Genome-Wide Identification and Expression Pattern Analysis of SBP Gene Family in Neolamarckia cadamba
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of NcSBP Genes in N. cadamba
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
2.3. Gene Structures, Conserved Motifs, and Domain Analysis
2.4. Chromosome Localization and Collinearity Analysis
2.5. Promoter cis-Acting Element Analysis
2.6. Expression Pattern of NcSBPs
3. Results
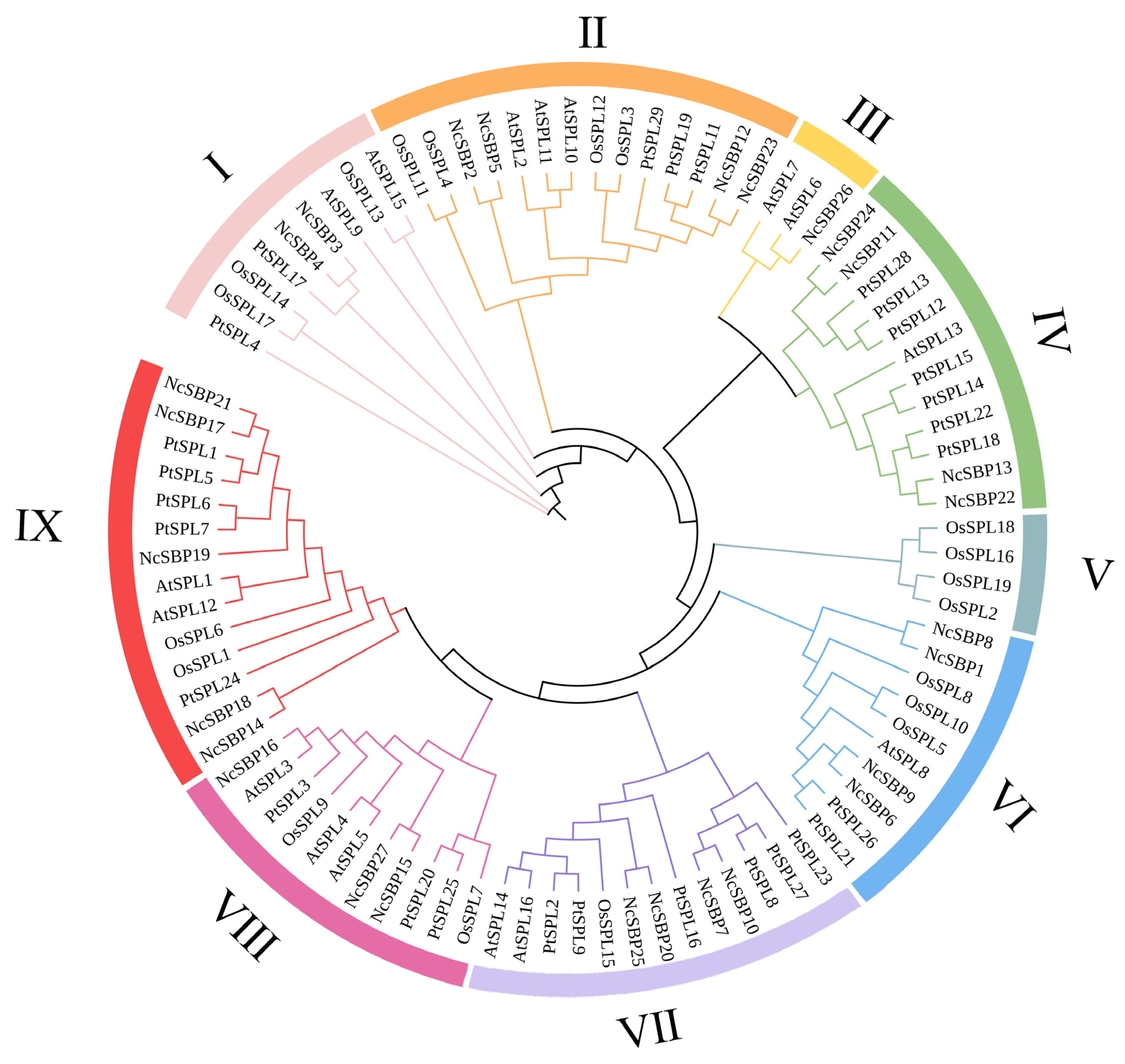
3.1. Identification and Phylogenetic Analysis of NcSBP Genes
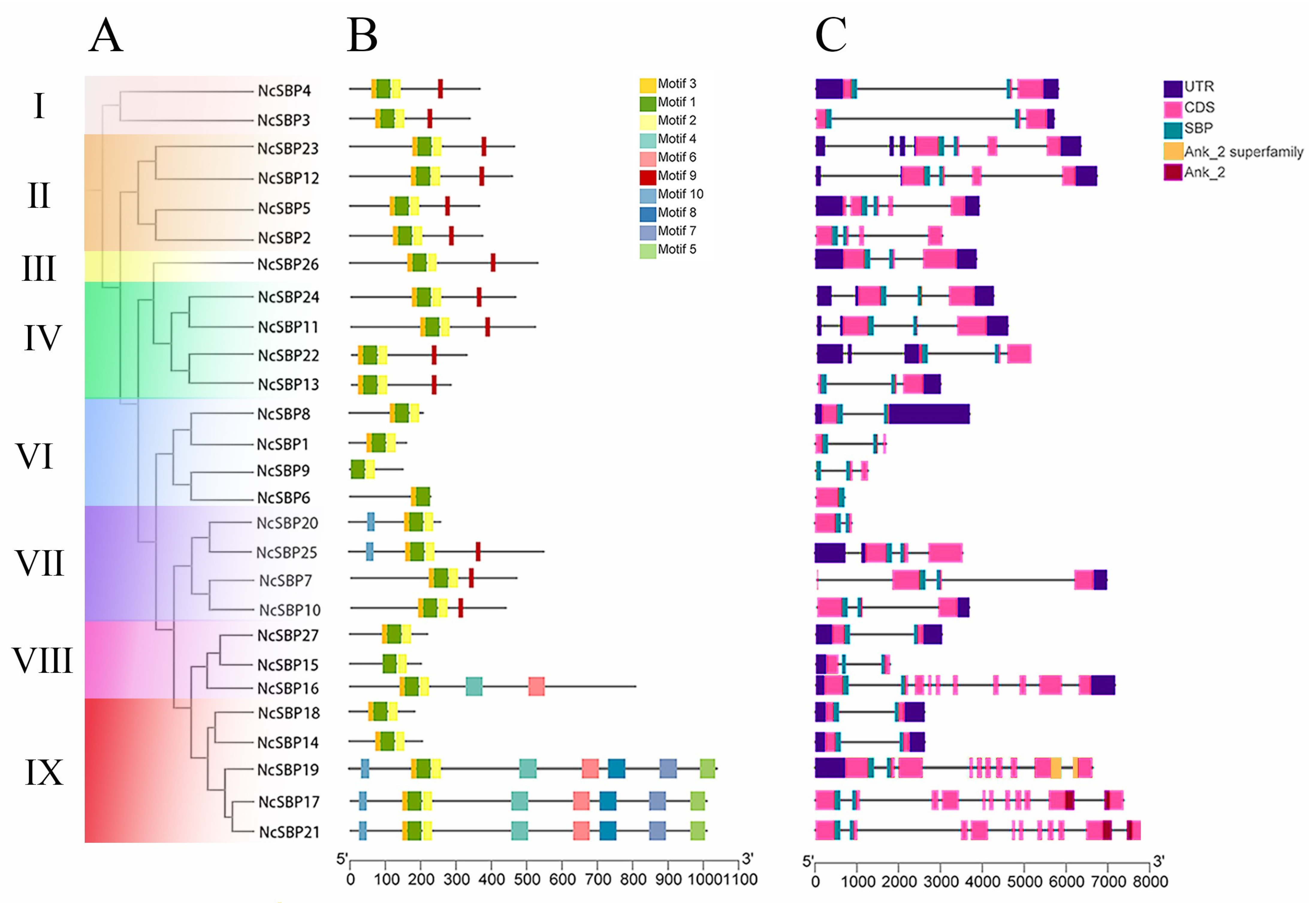
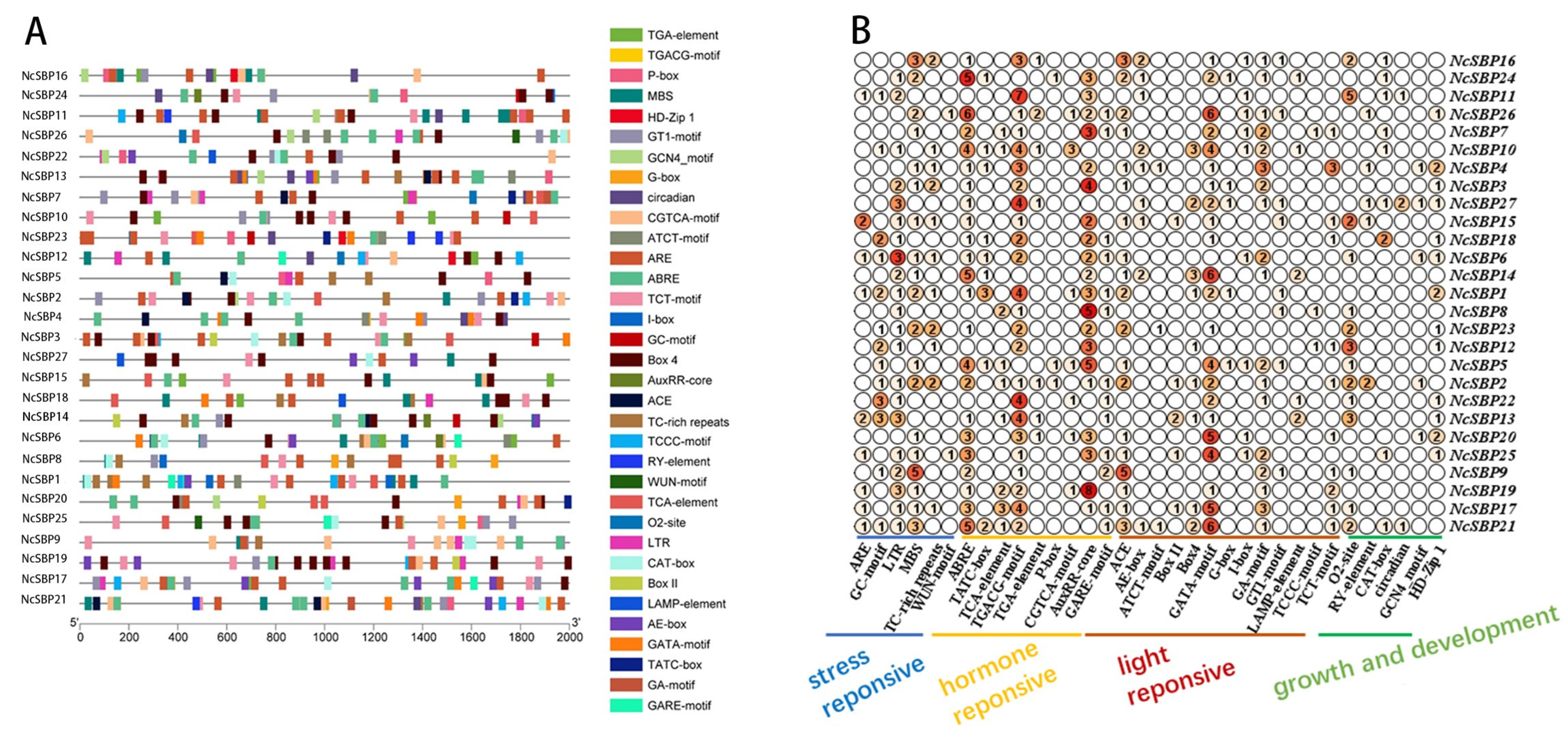
3.2. Conserved Motif and Gene Structure Analysis of NcSBPs
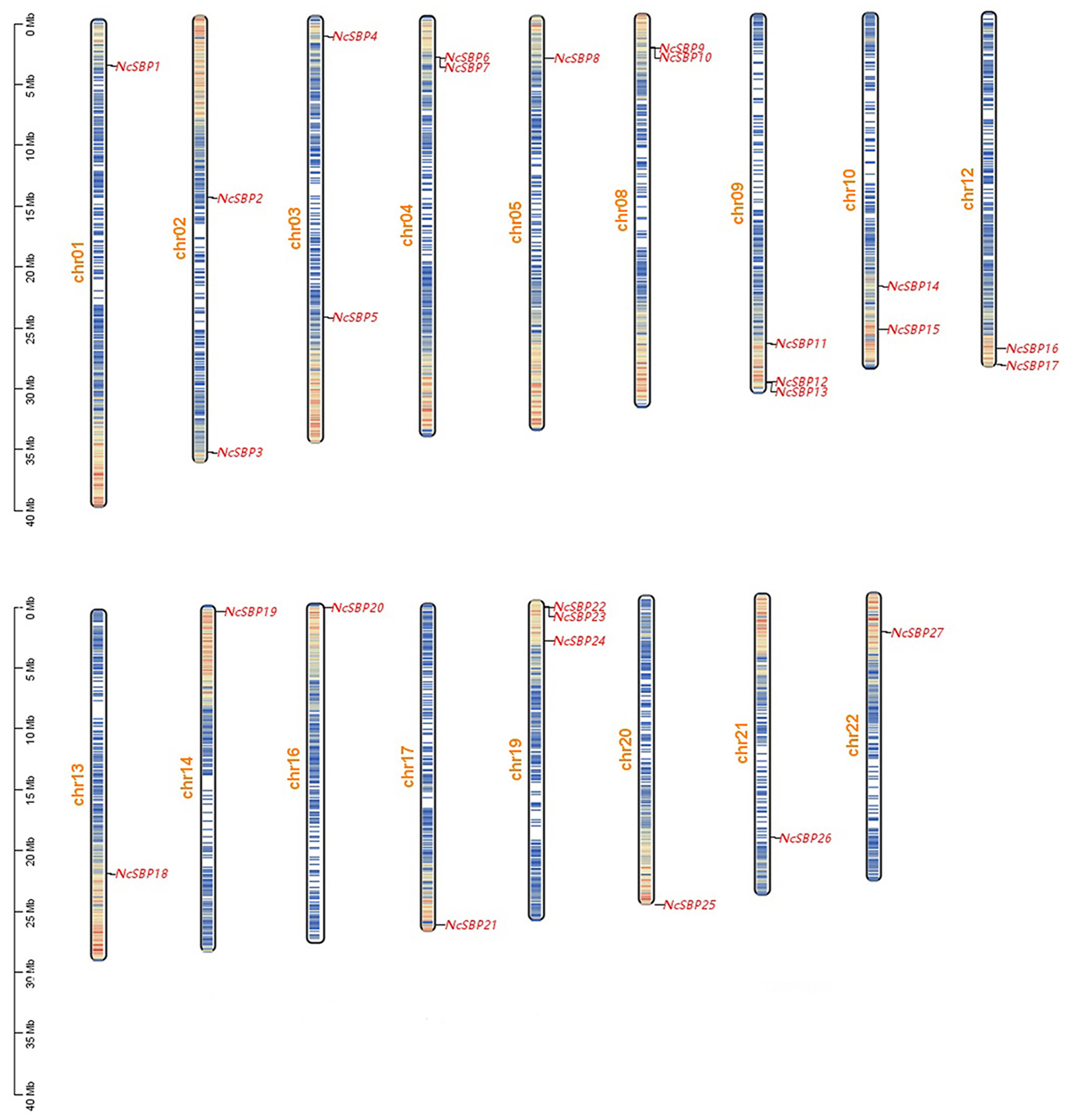
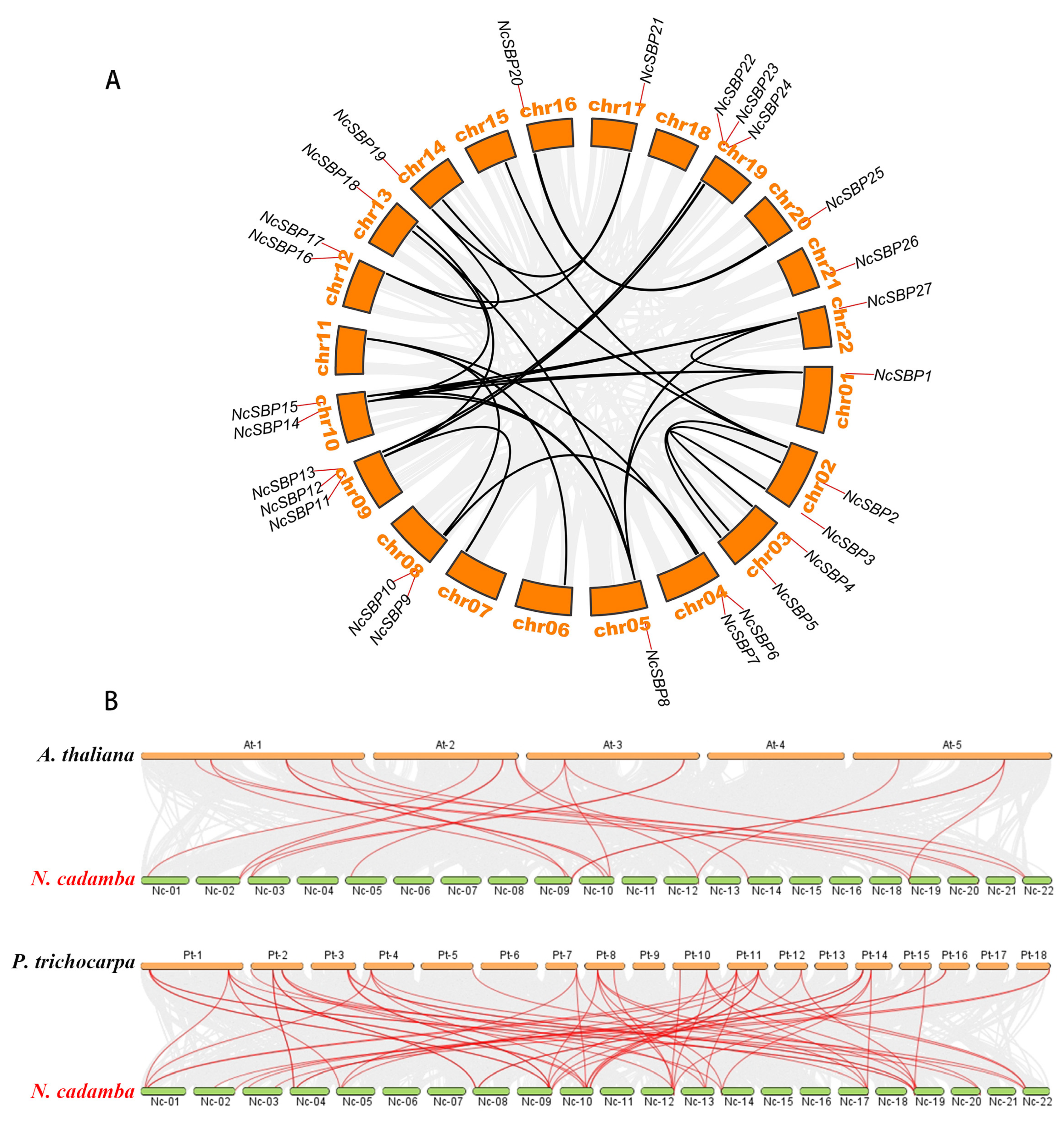
3.3. Chromosome Localization and Collinearity Analysis of NcSBPs
3.4. cis-Acting Element Analysis of NcSBP Promoters
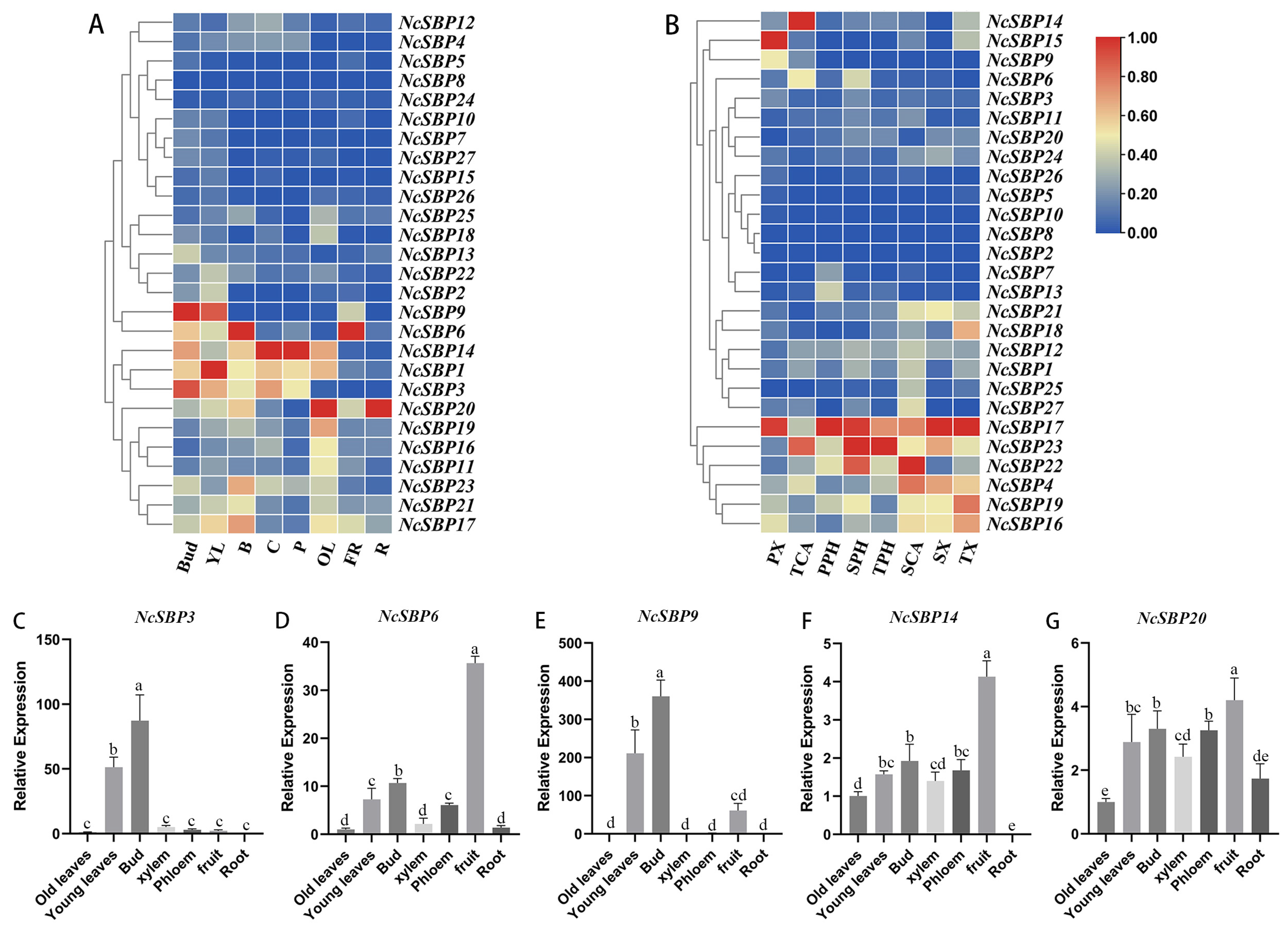
3.5. Expression Patterns of NcSBP Genes in Various Tissues
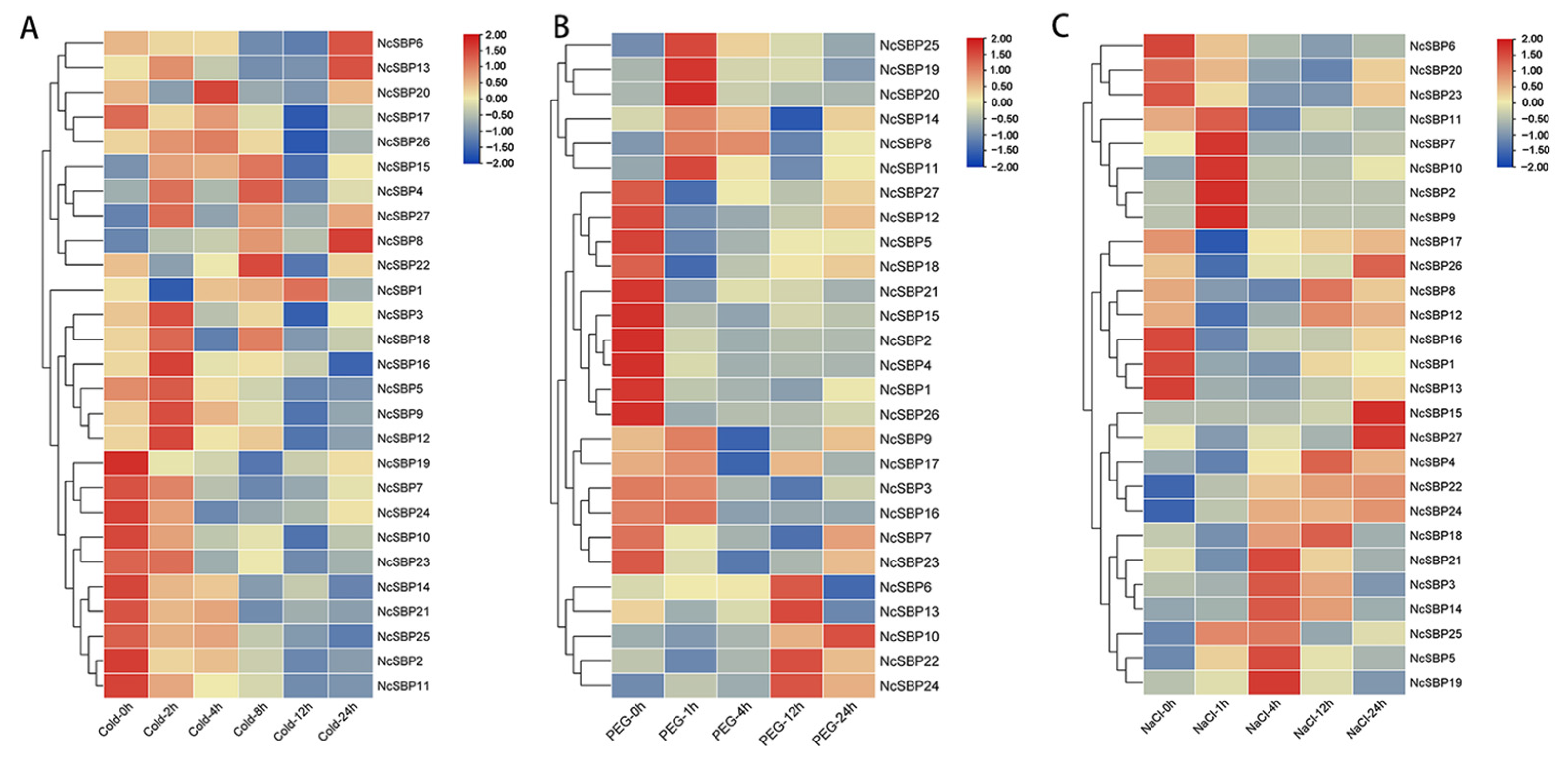
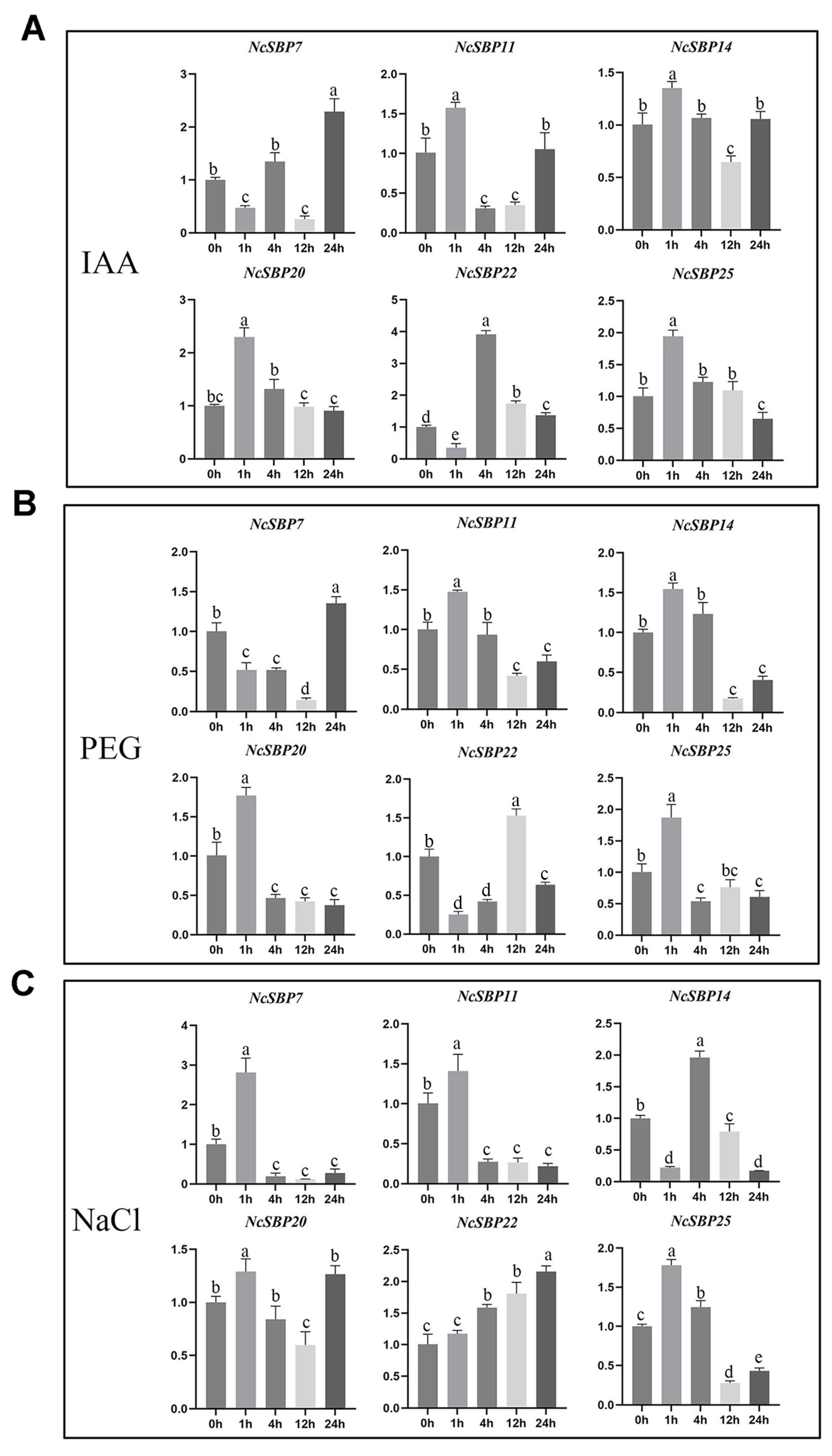
3.6. Expression Analysis of NcSBPs in Response to Hormones and Abiotic Stress Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Zhang, Z.; Liu, D.; Zhang, K.; Li, A.; Mao, L. SQUAMOSA promoter-binding protein-like transcription factors: Star players for plant growth and development. J. Integr. Plant Biol. 2010, 52, 946–951. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Jach, G.; Saedler, H.; Huijser, P. Functional dissection of the plant-specific SBP-domain: Overlap of the DNA-binding and nuclear localization domains. J. Mol. Biol. 2005, 352, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Nunokawa, E.; et al. A novel zinc-binding motif revealed by solution structures of DNA-binding domains of Arabidopsis SBP-family transcription factors. J. Mol. Biol. 2004, 337, 49–63. [Google Scholar] [CrossRef]
