Common Regulatory Mechanisms Mediated by Cuproptosis Genes in Inflammatory Bowel Disease and Major Depressive Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Identification of Differentially Expressed Cuproptosis Genes
2.3. Immune Cell Infiltration Analysis in CD, UC, and MDD
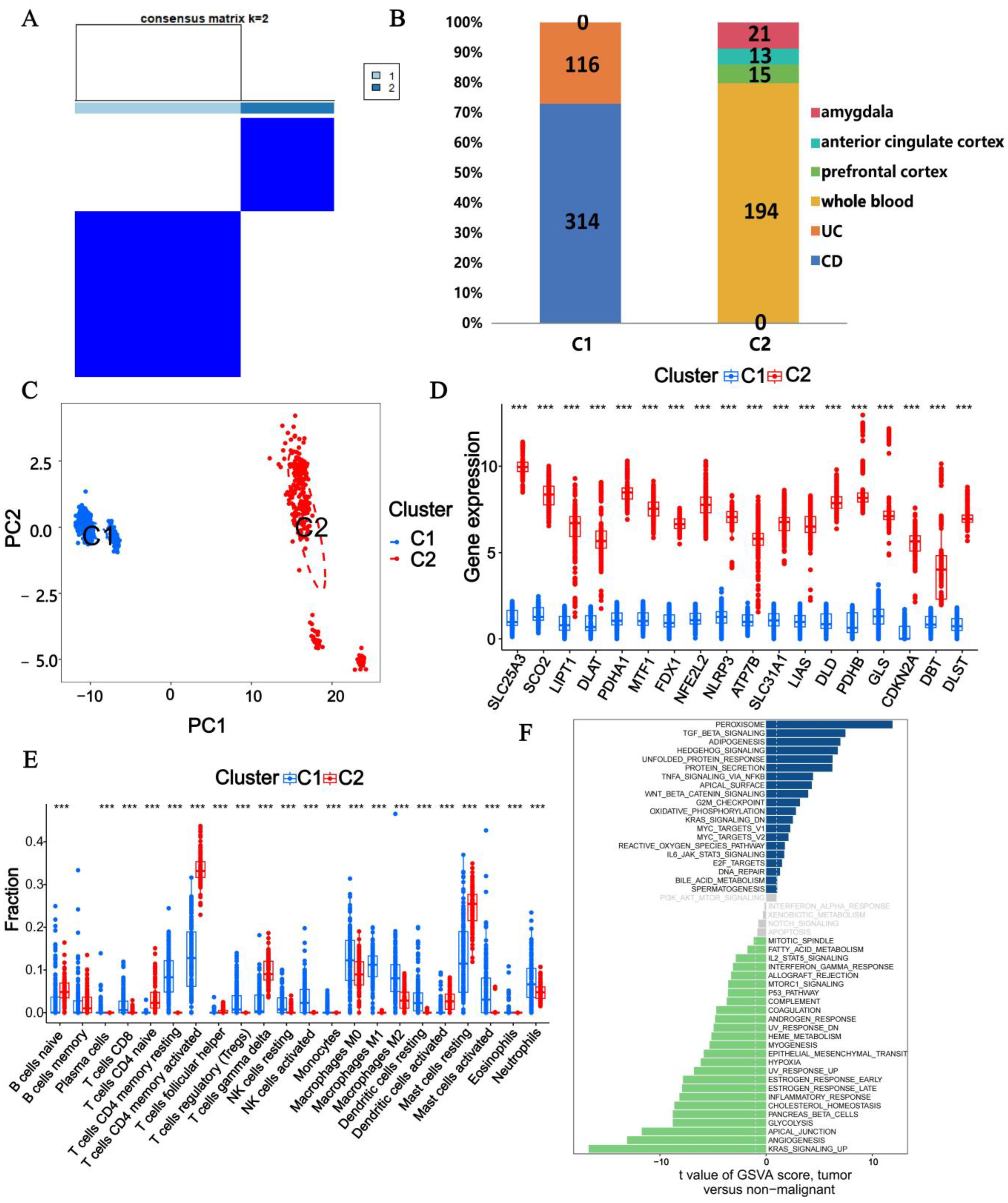
2.4. Identification and Exploration of CD, UC, and MDD Subtypes
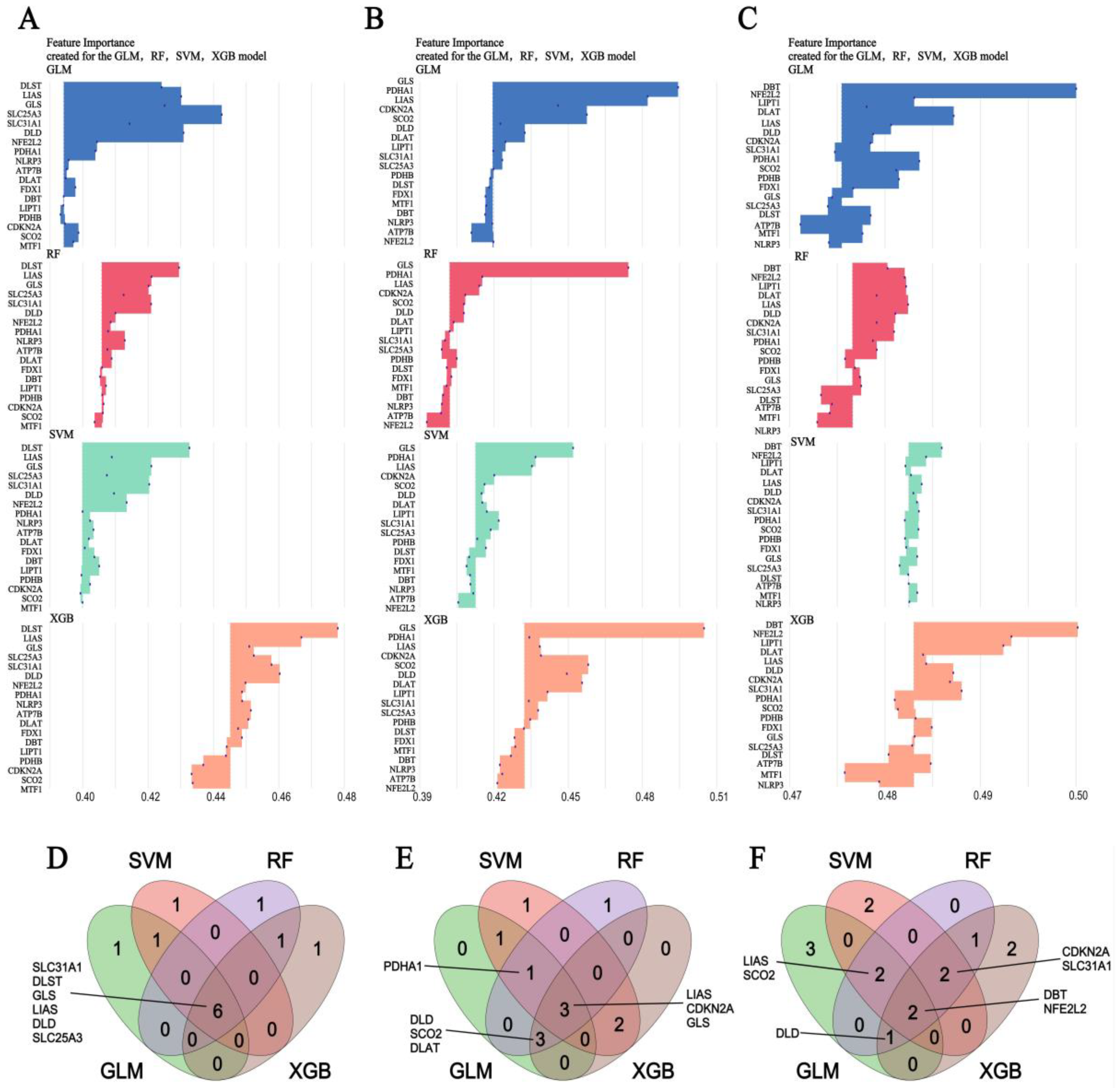
2.5. Machine Learning Screening of Candidate Important Genes
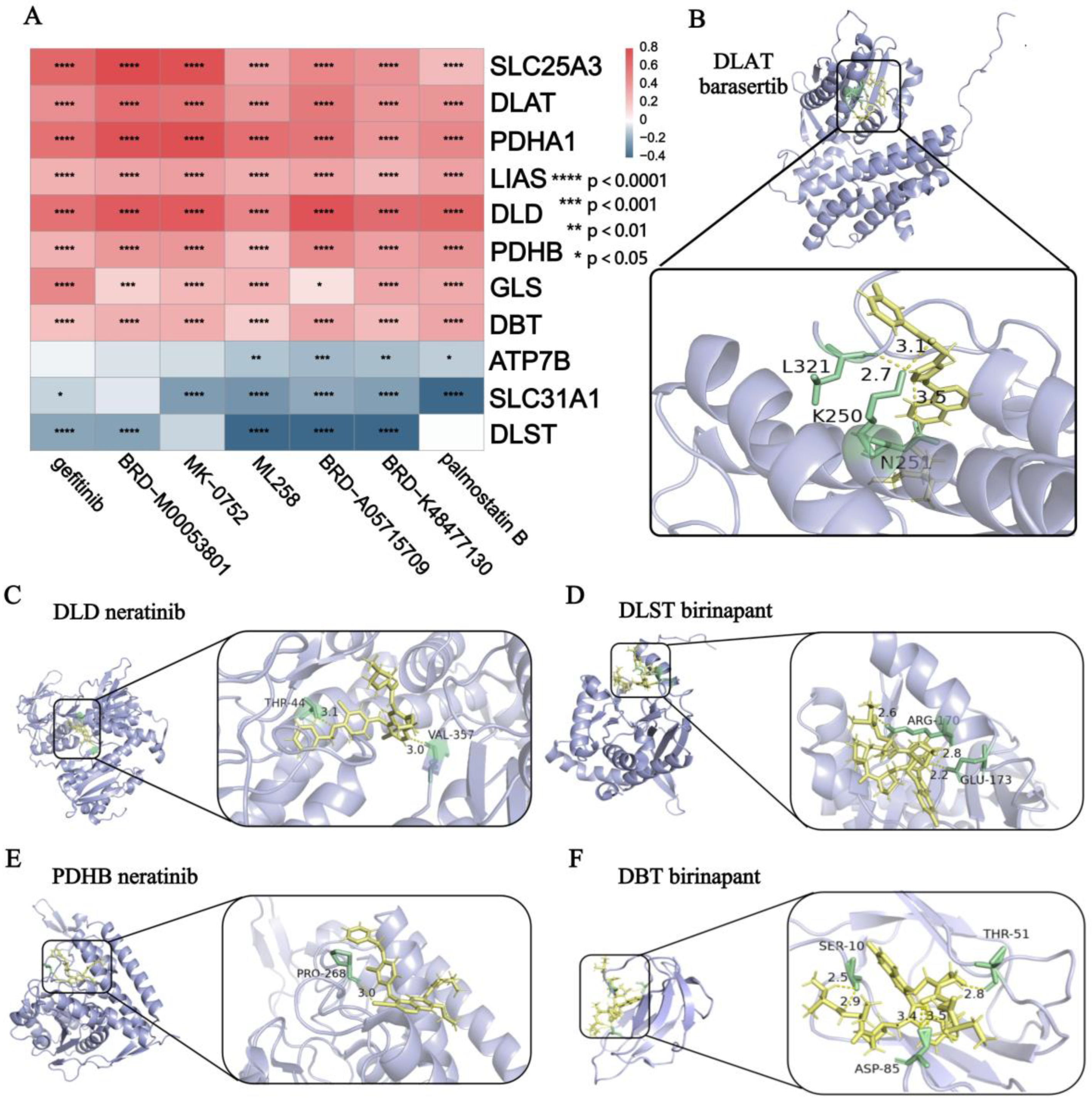
2.6. Drug Predict
2.7. Molecular Docking
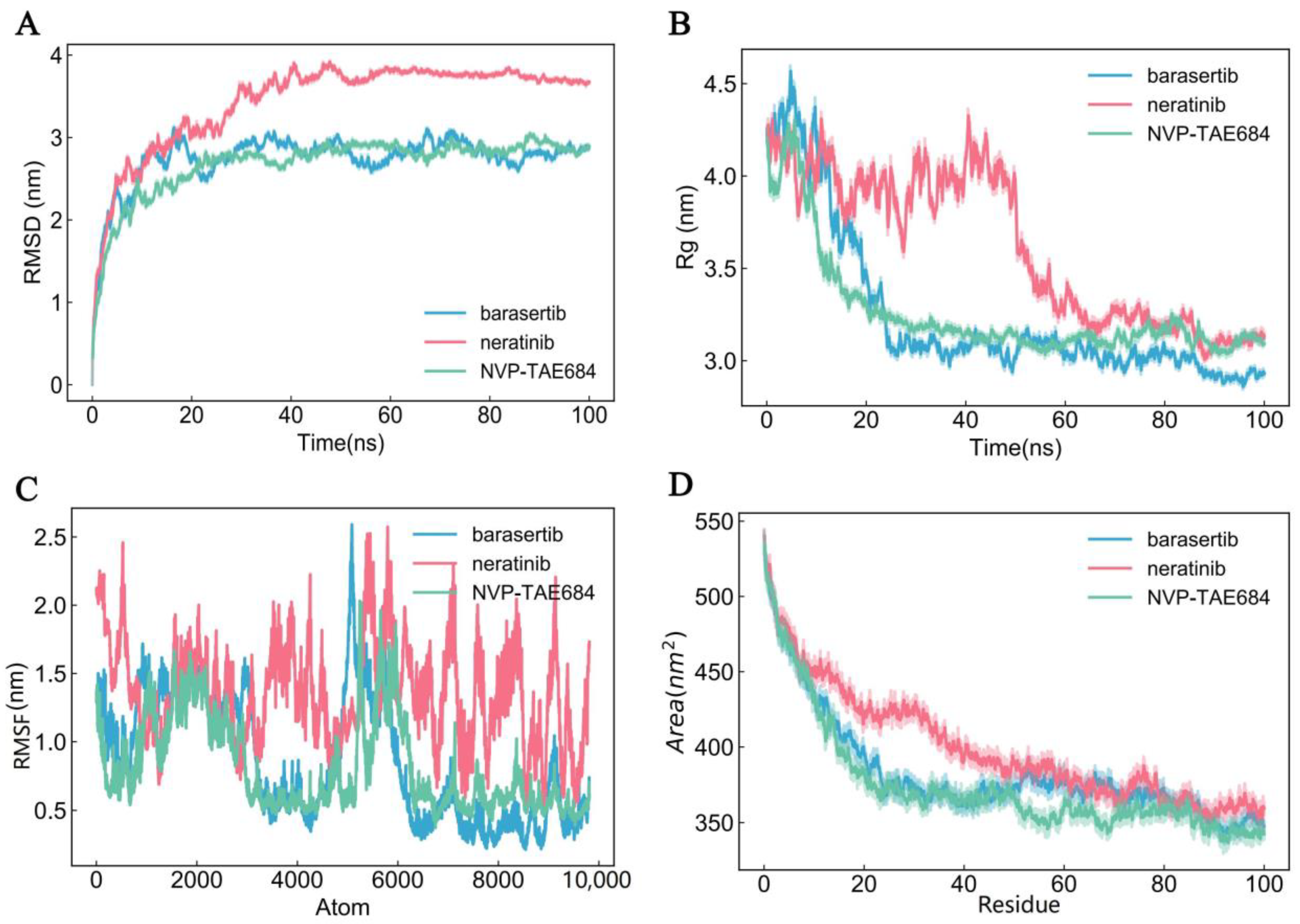
2.8. Molecular Dynamics Simulations
2.9. Phenome-Wide Association Studies
2.10. Statistical Analysis
3. Results
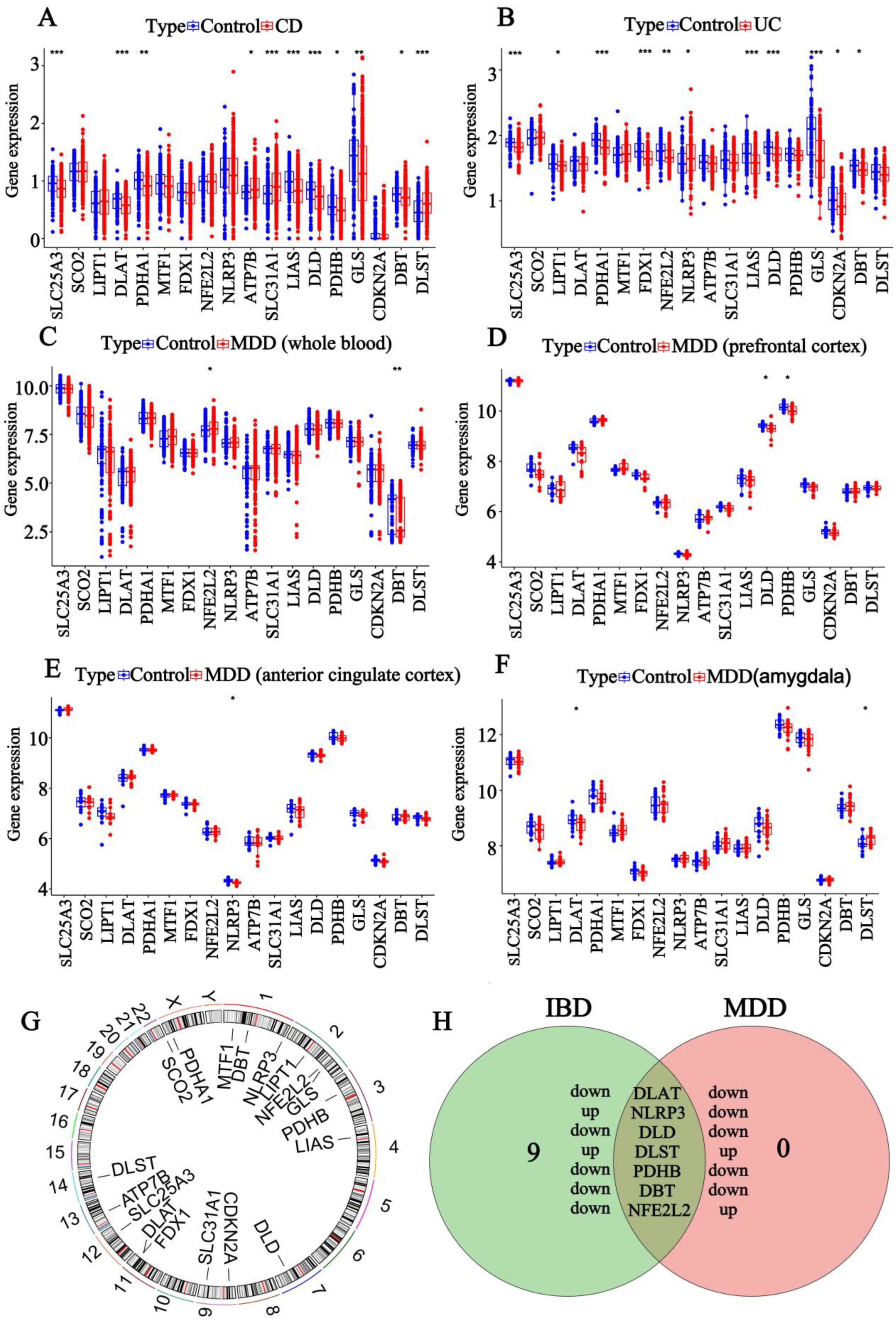
3.1. Screening of DEGs for IBD and MDD
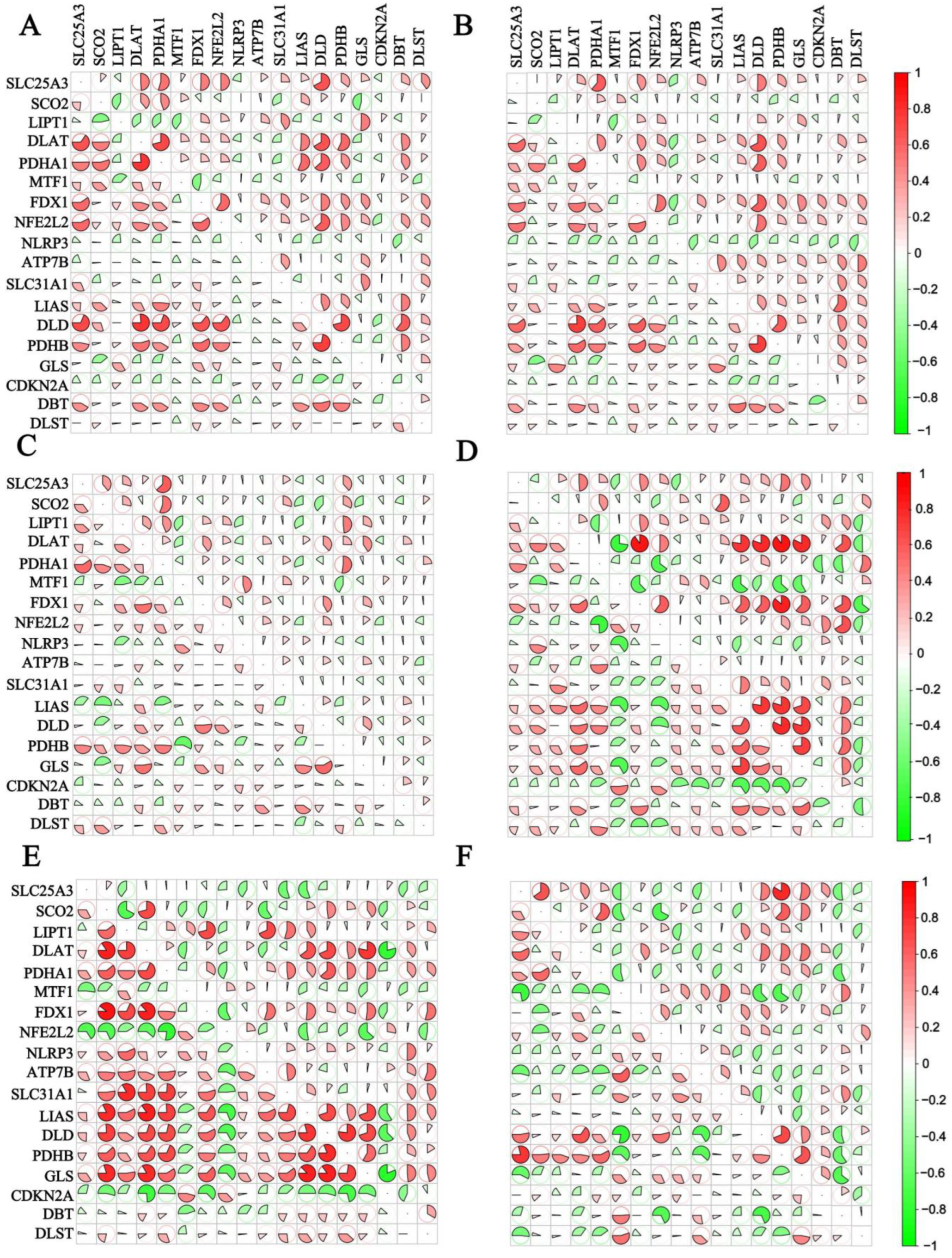
3.2. Correlation Analysis of the 18 CRGs
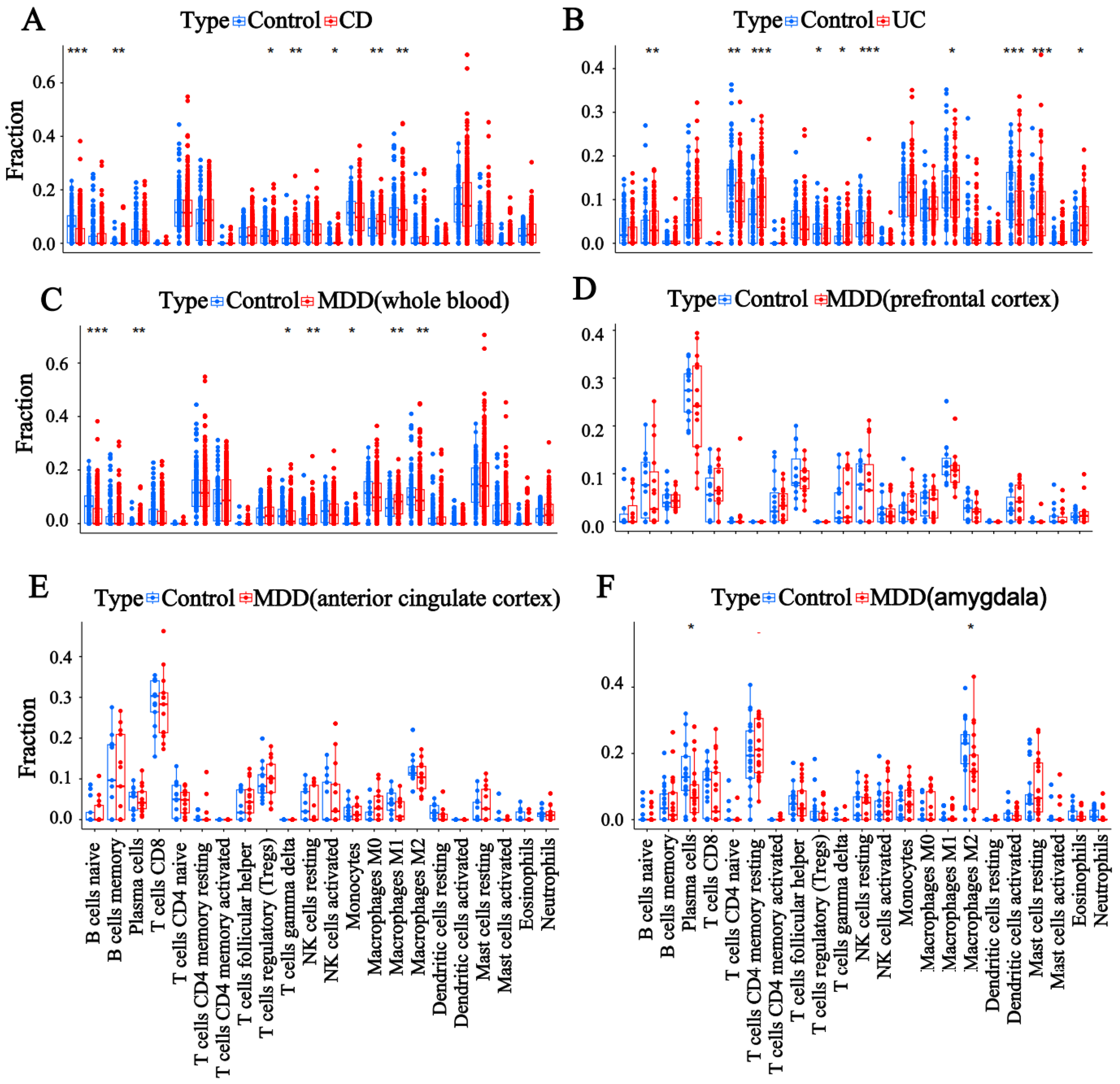
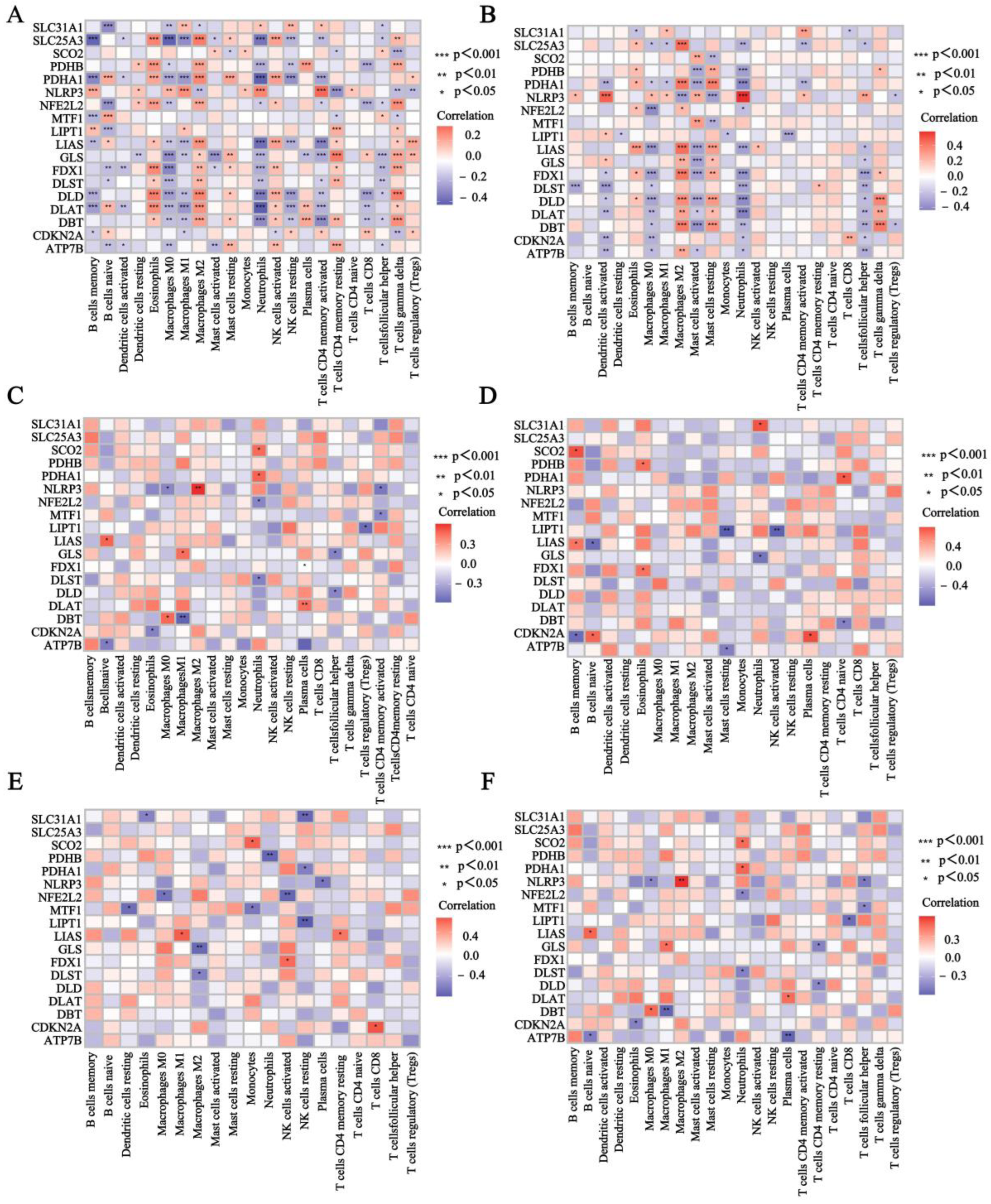
3.3. Evaluation of Immune Cell Infiltration
3.4. Subtype Analysis with CRGs
3.5. Machine Learning Screening for the Important Genes
3.6. Drug Prediction and Molecular Docking Analysis
3.7. Molecular Dynamics Simulation
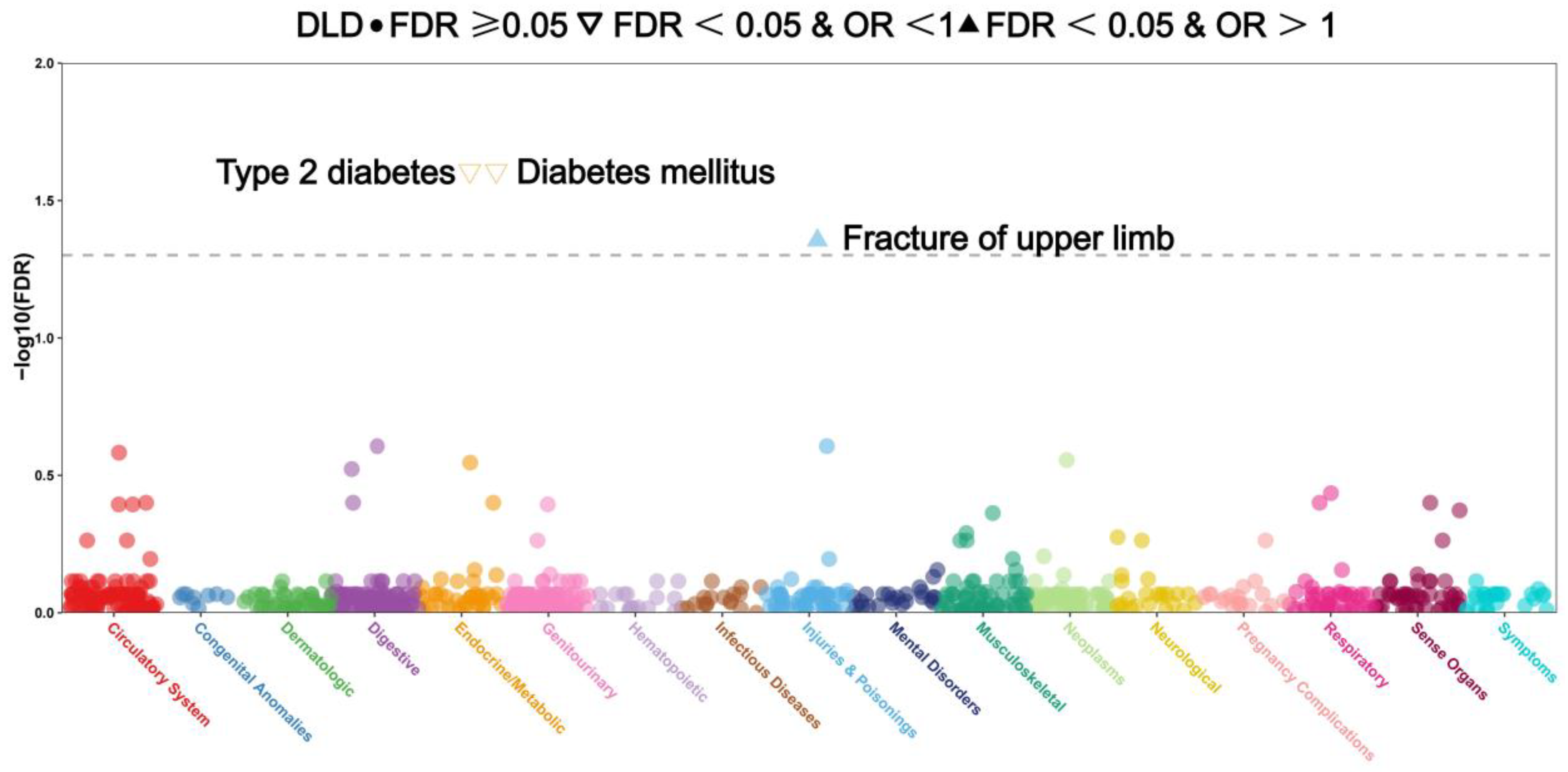
3.8. Analysis of Phenome-Wide Association Studies of 18 CRGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elford, A.T.; Leong, R.W.; Halmos, E.P.; Morgan, M.; Kilpatrick, K.; Lewindon, P.J.; Gearry, R.B.; Christensen, B. IBD Barriers across the Continents: A Continent-Specific Analysis—Australasia. Ther. Adv. Gastroenterol. 