparative morpho-anatomical studies of two sections of the genus Dendrobium sw. (Orchidaceae). Vegetos 2023, 36, 377–392. [Google Scholar] [CrossRef]
- Zhao, D.-K.; Mou, Z.-M.; Ruan, Y.-L. Orchids acquire fungal carbon for seed germination: Pathways and players. Trends Plant Sci. 2024, 29, 733–741. [Google Scholar] [CrossRef]
- Li, S.; Liang, C.; Deng, S.; Chen, C.; Yuan, L.; Liu, Z.; Wu, S.; Lan, S.; Tang, Z.; Liu, Z. Comparison of Orchid Conservation Between China and Other Countries. Diversity 2024, 16, 692. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.Y.; Zheng, P.J.; Li, K.L.; Liang, Y.M.; Chen, X.F.; Zhang, C.; Zhao, X.; He, X.; Zhang, G.Q. Dendrobium thyrsiflorum genome and its molecular insights into genes involved in important horticultural traits. Plant Biotechnol. J. 2024, 22, 2887. [Google Scholar] [CrossRef]
- Dawson-Glass, E.; Hargreaves, A.L. Does pollen limitation limit plant ranges? Evidence and implications. Philos. Trans. R. Soc. B 2022, 377, 20210014. [Google Scholar] [CrossRef]
- Söderquist, L.; Dahlgren, J.P.; Sletvold, N. Population viability of the orchid Gymnadenia conopsea increases with population size but is not related to genetic diversity. J. Ecol. 2023, 113, 635–648. [Google Scholar] [CrossRef]
- Molina, W.F.; de Almeida Vieira, F.; Fajardo, C.G. Status Quo and Orchid Conservation Challenges in the Neotropical Region. In Conservation Genetics in the Neotropics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 73–88. [Google Scholar] [CrossRef]
- Francisqueti, A.M.; Marin, R.R.; Hengling, M.M.; Hosomi, S.T.; Pritchard, H.W.; Custódio, C.C.; Machado-Neto, N.B. Orchid seeds are not always short lived in a conventional seed bank! Ann. Bot. 2024, 133, 941–952. [Google Scholar] [CrossRef]
- Pyati, A.N. In vitro Propagation of orchid (Dendrobium ovatum (L.) Kraenzl.) through Somatic Embryogenesis. Plant Tissue Cult. Biotechnol. 2022, 32, 53–66. [Google Scholar] [CrossRef]
- Neigel, E.R.; Harkess, R.L.; Fox, A.A. Mapping potential butterfly milkweed (Asclepias tuberosa) habitat in Mississippi using Species Distribution Modeling (SDM) and Geographic Information Systems (GIS). Nativ. Plants J. 2022, 23, 84–96. [Google Scholar] [CrossRef]
- Yang, F.; Guo, Y.; Li, J.; Lu, C.; Wei, Y.; Gao, J.; Xie, Q.; Jin, J.; Zhu, G. Genome-wide association analysis identified molecular markers and candidate genes for flower traits in Chinese orchid (Cymbidium sinense). Hortic. Res. 2023, 10, uhad206. [Google Scholar] [CrossRef]
- Freudenstein, J.V. Orchid phylogenetics and evolution: History, current status and prospects. Ann. Bot. 2024, mcae202. [Google Scholar] [CrossRef]
- Kolanowska, M.; Rewicz, A.; Nowak, S. Can global warming be beneficial for Arctic-alpine orchid species? Outcomes from ecological niche modeling for Chamorchis alpina (L.) Rich. (Orchidaceae). Sci. Total Environ. 2024, 943, 173616. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, Y.; Zhang, L.; Fu, H.; Tang, G.; Huang, G.; Li, W. Orchid2024: A cultivar-level dataset and methodology for fine-grained classification of Chinese Cymbidium Orchids. Plant Methods 2024, 20, 124. [Google Scholar] [CrossRef]
- Wong, D.C.; Wang, Z.; Perkins, J.; Jin, X.; Marsh, G.E.; John, E.G.; Peakall, R. The road less taken: Dihydroflavonol 4-reductase inactivation and delphinidin anthocyanin loss underpins a natural intraspecific flower colour variation. Mol. Ecol. 2024, e17334. [Google Scholar] [CrossRef]
- Gibert, A.; Schatz, B.; Buscail, R.; Nguyen, D.; Baguette, M.; Barthes, N.; Bertrand, J.A. Floral phenotypic divergence and genomic insights in an Ophrys orchid: Unraveling early speciation processes. New Phytol. 2025, 245, 849–868. [Google Scholar] [CrossRef]
- Gutiérrez–Rodríguez, B.E.; Dáttilo, W.; Villalobos, F.; Sosa, V. Areas of endemism of the orchids of Megamexico: Hotspots of biotic interactions with pollinators. J. Syst. Evol. 2024, 13119. [Google Scholar] [CrossRef]
- Miura, C.; Furui, Y.; Yamamoto, T.; Kanno, Y.; Honjo, M.; Yamaguchi, K.; Suetsugu, K.; Yagame, T.; Seo, M.; Shigenobu, S. Autoactivation of mycorrhizal symbiosis signaling through gibberellin deactivation in orchid seed germination. Plant Physiol. 2024, 194, 546–563. [Google Scholar] [CrossRef]
