Current Insights on Salivary Gland Adenoid Cystic Carcinoma: Related Genes and Molecular Pathways
Abstract
:1. Introduction
2. Materials and Methods
Inclusion and Exclusion Criteria
- Language: Articles written in English.
- Time frame: Studies published between 2015–2025
- Type of study: Original research articles
- Language: Articles written in a language other than English
- Time frame: Articles written before 2015
- Type of study: Non-original research articles
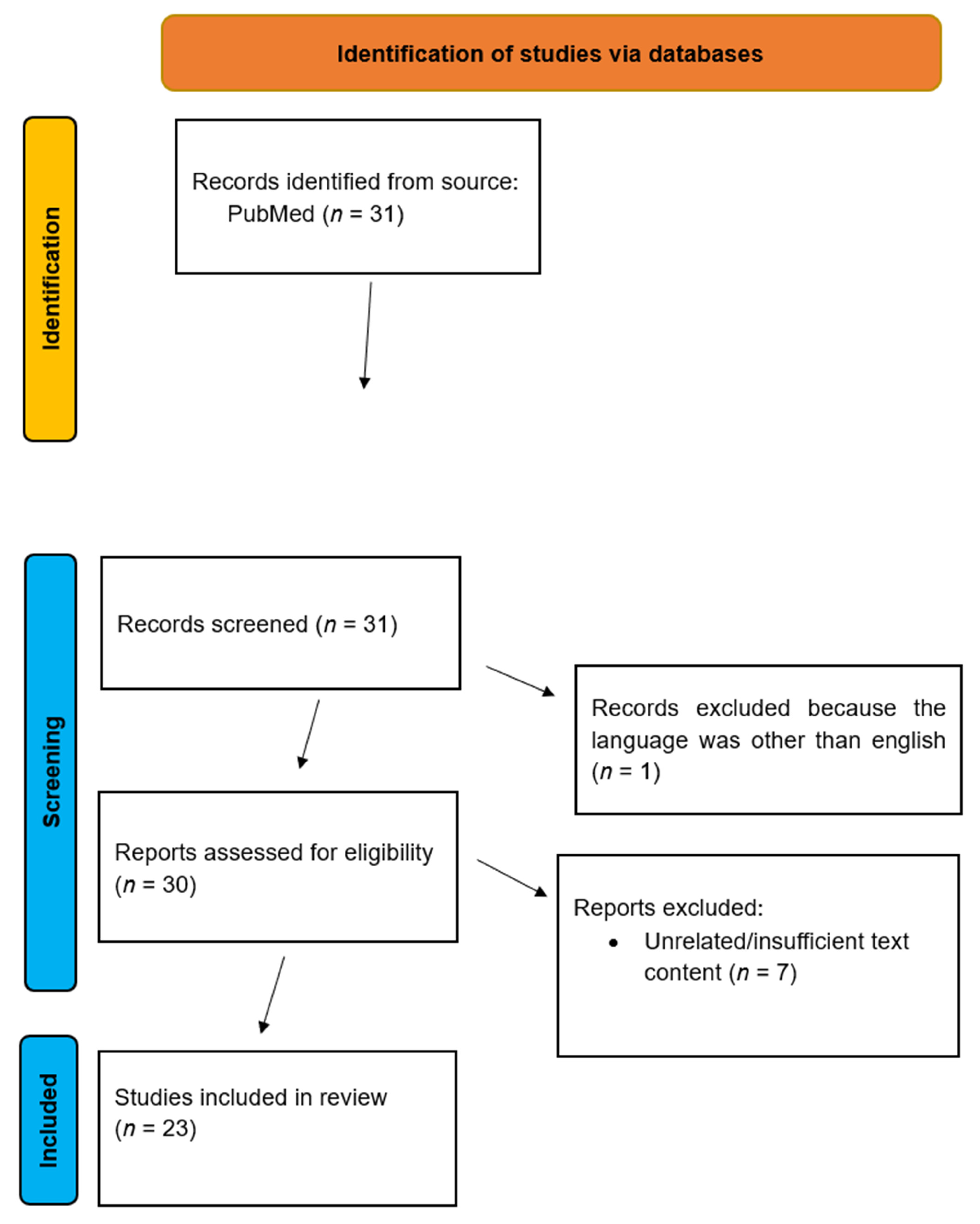
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Acronym |
| MYB | v-myb avian myelobastosis viral oncogene homolog |
| NFIB | nuclear factor I/B |
| MYC | myelocytoma |
| NOTCH | neurogenic locus notch homolog protein |
| AXL | anexelekto |
| MET | mesenchymal epithelial transition factor receptor |
| EGFR | epidermal growth factor receptor |
| ATRA | all-trans retinoic acid |
| DEG | differential gene expression |
| RPPA | reverse-phase protein array |
| scRNA-seq | single-cell RNA sequencing |
| SPEN | solid pseudopapillary epithelial neoplasms |
| IHC | immunohistochemistry |
| PCR | polymerase chain reaction |
| HEY1 | hes related family bHLH transcription factor with YRPW motif 1 |
| EMT | epithelial-to-mesenchymal transition |
| MMP | matrix metalloproteinase |
| HES | hairy and enhancer of split |
| ceRNA | competing endogenous RNA |
| NGS | next-generation sequencing |
| PIK3CA | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| ERBB2 | erythroblastic leukemia viral oncogene homologue 2 |
| HRAS | harvey rat sarcoma virus |
| TNF | tumor necrosis factor |
| ROS | proto-oncogene tyrosine-protein kinase |
| RAS | rat sarcoma |
| SOX | SRY-box transcription factor |
| AR | androgen receptor |
| SMAD | suppressor of mothers against decapentaplegic |
| MAPK | mitogen-activated protein kinase |
| RAD | radiation-sensitive protein |
| FGFR | fibroblast growth factor receptor |
| AKT | AKT serine/threonine kinase |
| FGF | fibroblast growth factor |
| PTEN | phosphatase and tensin homolog |
| TGF | transforming growth factor |
| mTOR | mechanistic target of rapamycin kinase |
| RAR | retinoic acid receptor |
| RXR | retinoid X receptor |
| ITGB | integrin subunit beta |
| FBN | fibrillin |
| LTBP | latent transforming growth factor beta binding protein |
| miRNA | micro ribonucleic acid |
| Wnt | wingless-type MMTV integration site |
| rt-PCR | reverse transcription polymerase chain reaction |
| PP2A | protein phosphatase 2 scaffold subunit Alpha |
| CIP2A | cellular inhibitor of protein phosphatase 2A (PP2A) |
| SET | SET nuclear proto-oncogene |
| EN1 | engrailed homeobox 1 |
| CTGF–new name CCN2 | cellular communication network factor |
| SH-SY5Y | thrice-subcloned cell line derived from the SK-N-SH neuroblastoma cell line |
| IGF | insulin-like growth factor |
| SLC22A3 | solute carrier family 22 member 3 |
| FOXP2 | forkhead box P2 |
| Cdc42EP3 | CDC42 effector protein 3 |
| COL27A1 | collagen type XXVII alpha 1 chain |
| DUSP1 | dual specificity phosphatase 1 |
| HSPB8 | heat shock protein family B (small) member 8 |
| Ki-67 | marker of proliferation |
| hsa_circRNA | circular ribonucleic acid |