2023, 16, 17562848231197509. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.M.; Zammitti, E.P.; Ward, B.W.; Wheaton, A.G.; Croft, J.B. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of Symptoms of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Weger, M.; Sandi, C. High Anxiety Trait: A Vulnerable Phenotype for Stress-Induced Depression. Neurosci. Biobehav. Rev. 2018, 87, 27–37. [Google Scholar] [CrossRef]
- Horwitz, A.V. How an Age of Anxiety Became an Age of Depression. Milbank Q. 2010, 88, 112–138. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Soto, M.; Herzog, C.; Pacheco, J.A.; Fujisaka, S.; Bullock, K.; Clish, C.B.; Kahn, C.R. Gut Microbiota Modulate Neurobehavior through Changes in Brain Insulin Sensitivity and Metabolism. Mol. Psychiatry 2018, 23, 2287–2301. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P.; et al. Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef] [PubMed]
- Lianos, G.D.; Frountzas, M.; Kyrochristou, I.D.; Sakarellos, P.; Tatsis, V.; Kyrochristou, G.D.; Bali, C.D.; Gazouli, M.; Mitsis, M.; Schizas, D. What Is the Role of the Gut Microbiota in Anastomotic Leakage After Colorectal Resection? A Scoping Review of Clinical and Experimental Studies. J. Clin. Med. 2024, 13, 6634. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Koola, J.; Dulai, P.S.; Prokop, L.J.; Sandborn, W.J.; Singh, S. Rate of Risk Factors for and Interventions to Reduce Hospital Readmission in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1939–1948.e7. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Mazzarella, C.; Dallio, M.; Romeo, M.; Pellegrino, R.; Durante, T.; Romano, M.; Loguercio, C.; Di Mauro, M.; Federico, A.; et al. The Lesson from the First Italian Lockdown: Impacts on Anxiety and Depressive Symptoms and Sleep Quality in Patients with Remission of Inflammatory Bowel Disease. Rev. Recent Clin. Trials 2022, 17, 109–119. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and Links with Cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef]
- Lutsenko, S. Human Copper Homeostasis: A Network of Interconnected Pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Lech, G. Analysis of the Nutrients and Food Products Intake of Polish Males with Ulcerative Colitis in Remission. Nutrients 2019, 11, 2333. [Google Scholar] [CrossRef]
- Ojuawo, A.; Keith, L. The Serum Concentrations of Zinc, Copper and Selenium in Children with Inflammatory Bowel Disease. Cent. Afr. J. Med. 2002, 48, 116–119. [Google Scholar]
- Poursadegh, F.; Ahadi, M.; Vosoughinia, H.; Salehi, M.; Beheshti Namdar, A.; Farzanehfar, M.R.; Memar, B.; Ziaolhagh, R. A STROBE Compliant Observational Study on Trace Elements in Patients with Ulcerative Colitis and Their Relationship with Disease Activity. Medicine 2018, 97, e13523. [Google Scholar] [CrossRef]