- Cardon, G.; Hohmann, S.; Klein, J.; Nettesheim, K.; Saedler, H.; Huijser, P. Molecular characterisation of the Arabidopsis SBP-box genes. Gene 1999, 237, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Saedler, H.; Huijser, P. A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Mol. Gen. Genet. 1996, 250, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.; Whipple, C.; Jackson, D.; Hake, S. The maize SBP-box transcription factor encoded by tasselsheath4 regulates bract development and the establishment of meristem boundaries. Development 2010, 137, 1243–1250. [Google Scholar] [CrossRef]
- Riese, M.; Zobell, O.; Saedler, H.; Huijser, P. SBP-domain transcription factors as possible effectors of cryptochrome-mediated blue light signalling in the moss Physcomitrella patens. Planta 2008, 227, 505–515. [Google Scholar] [CrossRef]
- Li, J.; Hou, H.; Li, X.; Xiang, J.; Yin, X.; Gao, H.; Zheng, Y.; Bassett, C.L.; Wang, X. Genome-wide identification and analysis of the SBP-box family genes in apple (Malus×domestica Borkh.). Plant Physiol. Biochem. 2013, 70, 100–114. [Google Scholar] [CrossRef]
- Zhang, S.D.; Ling, L.Z.; Yi, T.S. Evolution and divergence of SBP-box genes in land plants. BMC Genom. 2015, 16, 787. [Google Scholar] [CrossRef]