| FABP | fatty acid binding protein |
| VCAN | versican |
| KRAS | Kirsten rat sarcoma |
| BRAF | v-raf murine sarcoma viral oncogene homolog B |
References
- Tasoulas, J.; Divaris, K.; Theocharis, S.; Farquhar, D.; Shen, C.; Hackman, T.; Amelio, A.L. Impact of Tumor Site and Adjuvant Radiotherapy on Survival of Patients with Adenoid Cystic Carcinoma: A SEER Database Analysis. Cancers 2021, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R. Histologic grading and prognostic biomarkers in salivary gland carcinomas. Adv. Anat. Pathol. 2011, 18, 29–45. [Google Scholar] [PubMed]
- Seethala, R.R. An update on grading of salivary gland carcinomas. Head. Neck Pathol. 2009, 3, 69–77. [Google Scholar] [PubMed]
- Jaber, M.A.; Hassan, M.; Ingafou, M.; Elameen, A.M. Adenoid Cystic Carcinoma of the Minor Salivary Glands: A Systematic Review and Meta-Analysis of Clinical Characteristics and Management Strategies. J. Clin. Med. 2024, 13, 267. [Google Scholar] [CrossRef]
- Belulescu, I.C.; Margaritescu, C.; Dumitrescu, C.I.; Dăguci, L.; Munteanu, C.; Margaritescu, O.C. Adenoid Cystic Carcinoma of Salivary Gland: A Ten-Year Single Institute Experience. Curr. Health Sci. J. 2020, 46, 56–65. [Google Scholar]
- Moskaluk, C.A. Adenoid Cystic Carcinoma: Clinical and Molecular Features. Head. Neck Pathol. 2013, 7, 17–22. [Google Scholar]
- Balamucki, C.J.; Amdur, R.J.; Werning, J.W.; Vaysberg, M.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. Adenoid cystic carcinoma of the head and neck. Am. J. Otolaryngol. 2012, 33, 510–518. [Google Scholar]
- Khurram, S.A.; Barrett, A.W.; Speight, P.M. Diagnostic difficulties in lesions of the minor salivary glands. Diagn. Histopathol. 2017, 23, 250–259. [Google Scholar]
- Jo, V.Y.; Krane, J.F. Ancillary testing in salivary gland cytology: A practical guide. Cancer Cytopathol. 2018, 126, 627–642. [Google Scholar] [CrossRef]
- Dewenter, I.; Otto, S.; Kakoschke, T.K.; Smolka, W.; Obermeier, K.T. Recent Advances, Systemic Therapy, and Molecular Targets in Adenoid Cystic Carcinoma of the Head and Neck. J. Clin. Med. 2023, 12, 1463. [Google Scholar] [CrossRef]
- An, N.; Li, Y. Survival benefit added by adjuvant chemotherapy in adenoid cystic carcinoma of salivary gland. Sci. Rep. 2024, 14, 25746. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Russo, C.A.; Junn, J.C.; Yom, S.S.; Bakst, R.L. Radiation Therapy for Adenoid Cystic Carcinoma of the Head and Neck. Cancers 2021, 13, 6335. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Au, V.; Mokhtari, T.E.; Goss, D.; Faden, D.L.; Varvares, M.A. A Contemporary Review of Molecular Therapeutic Targets for Adenoid Cystic Carcinoma. Cancers 2022, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Mitani, Y.; McGrail, D.J.; Li, K.; Karpinets, T.V.; Bell, D.; Frank, S.J.; Song, X.; Kupferman, M.E.; Liu, B.; et al. Proteogenomic Analysis of Salivary Adenoid Cystic Carcinomas Defines Molecular Subtypes and Identifies Therapeutic Targets. Clin. Cancer Res. 2020, 27, 852–864. [Google Scholar]
- Zhou, M.-J.; Yang, J.-J.; Ma, T.-Y.; Feng, G.-X.; Wang, X.-L.; Wang, L.-Y.; Ge, Y.-Z.; Gao, R.; Liu, H.-L.; Shan, L.; et al. Increased retinoic acid signaling decreases lung metastasis in salivary adenoid cystic carcinoma by inhibiting the noncanonical Notch1 pathway. Exp. Mol. Med. 2023, 55, 597–611. [Google Scholar] [CrossRef]