- Lucena-Valera, A.; Ruz-Zafra, P.; Ampuero, J. Wilson’s Disease: Overview. Med. Clin. 2023, 160, 261–267. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s Disease and Other Neurological Copper Disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Günther, P.; Hermann, W. Mild cognitive impairment and depressive symptoms in patients with Wilson’s disease under long-term therapy. Fortschr. Neurol. Psychiatr. 2012, 80, 149–153. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhong, S.; Liao, X.; Lai, S.; Shan, Y.; Chen, J.; Zhang, L.; Lu, Q.; Shen, S.; et al. Alleviation of Cognitive Deficits and High Copper Levels by an NMDA Receptor Antagonist in a Rat Depression Model. Compr. Psychiatry 2020, 102, 152200. [Google Scholar] [CrossRef]
- Słupski, J.; Cubała, W.J.; Górska, N.; Gałuszko-Węgielnik, M.; Wiglusz, M.S. Role of Copper in Depression. Relationship with Ketamine Treatment. Med. Hypotheses 2018, 119, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Li, S.; Qiao, Z.; Ma, X.; Yang, M.; Zhang, J.; Cao, L.; Yao, S.; Yang, Z.; et al. Identification of Cuproptosis Hub Genes Contributing to the Immune Microenvironment in Ulcerative Colitis Using Bioinformatic Analysis and Experimental Verification. Front. Immunol. 2023, 14, 1113385. [Google Scholar] [CrossRef]
- Noble, C.L.; Abbas, A.R.; Lees, C.W.; Cornelius, J.; Toy, K.; Modrusan, Z.; Clark, H.F.; Arnott, I.D.; Penman, I.D.; Satsangi, J.; et al. Characterization of Intestinal Gene Expression Profiles in Crohn’s Disease by Genome-Wide Microarray Analysis. Inflamm. Bowel Dis. 2010, 16, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, B.; Zhu, W.; Xu, X.; Gong, Q.Q.; Morando, C.; Dassopoulos, T.; Newberry, R.D.; Hunt, S.R.; Li, E. An Ileal Crohn’s Disease Gene Signature Based on Whole Human Genome Expression Profiles of Disease Unaffected Ileal Mucosal Biopsies. PLoS ONE 2012, 7, e37139. [Google Scholar] [CrossRef]
- Keir, M.E.; Fuh, F.; Ichikawa, R.; Acres, M.; Hackney, J.A.; Hulme, G.; Carey, C.D.; Palmer, J.; Jones, C.J.; Long, A.K.; et al. Regulation and Role of αE Integrin and Gut Homing Integrins in Migration and Retention of Intestinal Lymphocytes during Inflammatory Bowel Disease. J. Immunol. Baltim. Md 1950 2021, 207, 2245–2254. [Google Scholar] [CrossRef]
- Bjerrum, J.T.; Hansen, M.; Olsen, J.; Nielsen, O.H. Genome-Wide Gene Expression Analysis of Mucosal Colonic Biopsies and Isolated Colonocytes Suggests a Continuous Inflammatory State in the Lamina Propria of Patients with Quiescent Ulcerative Colitis. Inflamm. Bowel Dis. 2010, 16, 999–1007. [Google Scholar] [CrossRef]
- Leday, G.G.R.; Vértes, P.E.; Richardson, S.; Greene, J.R.; Regan, T.; Khan, S.; Henderson, R.; Freeman, T.C.; Pariante, C.M.; Harrison, N.A.; et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol. Psychiatry 2018, 83, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Spijker, S.; Van Zanten, J.S.; De Jong, S.; Penninx, B.W.J.H.; van Dyck, R.; Zitman, F.G.; Smit, J.H.; Ylstra, B.; Smit, A.B.; Hoogendijk, W.J.G. Stimulated Gene Expression Profiles as a Blood Marker of Major Depressive Disorder. Biol. Psychiatry 2010, 68, 179–186. [Google Scholar] [CrossRef]
