IL-6 Affects Liver Metabolic Abnormalities Caused by Silicon Exposure by Regulating the PKC/YY1 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Clinical Data and Blood Samples
2.2. Experimental Animals and Materials
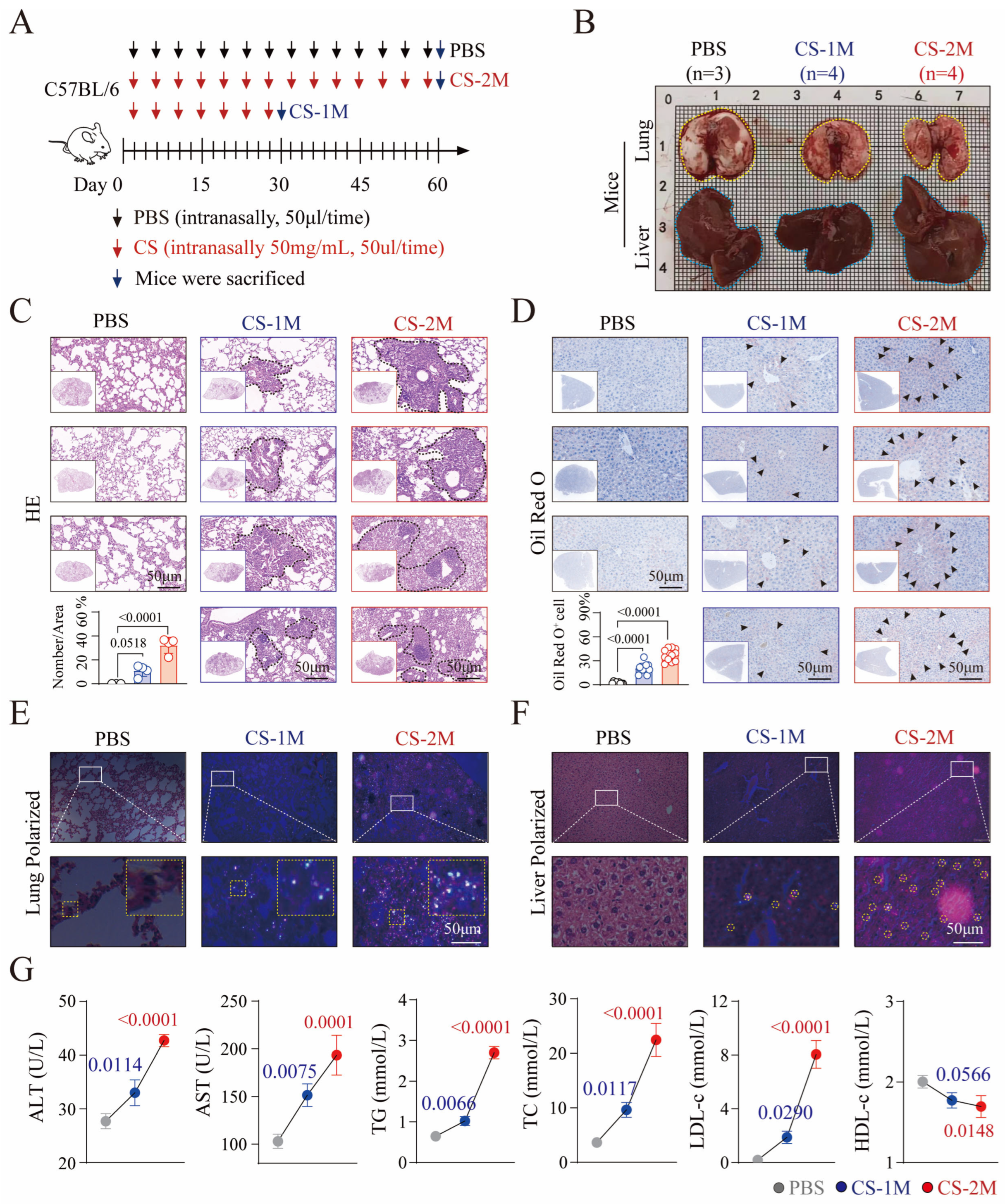
2.3. Silica Exposure Mouse Model Inducing Liver Injury Construction and Analysis