- Powell, S.; Kulakova, K.; Hanratty, K.; Khan, R.; Casserly, P.; Crown, J.; Walsh, N.; Kennedy, S. Molecular Analysis of Salivary and Lacrimal Adenoid Cystic Carcinoma. Cancers 2024, 16, 2868. [Google Scholar] [CrossRef]
- Liu, H.-B.; Huang, G.-J.; Luo, M.-S. Transcriptome analyses identify hub genes and potential mechanisms in adenoid cystic carcinoma. Medicine 2020, 99, e18676. [Google Scholar]
- Liu, B.; Mitani, Y.; Rao, X.; Zafereo, M.; Zhang, J.; Zhang, J.; Futreal, P.A.; Lozano, G.; El-Naggar, A.K. Spatio-Temporal Genomic Heterogeneity, Phylogeny, and Metastatic Evolution in Salivary Adenoid Cystic Carcinoma. JNCI J. Natl. Cancer Inst. 2017, 109, djx033. [Google Scholar]
- Xie, J.; Lin, L.-S.; Huang, X.-Y.; Gan, R.-H.; Ding, L.-C.; Su, B.-H.; Zhao, Y.; Lu, Y.-G.; Zheng, D.-L. The NOTCH1-HEY1 pathway regulates self-renewal and epithelial-mesenchymal transition of salivary adenoid cystic carcinoma cells. Int. J. Biol. Sci. 2020, 16, 598–610. [Google Scholar] [CrossRef]
- Huang, J.; Fehr, A.; Jäwert, F.; Nilsson, J.A.; Morris, L.G.; Stenman, G.; Andersson, M.K. MYB alternative promoter activity is increased in adenoid cystic carcinoma metastases and is associated with a specific gene expression signature. Oral. Oncol. 2024, 151, 106763. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Gan, R.-H.; Xie, J.; She, L.; Zhao, Y.; Ding, L.-C.; Su, B.-H.; Zheng, D.-L.; Lu, Y.-G. The oncogenic effects of HES1 on salivary adenoid cystic carcinoma cell growth and metastasis. BMC Cancer 2018, 18, 436. [Google Scholar] [CrossRef]
- Tang, Y.F.; Wu, W.J.; Zhang, J.Y.; Zhang, J. Reconstruction and analysis of the aberrant lncRNA-miRNA-mRNA network based on competitive endogenous RNA in adenoid cystic carcinoma of the salivary gland. Transl. Cancer Res. 2021, 10, 5133–5149. [Google Scholar]
- Lassche, G.; van Helvert, S.; Eijkelenboom, A.; Tjan, M.J.H.; Jansen, E.A.M.; van Cleef, P.H.J.; Verhaegh, G.W.; Kamping, E.J.; Grünberg, K.; Grunsven, A.C.H.v.E.-V.; et al. Identification of Fusion Genes and Targets for Genetically Matched Therapies in a Large Cohort of Salivary Gland Cancer Patients. Cancers 2022, 14, 4156. [Google Scholar] [CrossRef]
- Han, N.; Lu, H.; Zhang, Z.; Ruan, M.; Yang, W.; Zhang, C. Comprehensive and in-depth analysis of microRNA and mRNA expression profile in salivary adenoid cystic carcinoma. Gene 2018, 678, 349–360. [Google Scholar]
- Geng, S.; Chen, L.; Lin, W.; Wan, F.; Le, Z.; Hu, W.; Chen, H.; Liu, X.; Huang, Q.; Zhang, H.; et al. Exploring the Therapeutic Potential of Triptonide in Salivary Adenoid Cystic Carcinoma: A Comprehensive Approach Involving Network Pharmacology and Experimental Validation. Curr. Pharm. Des. 2024, 30, 2276–2289. [Google Scholar] [CrossRef]
- Saida, K.; Murase, T.; Ito, M.; Fujii, K.; Takino, H.; Masaki, A.; Kawakita, D.; Ijichi, K.; Tada, Y.; Kusafuka, K.; et al. Mutation analysis of the EGFR pathway genes, EGFR, RAS, PIK3CA, BRAF, and AKT1, in salivary gland adenoid cystic carcinoma. Oncotarget 2018, 9, 17043–17055. [Google Scholar]