- Chang, L.-C.; Jamain, S.; Lin, C.-W.; Rujescu, D.; Tseng, G.C.; Sibille, E. A Conserved BDNF, Glutamate- and GABA-Enriched Gene Module Related to Human Depression Identified by Coexpression Meta-Analysis and DNA Variant Genome-Wide Association Studies. PLoS ONE 2014, 9, e90980. [Google Scholar] [CrossRef]
- Liu, X.; Luo, B.; Wu, X.; Tang, Z. Cuproptosis and Cuproptosis-Related Genes: Emerging Potential Therapeutic Targets in Breast Cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 189013. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ge, J.; Chu, Y.; Cai, S.; Gong, A.; Wu, J.; Zhang, J. Identification of Hub Cuproptosis Related Genes and Immune Cell Infiltration Characteristics in Periodontitis. Front. Immunol. 2023, 14, 1164667. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinforma. Oxf. Engl. 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A Class Discovery Tool with Confidence Assessments and Item Tracking. Bioinforma. Oxf. Engl. 2010, 26, 1572–1573. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene Set Variation Analysis for Microarray and RNA-Seq Data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Valkenborg, D.; Rousseau, A.-J.; Geubbelmans, M.; Burzykowski, T. Support Vector Machines. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 754–757. [Google Scholar] [CrossRef]