- Wang, D.; Trimbos, K.B.; Gomes, S.I.; Jacquemyn, H.; Merckx, V.S. Metabarcoding read abundances of orchid mycorrhizal fungi are correlated to copy numbers estimated using ddPCR. New Phytol. 2024, 242, 1825–1834. [Google Scholar] [CrossRef]
- Newmarch, M.B.; Velde, M.; Menon, M.; Sarasan, V. Soil Studies for Fungal Diversity to Enable the Conservation Translocation of Green-Winged Orchid. Diversity 2024, 16, 327. [Google Scholar] [CrossRef]
- Hickerson, N.M.; Samuel, M.A. CRISPR-Cas9 Technology in Plant Genomics Research: A Review and Tutorial for Implementation with Considerations for Orchid Genetic Studies. In Orchid Propagation; Springer Protocols Handbooks; Humana: New York, NY, USA, 2024; pp. 269–303. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Zhang, S.; Wong, C.E.; Liang, Q.; Pang, S.; Wu, Y.; Zhao, M.; Yu, H. Pangeneric genome analyses reveal the evolution and diversity of the orchid genus Dendrobium. Nat. Plants 2025, 1–17. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, Y.-B.; Chen, W.-L.; Wu, K.-C.; Lin, Y.-W.; Sun, E.; Liu, C.-Y. The CWT IoT Device for Detecting Rare Events of Orchid Disease. IEEE Internet Things J. 2024, 11, 22830–22842. [Google Scholar] [CrossRef]
- Liu, C.; Leng, J.; Li, Y.; Ge, T.; Li, J.; Chen, Y.; Guo, C.; Qi, J. A spatiotemporal atlas of organogenesis in the development of orchid flowers. Nucleic Acids Res. 2022, 50, 9724–9737. [Google Scholar] [CrossRef]



| No. | Journal Name | TLCS | TGCS | Records |
|---|---|---|---|---|
| 1 | New Phytologist | 8030 | 17,854 | 233 |
| 2 | American Journal of Botany | 7370 | 14,107 | 316 |
| 3 | Botanical Journal of The Linnean Society | 5895 | 8676 | 338 |
| 4 | Annals of Botany | 5677 | 9846 | 258 |
| 5 | Plant Systematics and Evolution | 3839 | 6299 | 270 |
| 6 | Molecular Ecology | 2670 | 4455 | 70 |
| 7 | Phytochemistry | 2524 | 6911 | 171 |
| 8 | Phytotaxa | 2302 | 2927 | 724 |
| 9 | Evolution | 1794 | 3538 | 49 |
| 10 | Scientia Horticulturae | 1709 | 3412 | 164 |
| No. | Institution | TLCS | TGCS | Records | Country |
|---|---|---|---|---|---|
| 1 | Royal Botanic Gardens, Kew | 7223 | 15,371 | 387 | UK |
| 2 | Chinese Academy of Sciences | 5318 | 15,406 | 827 | China |
| 3 | University of Western Australia | 4775 | 10,130 | 180 | Australia |
| 4 | University of Florida | 3400 | 7158 | 257 | USA |
| 5 | Australian National University | 2874 | 5711 | 159 | Australia |
| 6 | University of Naples Federico II | 2562 | 4201 | 133 | Italy |
| 7 | University of Puerto Rico | 2282 | 3522 | 96 | USA |
| 8 | Academy Sinica | 2174 | 5082 | 170 | China |
| 9 | University of Bayreuth | 2121 | 3820 | 69 | Germany |
| 10 | University of KwaZulu Natal | 2096 | 5696 | 144 | SA |
| No. | Continent | Total Publications | Max Betweenness Centrality | Major Collaborating Countries |
|---|---|---|---|---|
| 1 | Asia | 8089 | 0.07 | China, Japan, India |
| 2 | Europe | 9224 | 0.17 | England, Germany, France |
| 3 | North America | 4695 | 0.19 | USA, Mexico, Canada |
| 4 | Oceania | 1083 | 0.09 | Australia, New Zealand, New Caledonia |
| 5 | South America | 2070 | 0.02 | Brazil, Argentina, Colombia |
| 6 | Africa | 747 | 0.05 | South Africa, Egypt, Nigeria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, J.; Jin, J.; Gao, J.; Xie, Q.; Lu, C.; Zhu, G.; Yang, F. Centenary Progress on Orchidaceae Research: A Bibliometric Analysis. Genes 2025, 16, 336. https://doi.org/10.3390/genes16030336
Wei Y, Li J, Jin J, Gao J, Xie Q, Lu C, Zhu G, Yang F. Centenary Progress on Orchidaceae Research: A Bibliometric Analysis. Genes. 2025; 16(3):336. https://doi.org/10.3390/genes16030336
Chicago/Turabian StyleWei, Yonglu, Jie Li, Jianpeng Jin, Jie Gao, Qi Xie, Chuqiao Lu, Genfa Zhu, and Fengxi Yang. 2025. "Centenary Progress on Orchidaceae Research: A Bibliometric Analysis" Genes 16, no. 3: 336. https://doi.org/10.3390/genes16030336
APA StyleWei, Y., Li, J., Jin, J., Gao, J., Xie, Q., Lu, C., Zhu, G., & Yang, F. (2025). Centenary Progress on Orchidaceae Research: A Bibliometric Analysis. Genes, 16(3), 336. https://doi.org/10.3390/genes16030336