- Liu, Z.; Gao, J.; Yang, Y.; Zhao, H.; Ma, C.; Yu, T. Potential targets identified in adenoid cystic carcinoma point out new directions for further research. Am. J. Transl. Res. 2021, 13, 1085. [Google Scholar]
- Brayer, K.J.; Frerich, C.A.; Kang, H.; Ness, S.A. Recurrent Fusions in MYB and MYBL1 Define a Common, Transcription Factor-Driven Oncogenic Pathway in Salivary Gland Adenoid Cystic Carcinoma. Cancer Discov. 2016, 6, 176–187. [Google Scholar]
- Humtsoe, J.O.; Kim, H.-S.; Leonard, B.; Ling, S.; Keam, B.; Marchionni, L.; Afsari, B.; Considine, M.; Favorov, A.V.; Fertig, E.J.; et al. Newly Identified Members of FGFR1 Splice Variants Engage in Cross-talk with AXL/AKT Axis in Salivary Adenoid Cystic Carcinoma. Cancer Res. 2021, 81, 1001–1013. [Google Scholar]
- Cao, Y.; Liu, H.; Gao, L.; Lu, L.; Du, L.; Bai, H.; Li, J.; Said, S.; Wang, X.-J.; Song, J.; et al. Cooperation Between Pten and Smad4 in Murine Salivary Gland Tumor Formation and Progression. Neoplasia 2018, 20, 764–774. [Google Scholar] [CrossRef]
- Viragova, S.; Aparicio, L.; Palmerini, P.; Zhao, J.; Salazar, L.E.V.; Schurer, A.; Dhuri, A.; Sahoo, D.; Moskaluk, C.A.; Rabadan, R.; et al. Inverse agonists of retinoic acid receptor/retinoid X receptor signaling as lineage-specific antitumor agents against human adenoid cystic carcinoma. JNCI J. Natl. Cancer Inst. 2023, 115, 838–852. [Google Scholar] [PubMed]
- Gomes, Á.N.d.M.; Oliveira, K.K.; Marchi, F.A.; Bettim, B.B.; Germano, J.N.; Filho, J.G.; Pinto, C.A.L.; Lourenço, S.V.; Coutinho-Camillo, C.M. TGFβ signaling pathway in salivary gland tumors. Arch. Oral. Biol. 2024, 162, 105943. [Google Scholar]
- Frerich, C.A.; Sedam, H.N.; Kang, H.; Mitani, Y.; El-Naggar, A.K.; Ness, S.A. N-Terminal Truncated Myb with New Transcriptional Activity Produced Through Use of an Alternative MYB Promoter in Salivary Gland Adenoid Cystic Carcinoma. Cancers 2019, 12, 45. [Google Scholar] [CrossRef]
- Meinrath, J.; Haak, A.; Igci, N.; Dalvi, P.; Arolt, C.; Meemboor, S.; Siebolts, U.; Eischeidt-Scholz, H.; Wickenhauser, C.; Grünewald, I.; et al. Expression profiling on subclasses of primary parotid gland carcinomas. Oncotarget 2020, 11, 4123–4137. [Google Scholar] [PubMed]
- Denaro, M.; Navari, E.; Ugolini, C.; Seccia, V.; Donati, V.; Casani, A.P.; Basolo, F. A microRNA signature for the differential diagnosis of salivary gland tumors. PLoS ONE 2019, 14, e0210968. [Google Scholar]
- Routila, J.; Mäkelä, J.; Luukkaa, H.; Leivo, I.; Irjala, H.; Westermarck, J.; Mäkitie, A.; Ventelä, S. Potential role for inhibition of protein phosphatase 2A tumor suppressor in salivary gland malignancies. Genes Chromosom. Cancer 2015, 55, 69–81. [Google Scholar] [PubMed]
- Frerich, C.A.; Brayer, K.J.; Painter, B.M.; Kang, H.; Mitani, Y.; El-Naggar, A.K.; Ness, S.A. Transcriptomes define distinct subgroups of salivary gland adenoid cystic carcinoma with different driver mutations and outcomes. Oncotarget 2017, 9, 7341–7358. [Google Scholar]
- Brayer, K.J.; Kang, H.; El-Naggar, A.K.; Andreasen, S.; Homøe, P.; Kiss, K.; Mikkelsen, L.; Heegaard, S.; Pelaez, D.; Moeyersoms, A.; et al. Dominant Gene Expression Profiles Define Adenoid Cystic Carcinoma (ACC) from Different Tissues: Validation of a Gene Signature Classifier for Poor Survival in Salivary Gland ACC. Cancers 2023, 15, 1390. [Google Scholar] [CrossRef]