- Wang, J.W.; Schwab, R.; Czech, B.; Mica, E.; Weigel, D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell 2008, 20, 1231–1243. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Spector, D.L. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 2007, 17, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Cai, W.J.; Wang, S.; Shan, C.M.; Wang, L.J.; Chen, X.Y. Temporal control of trichome distribution by microRNA156-targeted SPL genes in Arabidopsis thaliana. Plant Cell 2010, 22, 2322–2335. [Google Scholar] [CrossRef]
- Schwarz, S.; Grande, A.V.; Bujdoso, N.; Saedler, H.; Huijser, P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol. Biol. 2008, 67, 183–195. [Google Scholar] [CrossRef]
- Shikata, M.; Koyama, T.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis SBP-box genes SPL10, SPL11 and SPL2 control morphological change in association with shoot maturation in the reproductive phase. Plant Cell Physiol. 2009, 50, 2133–2145. [Google Scholar] [CrossRef]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Quodt, V.; Chandler, J.; Höhmann, S.; Berndtgen, R.; Huijser, P. SPL8 Acts together with the Brassinosteroid-Signaling component BIM1 in controlling Arabidopsis thaliana male fertility. Plants 2013, 2, 416–428. [Google Scholar] [CrossRef]
- Xie, K.; Wu, C.; Xiong, L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like Transcription factors and microRNA156 in Rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef]
- Cao, R.; Guo, L.; Ma, M.; Zhang, W.; Liu, X.; Zhao, H. Identification and functional characterization of squamosa promoter binding protein-like gene TaSPL16 in Wheat (Triticum aestivum L.). Front. Plant Sci. 2019, 10, 212. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Liu, X.; Mao, X.; Li, A.; Wang, J.; Chang, X.; Zhang, X.; Jing, R. Functional conservation and divergence among homoeologs of TaSPL20 and TaSPL21, two SBP-Box genes governing Yield-Related traits in Hexaploid Wheat. Plant Physiol. 2017, 174, 1177–1191. [Google Scholar] [CrossRef]