2.4. Hematoxylin and Eosin (HE) Staining and Oil Red O Staining
2.5. Assessment and Analysis of Lipid Levels in Mice
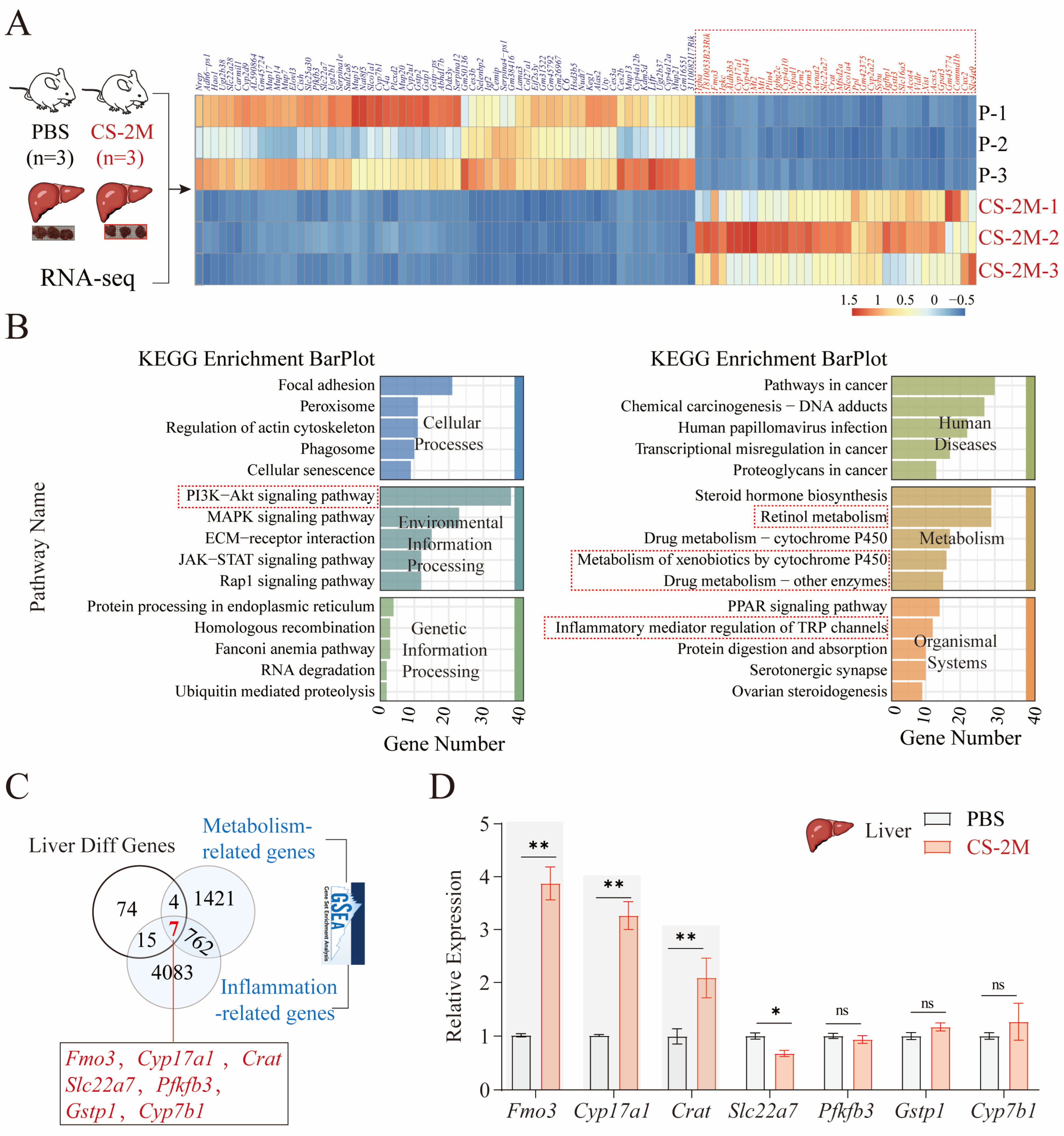
2.6. Mouse Liver Tissue RNA Sequencing and Analysis
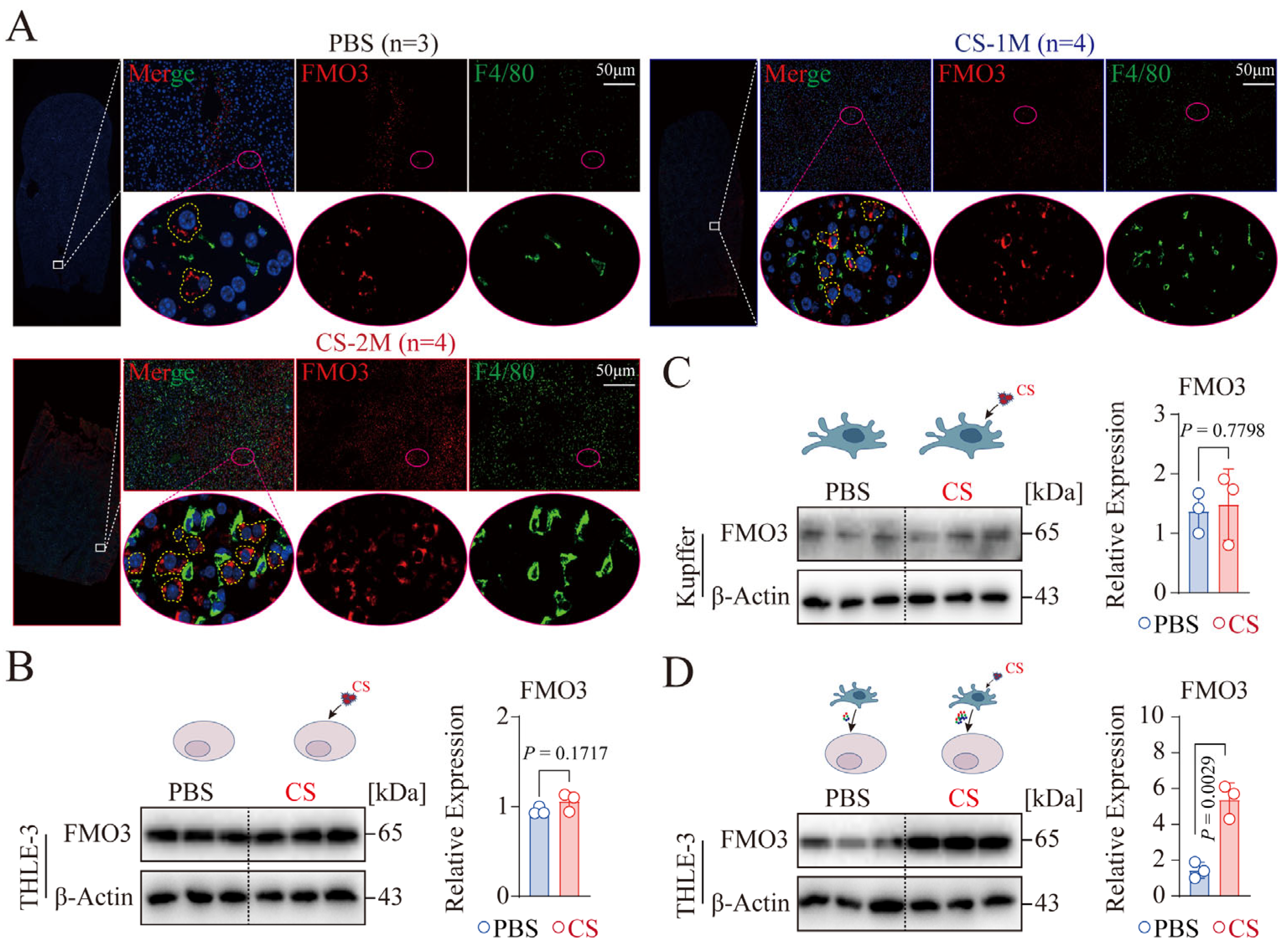
2.7. Analysis of Immunofluorescent Staining