- Tang, Y.-F.; An, P.-G.; Gu, B.-X.; Yi, S.; Hu, X.; Wu, W.-J.; Zhang, J. Transcriptomic insights into adenoid cystic carcinoma via RNA sequencing. Front. Genet. 2023, 14, 1144945. [Google Scholar]
- Sisto, M.; Ribatti, D.; Lisi, S. SMADS-Mediate Molecular Mechanisms in Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 3203. [Google Scholar] [CrossRef]
- Woods, L.T.; Camden, J.M.; El-Sayed, F.G.; Khalafalla, M.G.; Petris, M.J.; Erb, L.; Weisman, G.A. Increased Expression of TGF-β Signaling Components in a Mouse Model of Fibrosis Induced by Submandibular Gland Duct Ligation. PLoS ONE 2015, 10, e0123641. [Google Scholar]
- Freudlsperger, C.; Bian, Y.; Wise, S.C.; Burnett, J.; Coupar, J.; Yang, X.; Chen, Z.; Van Waes, C. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 2013, 32, 1549–1559. [Google Scholar] [PubMed]
- Cucu, I.; Nicolescu, M.I. A Synopsis of Signaling Crosstalk of Pericytes and Endothelial Cells in Salivary Gland. Dent. J. 2021, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.G.; Ribbat, J.; Berndt, A.; Richter, P.; Kosmehl, H.; Benedek, G.A.; Jacobsen, H.C.; Trenkle, T.; Sieg, P.; Rades, D. Expression of Wnt-1, TGF-β and related cell—cell adhesion components following radiotherapy in salivary glands of patients with manifested radiogenic xerostomia. Radiother. Oncol. 2011, 101, 93–99. [Google Scholar]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar]
- Wysocki, P.T.; Izumchenko, E.; Meir, J.; Ha, P.K.; Sidransky, D.; Brait, M. Adenoid cystic carcinoma: Emerging role of translocations and gene fusions. Oncotarget 2016, 7, 66239–66254. [Google Scholar]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.T.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar]
- Ferrarotto, R.; Mishra, V.; Herz, E.; Yaacov, A.; Solomon, O.; Rauch, R.; Mondshine, A.; Motin, M.; Leibovich-Rivkin, T.; Davis, M.; et al. AL101, a gamma-secretase inhibitor, has potent antitumor activity against adenoid cystic carcinoma with activated NOTCH signaling. Cell Death Dis. 2022, 13, 678. [Google Scholar]
- Naik, P.P.; Das, D.N.; Panda, P.K.; Mukhopadhyay, S.; Sinha, N.; Praharaj, P.P.; Agarwal, R.; Bhutia, S.K. Implications of cancer stem cells in developing therapeutic resistance in oral cancer. Oral. Oncol. 2016, 62, 122–135. [Google Scholar]
- Leivo, I. Insights into a complex group of neoplastic disease: Advances in histopathologic classification and molecular pathology of salivary gland cancer. Acta Oncol. 2006, 45, 662–668. [Google Scholar]
- Jia, Y.; Liu, Y.; Yang, H.; Yao, F. Adenoid cystic carcinoma: Insights from molecular characterization and therapeutic advances. Medcomm 2024, 5, e734. [Google Scholar] [PubMed]
- Hernole, J.; Narayanappa, R. Interactions between Notch and matrix metalloproteinases: The role in cancer. Nowotw. J. Oncol. 2023, 73, 354–361. [Google Scholar]
- Li, X.; Liu, W.; Geng, C.; Li, T.; Li, Y.; Guo, Y.; Wang, C. Ginsenoside Rg3 Suppresses Epithelial-Mesenchymal Transition via Downregulating Notch-Hes1 Signaling in Colon Cancer Cells. Am. J. Chin. Med. 2020, 49, 217–235. [Google Scholar] [PubMed]