- Hou, H.; Li, J.; Gao, M.; Singer, S.D.; Wang, H.; Mao, L.; Fei, Z.; Wang, X. Genomic organization, phylogenetic comparison and differential expression of the SBP-box family genes in grape. PLoS ONE 2013, 8, e59358. [Google Scholar] [CrossRef]
- Chen, W.; Kong, J.; Lai, T.; Manning, K.; Wu, C.; Wang, Y.; Qin, C.; Li, B.; Yu, Z.; Zhang, X.; et al. Tuning LeSPL-CNR expression by SlymiR157 affects tomato fruit ripening. Sci. Rep. 2015, 5, 7852. [Google Scholar] [CrossRef]
- Yu, S.; Galvão, V.C.; Zhang, Y.; Horrer, D.; Zhang, T.; Hao, Y.; Feng, Y.; Wang, S.; Schmid, M.; Wang, J. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA PROMOTER BINDING–LIKE transcription factors. Plant Cell 2012, 24, 3320–3332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Chen, S.; Huang, H.; Jiang, J.; Yuan, H.; Li, H. Molecular characterization and expression analysis of the SPL gene family with BpSPL9 transgenic lines found to confer tolerance to abiotic stress in Betula platyphylla Suk. Plant Cell Tissue Organ Cult. 2017, 130, 469–481. [Google Scholar] [CrossRef]
- Hou, H.; Jia, H.; Yan, Q.; Wang, X. Overexpression of a SBP-Box gene (VpSBP16) from chinese wild vitis species in Arabidopsis Improves Salinity and Drought Stress Tolerance. Int. J. Mol. Sci. 2018, 19, 940. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Mermod, M.; Yamasaki, H.; Kamiya, T.; Fujiwara, T.; Shikanai, T. SPL7 locally regulates copper-homeostasis-related genes in Arabidopsis. J. Plant Physiol. 2018, 224–225, 137–143. [Google Scholar] [CrossRef]