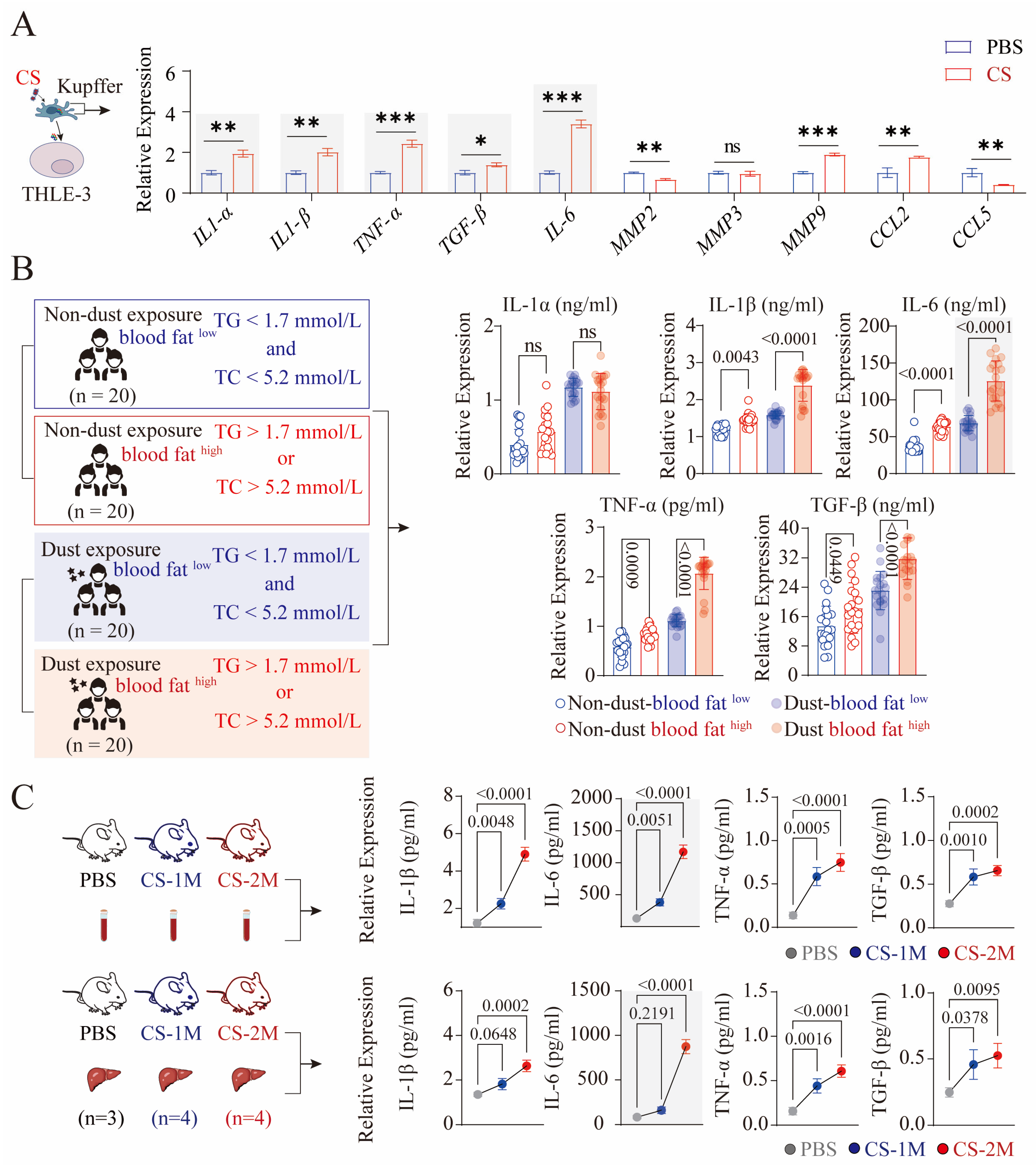
2.8. RT-qPCR
2.9. Cell Culture and Treatment
2.10. Western Blot Experiment
2.11. ELISA Detection of Inflammatory Cytokines in Human and Mouse Serum
2.12. GSEA and TRRUST Databases
2.13. Statistical Analysis
3. Results
3.1. Characterization of Dust-Exposed Workers