- Tomecka, P.; Kunachowicz, D.; Górczyńska, J.; Gebuza, M.; Kuźnicki, J.; Skinderowicz, K.; Choromańska, A. Factors Determining Epithelial-Mesenchymal Transition in Cancer Progression. Int. J. Mol. Sci. 2024, 25, 8972. [Google Scholar] [CrossRef]
- Liu, S.; Ye, D.; Xu, D.; Liao, Y.; Zhang, L.; Liu, L.; Yu, W.; Wang, Y.; He, Y.; Hu, J.; et al. Autocrine epiregulin activates EGFR pathway for lung metastasis via EMT in salivary adenoid cystic carcinoma. Oncotarget 2016, 7, 25251–25263. [Google Scholar]
- da Silva, F.J.; Carvalho de Azevedo, J.; Ralph, A.C.L.; Pinheiro, J.d.J.V.; Freitas, V.M.; Calcagno, D.Q. Salivary glands adenoid cystic carcinoma: A molecular profile update and potential implications. Front. Oncol. 2023, 13, 1191218. [Google Scholar]
- Romani, C.; Lorini, L.; Bozzola, A.; Bignotti, E.; Tomasoni, M.; Ardighieri, L.; Bugatti, M.; Battocchio, S.; Ravaggi, A.; Tomasini, D.; et al. Functional profiles of curatively treated adenoid cystic carcinoma unveil prognostic features and potentially targetable pathways. Sci. Rep. 2023, 13, 1809. [Google Scholar]
- Grünewald, I.; Vollbrecht, C.; Meinrath, J.; Meyer, M.F.; Heukamp, L.C.; Drebber, U.; Quaas, A.; Beutner, D.; Hüttenbrink, K.-B.; Wardelmann, E.; et al. Targeted next generation sequencing of parotid gland cancer uncovers genetic heterogeneity. Oncotarget 2015, 6, 18224–18237. [Google Scholar]
- Chen, G.; Wang, J.; Liu, Z.; Kornmann, M. Exon III splicing of fibroblast growth factor receptor 1 is modulated by growth factors and cyclin D1. Pancreas 2008, 37, 159–164. [Google Scholar]
- Druillennec, S.; Dorard, C.; Eychène, A. Alternative Splicing in Oncogenic Kinases: From Physiological Functions to Cancer. J. Nucleic Acids 2011, 2012, 639062. [Google Scholar]
- Gong, S. Isoforms of Receptors of Fibroblast Growth Factors. J. Cell. Physiol. 2014, 229, 1887–1895. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, L.K.; Duan, K.; Ellies, L.G.; Seaman, W.T.; Burger-Calderon, R.A.; Diatchenko, L.B.; Webster-Cyriaque, J. Correlation of transcription of MALAT-1, a novel noncoding RNA, with deregulated expression of tumor suppressor p53 in small DNA tumor virus models. J. Cancer Ther. 2013, 04, 774–786. [Google Scholar] [CrossRef]
- Cheng, J.; DeCaprio, J.A.; Fluck, M.M.; Schaffhausen, B.S. Cellular transformation by Simian Virus 40 and Murine Polyoma Virus T antigens. Semin. Cancer Biol. 2009, 19, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Ishii, K.; Sugiyama, G.; Kamata, Y.; Suzuki, A.; Kumamaru, W.; Ohyama, Y.; Nakano, H.; Kiyoshima, T.; Sumida, T.; et al. Regulation of β-Catenin Phosphorylation by PR55β in Adenoid Cystic Carcinoma. Cancer Genom. Proteom. 2018, 15, 53–60. [Google Scholar]
- Jin, Y.; Mertens, F.; Kullendorff, C.-M.; Panagopoulos, L. Fusion of the Tumor-Suppressor Gene CHEK2 and the Gene for the Regulatory Subunit B of Protein Phosphatase 2 PPP2R2A in Childhood Teratoma. Neoplasia 2006, 8, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Sun, J.-J.; Xi, S.-Y.; Jiang, Z.-M.; Xie, D.-R.; Yang, Q.; Zhang, X.-C. Malignant Salivary Gland Neoplasm of the Tongue Base with EWSR1::BEND2 Fusion: An Unusual Case with Literature Review. Head. Neck Pathol. 2024, 18, 118. [Google Scholar] [CrossRef]