- Hu, J.; Szymczak, S. A Review on Longitudinal Data Analysis with Random Forest. Brief. Bioinform. 2023, 24, bbad002. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lee, K.-Y. Generalized Functional Linear Model with a Point Process Predictor. Stat. Med. 2024, 43, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Subha Ramakrishnan, M.; Ganapathy, N. Extreme Gradient Boosting Based Improved Classification of Blood-Brain-Barrier Drugs. Stud. Health Technol. Inform. 2022, 294, 872–873. [Google Scholar] [CrossRef]
- Maeser, D.; Gruener, R.F.; Huang, R.S. oncoPredict: An R Package for Predicting in Vivo or Cancer Patient Drug Response and Biomarkers from Cell Line Screening Data. Brief. Bioinform. 2021, 22, bbab260. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.P.; Maurya, S.K.; Singh, A.; Prajapati, K.S.; Singh, A.K.; Shuaib, M.; Kumar, S. Bulbine Frutescens Phytochemicals as Novel ABC-Transporter Inhibitor: A Molecular Docking and Molecular Dynamics Simulation Study. J. Cancer Metastasis Treat. 2021, 7, 2. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, A.K.; Kushwaha, P.P.; Prajapati, K.S.; Shuaib, M.; Senapati, S.; Kumar, S. Identification of Potential Natural Inhibitors of SARS-CoV2 Main Protease by Molecular Docking and Simulation Studies. J. Biomol. Struct. Dyn. 2021, 39, 4334–4345. [Google Scholar] [CrossRef]
- In-Silico Methods of Drug Design: Molecular Simulations and Free Energy Calculations|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-981-13-6920-9_28 (accessed on 31 January 2025).