3.2. Silicon Exposure-Induced Dyslipidemia and Liver Dysfunction in Mice
3.3. Key Genes Associated with the Occurrence of Hepatic Metabolic Dysfunction in Silicon-Exposed Mice
3.4. CS-Activated Macrophages Promote the Expression of Hepatocyte FMO3 In Vitro
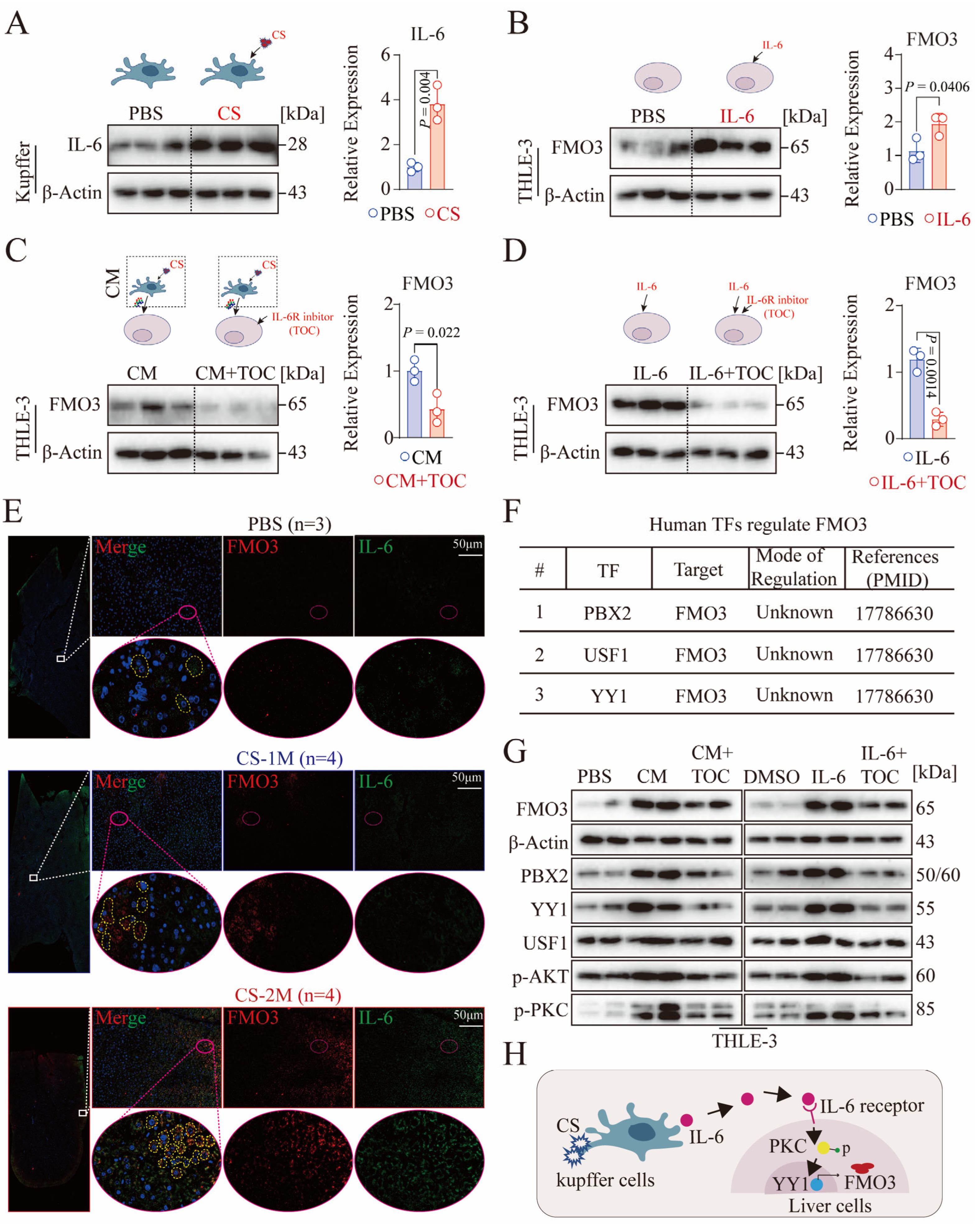
3.5. CS-Activated Macrophages Secrete a Large Amount of IL-6
3.6. Activation of Macrophages Facilitates Hepatocyte FMO3 Expression via the IL-6/PKC/YY1 Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese guidelines for lipid management (2023). Zhonghua Xin Xue Guan Bing Za Zhi 2023, 51, 221–255.