- Gielen, H.; Remans, T.; Vangronsveld, J.; Cuypers, A. Toxicity responses of Cu and Cd: The involvement of miRNAs and the transcription factor SPL7. BMC Plant Biol. 2016, 16, 145. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, C.; Meng, L.; Mao, D.; Peng, C.; Zhu, Y.; Huang, D.; Tan, Z.; Chen, C.; Liu, C.; et al. Overexpression of OsSPL9 enhances accumulation of Cu in rice grain and improves its digestibility and metabolism. J. Genet. Genom. 2016, 43, 673–676. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, K.; Chen, X. High frequency plant regeneration from leaf culture of Neolamarckia cadamba. Plant Biotechnol. 2019, 36, 13–19. [Google Scholar] [CrossRef]
- Salinas, M.; Xing, S.; Hohmann, S.; Berndtgen, R.; Huijser, P. Genomic organization, phylogenetic comparison and differential expression of the SBP-box family of transcription factors in tomato. Planta 2012, 235, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, J.W.; Bade, R.; Men, Z.H.; Hasi, A. Genome-wide identification and phylogenetic analysis of the SBP-box gene family in melons. Genet. Mol. Res. 2014, 13, 8794–8806. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.; Goel, R.; Kumari, S.; Dahuja, A. Genomic organization, phylogenetic comparison, and expression profiles of the SPL family genes and their regulation in soybean. Dev. Genes. Evol. 2017, 227, 101–119. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Fang, H.; Wen, Y.; Wang, Y.; Zhang, J.; Peng, C.; Long, J. Genome-wide identification and expression analysis of WRKY gene family in Neolamarckia cadamba. Int. J. Mol. Sci. 2023, 24, 7537. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, X.; Ouyang, K.; Yang, J.; Que, Q.; Long, J.; Zhang, J.; Zhang, T.; Wang, X.; Gao, J.; et al. Chromosome-level assembly of the Neolamarckia cadamba genome provides insights into the evolution of cadambine biosynthesis. Plant J. 2022, 109, 891–908. [Google Scholar] [CrossRef]
- Yi, N.; Yang, H.; Zhang, X.; Pian, R.; Li, H.; Zeng, W.; Wu, A.M. The physiological and transcriptomic study of secondary growth in Neolamarckia cadamba stimulated by the ethylene precursor ACC. Plant Physiol. Biochem. 2022, 190, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.J.; Zhang, M.J.; Bao, Y.T.; Yang, X.; Xu, W.Y.; Quyang, K.X.; Chen, X.Y. Selection and validation of reference genes for quantitative RT-PCR analysis in Neolamarckia cadamba. Chin. Bull. Bot. 2018, 53, 829–839, (In Chinese with English abstract). [Google Scholar]
- Yang, Z.; Wang, X.; Gu, S.; Hu, Z.; Xu, H.; Xu, C. Comparative study of SBP-box gene family in Arabidopsis and rice. Gene 2008, 407, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Long, J.; Dong, T.; Zheng, D.; Zhang, L.; Peng, C. Establishment of vascular tissue cells capture system by laser microdissection in Neolamarckia cadamba. Guihaia 2021, 41, 1226–1236, (In Chinese with English abstract). [Google Scholar]
- Song, N.; Cheng, Y.; Peng, W.; Peng, E.; Zhao, Z.; Liu, T.; Yi, T.; Dai, L.; Wang, B.; Hong, Y. Genome-wide characterization and expression analysis of the SBP-Box gene family in sweet orange (Citrus sinensis). Int. J. Mol. Sci. 2021, 22, 8918. [Google Scholar] [CrossRef]