- In Silico Updates on Lead Identification for Obesity and Cancer|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-981-16-1846-8_13 (accessed on 31 January 2025).
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Showalter, S.A.; Brüschweiler, R. Validation of Molecular Dynamics Simulations of Biomolecules Using NMR Spin Relaxation as Benchmarks: Application to the AMBER99SB Force Field. J. Chem. Theory Comput. 2007, 3, 961–975. [Google Scholar] [CrossRef]
- Zhou, W.; Nielsen, J.B.; Fritsche, L.G.; Dey, R.; Gabrielsen, M.E.; Wolford, B.N.; LeFaive, J.; VandeHaar, P.; Gagliano, S.A.; Gifford, A.; et al. Efficiently Controlling for Case-Control Imbalance and Sample Relatedness in Large-Scale Genetic Association Studies. Nat. Genet. 2018, 50, 1335–1341. [Google Scholar] [CrossRef]
- Zheng, J.; Haberland, V.; Baird, D.; Walker, V.; Haycock, P.C.; Hurle, M.R.; Gutteridge, A.; Erola, P.; Liu, Y.; Luo, S.; et al. Phenome-Wide Mendelian Randomization Mapping the Influence of the Plasma Proteome on Complex Diseases. Nat. Genet. 2020, 52, 1122–1131. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Z.; Noordam, R.; van Heemst, D.; Li-Gao, R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-Sample Mendelian Randomization Study. J. Crohns Colitis 2022, 16, 633–642. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper Induces Cell Death by Targeting Lipoylated TCA Cycle Proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tian, Z.; Zhang, P.; Zhen, L.; Meng, Q.; Sun, B.; Xu, X.; Jia, T.; Li, S. The Molecular Mechanisms of Cuproptosis and Its Relevance to Cardiovascular Disease. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 163, 114830. [Google Scholar] [CrossRef] [PubMed]
- Dreishpoon, M.B.; Bick, N.R.; Petrova, B.; Warui, D.M.; Cameron, A.; Booker, S.J.; Kanarek, N.; Golub, T.R.; Tsvetkov, P. FDX1 Regulates Cellular Protein Lipoylation through Direct Binding to LIAS. J. Biol. Chem. 2023, 299, 105046. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Słupski, J.; Cubała, W.J.; Górska, N.; Słupska, A.; Gałuszko-Węgielnik, M. Copper and Anti-Anhedonic Effect of Ketamine in Treatment-Resistant Depression. Med. Hypotheses 2020, 144, 110268. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.N.; Ingram, R.J.M.; Zhang, Z.; Taylor, L.M.; Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C.; Ghosh, S.; Raman, M. The Role of Precision Nutrition in the Modulation of Microbial Composition and Function in People with Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2021, 6, 754–769. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. Edinb. Scotl. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Wang, F.; Zhang, B.; Tang, X. Comprehensive Analysis of Cuproptosis-Related Genes in Immune Infiltration and Diagnosis in Ulcerative Colitis. Front. Immunol. 2022, 13, 1008146. [Google Scholar] [CrossRef]
- Tassi, A.; Mavromatis, I.; Piechocki, R.J.R. A Dataset of Full-Stack ITS-G5 DSRC Communications over Licensed and Unlicensed Bands Using a Large-Scale Urban Testbed. Data Brief 2019, 25, 104368. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Cenni, S.; Sesenna, V.; Boiardi, G.; Casertano, M.; Di Nardo, G.; Esposito, S.; Strisciuglio, C. The Mediterranean Diet in Paediatric Gastrointestinal Disorders. Nutrients 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and Challenges in Integrating Nutritional Care into Oncology Practice: Results from a National Survey on Behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Brizzi, A.; Landi, M.; Incrocci, L.; Pardossi, A.; Guidi, L. Evaluation of Major Minerals and Trace Elements in Wild and Domesticated Edible Herbs Traditionally Used in the Mediterranean Area. Biol. Trace Elem. Res. 2021, 199, 3553–3561. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper Homeostasis and Copper-Induced Cell Death in the Pathogenesis of Cardiovascular Disease and Therapeutic Strategies. Cell Death Dis. 2023, 14, 105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Wu, Q.; Sang, M.; Mao, L. Common Regulatory Mechanisms Mediated by Cuproptosis Genes in Inflammatory Bowel Disease and Major Depressive Disorder. Genes 2025, 16, 339. https://doi.org/10.3390/genes16030339
Shi J, Wu Q, Sang M, Mao L. Common Regulatory Mechanisms Mediated by Cuproptosis Genes in Inflammatory Bowel Disease and Major Depressive Disorder. Genes. 2025; 16(3):339. https://doi.org/10.3390/genes16030339
Chicago/Turabian StyleShi, Jiyuan, Qianyi Wu, Mengmeng Sang, and Liming Mao. 2025. "Common Regulatory Mechanisms Mediated by Cuproptosis Genes in Inflammatory Bowel Disease and Major Depressive Disorder" Genes 16, no. 3: 339. https://doi.org/10.3390/genes16030339
APA StyleShi, J., Wu, Q., Sang, M., & Mao, L. (2025). Common Regulatory Mechanisms Mediated by Cuproptosis Genes in Inflammatory Bowel Disease and Major Depressive Disorder. Genes, 16(3), 339. https://doi.org/10.3390/genes16030339