- Ren, M.X.; Tian, H.E.; Ma, L.; Zhou, L.P.; Wang, Y. Comparison of Occupational Stress and Its Factors of Workers in An Oil Refinery. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2018, 36, 422–425. [Google Scholar]
- Chen, W.; Liu, Y.; Wang, H.; Hnizdo, E.; Sun, Y.; Su, L.; Zhang, X.; Weng, S.; Bochmann, F.; Hearl, F.J.; et al. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: A cohort study. PLoS Med. 2012, 9, e1001206. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Dai, J.; Wang, Y.; Shi, P.; Zou, Y.; Pei, J.; Tian, Y.; Zhang, J.; Buranasudja, V.C.; Chen, J.; et al. Blocking Caspase-1/Gsdmd and Caspase-3/-8/Gsdme pyroptotic pathways rescues silicosis in mice. PLoS Genet. 2022, 18, e1010515. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Cai, X.; Zha, H.; Zhou, Z.; Sun, X.; Rong, F.; Tang, J.; Zhu, C.; Liu, X.; et al. Arginine monomethylation by PRMT7 controls MAVS-mediated antiviral innate immunity. Mol. Cell 2021, 81, 3171–3186.e78. [Google Scholar] [CrossRef]
- Neumann, E.; Hasseli, R.; Ohl, S.; Lange, U.; Frommer, K.W.; Müller-Ladner, U. Adipokines and Autoimmunity in Inflammatory Arthritis. Cells 2021, 10, 216. [Google Scholar] [CrossRef]
- Wei, Y.; You, Y.; Zhang, J.; Ban, J.; Min, H.; Li, C.; Chen, J. Crystalline silica-induced macrophage pyroptosis interacting with mitophagy contributes to pulmonary fibrosis via modulating mitochondria homeostasis. J. Hazard. Mater. 2023, 454, 131562. [Google Scholar] [CrossRef]
- Liu, X.; Wei, W.; Liu, Z.; Song, E.; Lou, J.; Feng, L.; Huang, R.; Chen, C.; Ke, P.C.; Song, Y. Serum apolipoprotein A-I depletion is causative to silica nanoparticles-induced cardiovascular damage. Proc. Natl. Acad. Sci. USA 2021, 118, e2108131118. [Google Scholar] [CrossRef]
- Duan, J.; Liang, S.; Feng, L.; Yu, Y.; Sun, Z. Silica nanoparticles trigger hepatic lipid-metabolism disorder in vivo and in vitro. Int. J. Nanomed. 2018, 13, 7303–7318. [Google Scholar] [CrossRef]
- Bissé, E.; Epting, T.; Beil, A.; Lindinger, G.; Lang, H.; Wieland, H. Reference values for serum silicon in adults. Anal. Biochem. 2005, 337, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Li, Y.; Guo, C.; Fan, Z.; Li, Y.; Yang, M.; Zhou, X.; Sun, Z.; Wang, J. Integrative proteomics and metabolomics approach to elucidate metabolic dysfunction induced by silica nanoparticles in hepatocytes. J. Hazard. Mater. 2022, 434, 128820. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Abulikemu, A.; Qi, Y.; Liu, X.; Wang, J.; Xu, K.; Guo, C.; Li, Y. Proteomics revealed composition- and size-related regulators for hepatic impairments induced by silica nanoparticles. Sci. Total Environ. 2024, 922, 170584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tao, H.; Fu, J.; Hou, W.; Hu, C.; Liu, Y.; Ding, X.; Hu, D.; Dai, Y. Cross-sectional analysis of dyslipidemia risk in coal mine workers: From epidemiology to animal models. Sci. Rep. 2024, 14, 26894. [Google Scholar] [CrossRef]