- Xie, X.; Yue, S.; Shi, B.; Li, H.; Cui, Y.; Wang, J.; Yang, P.; Li, S.; Li, X.; Bian, S. Comprehensive analysis of the SBP family in Blueberry and their regulatory mechanism controlling chlorophyll Accumulation. Front. Plant Sci. 2021, 12, 703994. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, D.; Yin, J.; Yang, L.; He, Y.; Zhu, Z.; Tong, H.; Chen, L.; Zhu, G.; Liu, Y.; et al. Genome-wide characterization and expression profiling of squamosa promoter Binding Protein-Like (SBP) transcription factors in Wheat (Triticum aestivum L.). Agronomy 2019, 9, 527. [Google Scholar] [CrossRef]
- Wang, P.; Chen, D.; Zheng, Y.; Jin, S.; Yang, J.; Ye, N. Identification and expression analyses of SBP-Box genes reveal their involvement in abiotic stress and hormone response in tea plant (Camellia sinensis). Int. J. Mol. Sci. 2018, 19, 3404. [Google Scholar] [CrossRef]
- Li, C.; Lu, S. Molecular characterization of the SPL gene family in Populus trichocarpa. BMC Plant Biol. 2014, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhao, K.; Jiang, G.; Sun, S.; Li, J.; Tu, M.; Wang, L.; Xie, H.; Chen, D. Genome-wide identification and expression analysis of the SBP-Box gene family in Loquat fruit development. Genes 2023, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Czech, B.; Weigel, D. MiR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Jung, J.; Seo, P.J.; Kang, S.K.; Park, C. MiR172 signals are incorporated into the miR156 signaling pathway at the SPL3/4/5 genes in Arabidopsis developmental transitions. Plant Mol. Biol. 2011, 76, 35–45. [Google Scholar] [CrossRef]