- Skálová, A.; Banečkova, M.; Thompson, L.D.; Ptáková, N.M.; Stevens, T.M.; Brcic, L.; Hyrcza, M.; Simpson, R.H.; Santana, T.D.; Michal, M.J.; et al. Expanding the Molecular Spectrum of Secretory Carcinoma of Salivary Glands with a Novel VIM-RET Fusion. Am. J. Surg. Pathol. 2020, 44, 1295–1307. [Google Scholar] [CrossRef]
- Persson, F.; Winnes, M.; Andrén, Y.; Wedell, B.; Dahlenfors, R.; Asp, J.; Mark, J.; Enlund, F.; Stenman, G. High-resolution array CGH analysis of salivary gland tumors reveals fusion and amplification of the FGFR1 and PLAG1 genes in ring chromosomes. Oncogene 2007, 27, 3072–3080. [Google Scholar] [CrossRef]
- Ju, R.; Huang, Y.; Guo, Z.; Han, L.; Ji, S.; Zhao, L.; Long, J. The circular RNAs differential expression profiles in the metastasis of salivary adenoid cystic carcinoma cells. Mol. Cell. Biochem. 2020, 476, 1269–1282. [Google Scholar] [CrossRef]
- Phuchareon, J.; Overdevest, J.B.; McCormick, F.; Eisele, D.W.; van Zante, A.; Tetsu, O. Fatty Acid Binding Protein 7 Is a Molecular Marker in Adenoid Cystic Carcinoma of the Salivary Glands: Implications for Clinical Significance. Transl. Oncol. 2014, 7, 780–787. [Google Scholar] [PubMed]
- Keerthikumar, S.; Gangoda, L.; Liem, M.; Fonseka, P.; Atukorala, I.; Ozcitti, C.; Mechler, A.; Adda, C.G.; Ang, C.-S.; Mathivanan, S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015, 6, 15375–15396. [Google Scholar] [PubMed]
- Niles, R.M. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat. Res. Mol. Mech. Mutagen. 2004, 555, 97–105. [Google Scholar]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
| 41 articles through keywords | |
| Time frame 2015–2025 | |
| 31 articles remained | 10 articles excluded |
| The rest of the inclusion criteria | |
| 23 articles remained | 8 articles excluded |
| Study | Country | Sample Size | Method | Results |
|---|---|---|---|---|
| [14] | USA | 54/38 | RNA/DNA sequencing (54)/ RPPA (38) | ACC-I (37%) and ACC-II (63%). ACC-I: worse prognosis. ACC-II: better prognosis. |
| [15] | China | 79 | scRNA-seq and exome target capture sequencing | ATRA suppresses lung metastasis due to aberrant NOTCH1 or MYB expression. |
| [16] | Ireland | 11 | In situ hybridization, DNA sequencing, and bioinformatic analysis | Three-quarters of the tumors displayed NFIB-MYB rearrangement at the 6q23.3 locus; one-third displayed NOTCH mutations. |
| [17] | China | 15 | Gene set enrichment analysis | Identified 382 DEGs: 119 upregulated and 263 downregulated genes. |
| [18] | USA | 34 | Whole-genome sequencing and variant analyses | Mutations in NOTCH/SPEN and t(6;9) associated gene fusions. |
| [19] | China | 87 | IHC, PCR, Cell assay, xenograft tumor model. | NOTCH1–HEY1 pathway upregulated in ACC and increased expression of EMT-related genes and MMPs. |
| [20] | Sweden | 26 | RNA sequencing and PCR | MYB TSS2 activity higher in ACC metastases (p = 0.0003). |
| [21] | China | 60 | RNA sequencing and IHC | HES1 linked to NOTCH. Silencing HES1 expression leads to suppression of cell metastasis and invasion. |
| [22] | China | 3 | Pathway enrichment analysis | Identified 27 upregulated and 54 downregulated mRNAs. |