- Liang, C.; Bai, Y.; Miao, R.; Yang, X.; Gao, L.; Liu, Y.; Zhou, J.; Guo, J.; Hu, D.; Wu, J. Celastrol as a candidate drug for silicosis: From bioinformatics and network pharmacology to experimental validation. Int. Immunopharmacol. 2023, 125, 111068. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals, 2020 ed.; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Liang, R.Y.; Dong, C.Q.; Yuan, L.; Jiang, B.Y.; Wang, D.M.; Chen, W.H. Progress in the epidemiological studies on coal mine dust exposure with workers’ health damage. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2022, 40, 476–480. [Google Scholar]
- Sun, M.; Zhang, J.; Liang, S.; Du, Z.; Liu, J.; Sun, Z.; Duan, J. Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotoxicol. Environ. Saf. 2021, 208, 11496. [Google Scholar] [CrossRef]
- Longest, P.W.; Holbrook, L.T. In silico models of aerosol delivery to the respiratory tract—Development and applications. Adv. Drug Deliv. Rev. 2012, 64, 296–311. [Google Scholar] [CrossRef]
- Xu, S.; Wu, X.; Wang, S.; Xu, M.; Fang, T.; Ma, X.; Chen, M.; Fu, J.; Guo, J.; Tian, S.; et al. TRIM56 protects against nonalcoholic fatty liver disease by promoting the degradation of fatty acid synthase. J. Clin. Investig. 2024, 134, e166149. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Mathis, B.J.; Tanaka, K.; Hiramatsu, Y. Metabolically Healthy Obesity: Are Interventions Useful? Curr. Obes. Rep. 2023, 12, 36–60. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Phillips, I.R.; Shephard, E.A. Flavin-containing monooxygenase 3 (FMO3): Genetic variants and their consequences for drug metabolism and disease. Xenobiotica 2020, 50, 19–33. [Google Scholar] [CrossRef]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, Q.; Jiang, X.; Hu, J.; Jiang, Q.; Sheng, L.; Peng, X.; Wang, S.; Chen, Y.; Wan, Y.; et al. Trimethylamine N-oxide impairs β-cell function and glucose tolerance. Nat. Commun. 2024, 15, 2526. [Google Scholar] [CrossRef]
- Li, L.; Cui, L.; Lin, P.; Liu, Z.; Bao, S.; Ma, X.; Nan, H.; Zhu, W.; Cen, J.; Mao, Y.; et al. Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers. Cell Stem Cell 2023, 30, 283–299.e9. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Guo, R.; Jiang, M.; Wang, G.; Li, B.; Jia, X.; Ai, Y.; Chen, S.; Tang, P.; Liu, A.; Yuan, Q.; et al. IL6 supports long-term expansion of hepatocytes in vitro. Nat. Commun. 2022, 13, 7345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wan, M.; Shi, J.; Horita, D.A.; Miller, L.D.; Kute, T.E.; Kridel, S.J.; Kulik, G.; Sui, G. Yin Yang 1 promotes mTORC2-mediated AKT phosphorylation. J. Mol. Cell Biol. 2016, 8, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mu, M.; Wang, B.; Zhang, H.; Liu, Y.; Yu, L.; Zhou, M.; Ma, J.; Wang, D.; Chen, W. Associations of coal mine dust exposure with arterial stiffness and atherosclerotic cardiovascular disease risk in chinese coal miners. Int. Arch. Occup. Environ. Health 2024, 97, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Petsonk, E.L.; Rose, C.; Cohen, R. Coal mine dust lung disease. New lessons from old exposure. Am. J. Respir. Crit. Care Med. 2013, 187, 1178–1185. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, H.; Wang, X.; Chen, Z.; Zhang, L.; Kang, Y.; Yang, Y.; Chen, L.; Zhou, H.; Cai, J.; et al. Left ventricular diastolic dysfunction and cardiovascular disease in different ambient air pollution conditions: A prospective cohort study. Sci. Total Environ. 2022, 831, 154872. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Diez Roux, A.V.; Gassett, A.J.; Jacobs, D.R., Jr.; et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016, 388, 696–704. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Maiseyeu, A.; Gopalakrishnan, B.; Villamena, F.A.; Chen, L.C.; Harkema, J.R.; Sun, Q.; Rajagopalan, S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 2014, 115, 770–780. [Google Scholar] [CrossRef]
- Li, H.; Cai, J.; Chen, R.; Zhao, Z.; Ying, Z.; Wang, L.; Chen, J.; Hao, K.; Kinney, P.L.; Chen, H.; et al. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation 2017, 136, 618–627. [Google Scholar] [CrossRef]
- Item, F.; Wueest, S.; Lemos, V.; Stein, S.; Lucchini, F.C.; Denzler, R.; Fisser, M.C.; Challa, T.D.; Pirinen, E.; Kim, Y.; et al. Fas cell surface death receptor controls hepatic lipid metabolism by regulating mitochondrial function. Nat. Commun. 2017, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Skroblin, P.; Kiechl, S.; Fernández-Hernando, C.; Mayr, M. Liver microRNAs: Potential mediators and biomarkers for metabolic and cardiovascular disease? Eur. Heart J. 2016, 37, 3260–3266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, R. ER stress and hepatic lipid metabolism. Front. Genet. 2014, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Koukouritaki, S.B.; Simpson, P.; Yeung, C.K.; Rettie, A.E.; Hines, R.N. Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr. Res. 2002, 51, 236–243. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Wenfeng, Z.; Yakun, W.; Di, M.; Jianping, G.; Chuanxin, W.; Chun, H. Kupffer cells: Increasingly significant role in nonalcoholic fatty liver disease. Ann. Hepatol. 2014, 13, 489–495. [Google Scholar] [CrossRef]
- Baffy, G. Kupffer cells in non-alcoholic fatty liver disease: The emerging view. J. Hepatol. 2009, 51, 212–223. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Tao, H.; Gao, J.; Wang, J.; Hui, G.; Zhu, Y.; Wang, J.; Ding, X.; Dai, Y. IL-6 Affects Liver Metabolic Abnormalities Caused by Silicon Exposure by Regulating the PKC/YY1 Signaling Pathway. Genes 2025, 16, 456. https://doi.org/10.3390/genes16040456
Zhao H, Tao H, Gao J, Wang J, Hui G, Zhu Y, Wang J, Ding X, Dai Y. IL-6 Affects Liver Metabolic Abnormalities Caused by Silicon Exposure by Regulating the PKC/YY1 Signaling Pathway. Genes. 2025; 16(4):456. https://doi.org/10.3390/genes16040456
Chicago/Turabian StyleZhao, Hui, Huihui Tao, Jian Gao, Jingjing Wang, Guangliang Hui, Ye Zhu, Jialin Wang, Xuansheng Ding, and Yong Dai. 2025. "IL-6 Affects Liver Metabolic Abnormalities Caused by Silicon Exposure by Regulating the PKC/YY1 Signaling Pathway" Genes 16, no. 4: 456. https://doi.org/10.3390/genes16040456
APA StyleZhao, H., Tao, H., Gao, J., Wang, J., Hui, G., Zhu, Y., Wang, J., Ding, X., & Dai, Y. (2025). IL-6 Affects Liver Metabolic Abnormalities Caused by Silicon Exposure by Regulating the PKC/YY1 Signaling Pathway. Genes, 16(4), 456. https://doi.org/10.3390/genes16040456