| [23] | Netherlands | 46 | NGS | Aberrations in PIK3CA (n = 18, 15%), ERBB2 (n = 15, 12%), HRAS and NOTCH1. Fusion transcripts were detected in 50% of the cases. |
| [24] | China | 6 | NGS, IHC, PCR, and bioinformatics | SCUBE3, CA6, hsa-miR-885-5p play an essential role in the development of ACC. |
| [25] | China | - | Molecular assays | Triptonide activates the TNF signaling pathway. |
| [26] | Japan | 70 | Single-base extension multiplex assay | EGFR mutations in 13 cases (18.6%): RAS mutations in 10 (14.3%), EGFR in one (1.4%), and PIK3CA in 5 (7.1%). Concurrent gene mutations were found in three cases (4.3%). RAS mutations associated with shorter disease-free and overall survival; (p = 0.010) and (p = 0.024), respectively. |
| [27] | China | - | Functional annotation analysis | Identified 36 potential target miRNAs. |
| [28] | USA | 20 | RNA-sequencing | Frequency of translocations: t(6;9) > t(8;9) > t(8;14) |
| [29] | USA | - | RNA-seq | Three novel, truncated, domain-lacking FGFR1 variants cooperate with AXL and desensitize cells to FGFR1 inhibitor. |
| [30] | China, USA | 55 | Mouse model development, qRT-PCR, IHC | Deletion of both PTEN and SMAD4 may lead to ACC, mTOR activation and TGFβ1 overexpression (correlated with the aggressive solid ACC form) |
| [31] | USA | - | Single-cell RNA sequencing | Myoepithelial-to-ductal differentiation is promoted by RAR/RXR signaling. |
| [32] | Brazil | 13 | Rt-PCR | ACC cases exhibited elevated expressions of ITGB6, SMAD2, SMAD4, FBN1, LTBP1, and c-MYC. |
| [33] | USA | - | RNA-sequencing | ACC tumors utilize an alternative MYB promoter. |
| [34] | Germany | 14 | RNA-sequencing | Dysregulation of the Wnt signaling pathway and activation of the PI3K pathway were noticed in ACC. |
| [35] | Italy | 4 | DNA Intelligent Analysis | Forty-six miRNAs were differentially expressed between malignant and benign lesions. |
| [36] | Finland | 38 | IHC, rt-PCR, Genome-wide microarray | Two PP2A inhibitors were highly expressed in both dysplasia and malignancy, whereas positive p-S6 staining revealed activation of mTOR pathway. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zisis, V.; Poulopoulos, K.; Shinas, N.; Charisi, C.; Poulopoulos, A. Current Insights on Salivary Gland Adenoid Cystic Carcinoma: Related Genes and Molecular Pathways. Genes 2025, 16, 370. https://doi.org/10.3390/genes16040370
Zisis V, Poulopoulos K, Shinas N, Charisi C, Poulopoulos A. Current Insights on Salivary Gland Adenoid Cystic Carcinoma: Related Genes and Molecular Pathways. Genes. 2025; 16(4):370. https://doi.org/10.3390/genes16040370
Chicago/Turabian StyleZisis, Vasileios, Konstantinos Poulopoulos, Nikolaos Shinas, Christina Charisi, and Athanasios Poulopoulos. 2025. "Current Insights on Salivary Gland Adenoid Cystic Carcinoma: Related Genes and Molecular Pathways" Genes 16, no. 4: 370. https://doi.org/10.3390/genes16040370
APA StyleZisis, V., Poulopoulos, K., Shinas, N., Charisi, C., & Poulopoulos, A. (2025). Current Insights on Salivary Gland Adenoid Cystic Carcinoma: Related Genes and Molecular Pathways. Genes, 16(4), 370. https://doi.org/10.3390/genes16040370