- Yamasaki, H.; Hayashi, M.; Fukazawa, M.; Kobayashi, Y.; Shikanai, T. SQUAMOSA promoter binding protein–like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 2009, 21, 347–361. [Google Scholar] [CrossRef]
- Xing, S.; Salinas, M.; Höhmann, S.; Berndtgen, R.; Huijser, P. MiR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis. Plant Cell 2010, 22, 3935–3950. [Google Scholar] [CrossRef]
- Stone, J.M.; Liang, X.; Nekl, E.R.; Stiers, J.J. Arabidopsis AtSPL14, a plant-specific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1. Plant J. 2005, 41, 744–754. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, L.; Zhou, Y.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. Identification and expression analysis of the SQUAMOSA promoter-binding protein (SBP)-box gene family in Prunus mume. Mol. Genet. Genom. MGG 2015, 290, 1701–1715. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Wang, X.; Ye, B.; Jin, M.; Chen, W.; Wang, Y.; Zhou, Y.; Blanks, A.M.; Gu, M.; Zhang, P.; et al. Molecular and functional characterization of the SBP-box transcription factor SPL-CNR in tomato fruit ripening and cell death. J. Exp. Bot. 2020, 71, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, C.; Lai, Y.; Wang, Y.; Kang, L.; Liu, A.; Lan, C.; Su, H.; Gao, Y.; Li, Z.; et al. Asymmetric gene expression and cell-type-specific regulatory networks in the root of bread wheat revealed by single-cell multiomics analysis. Genome Biol. 2023, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. ThemiR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014, 80, 1108–1117. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Liu, M.; Miao, J.; Ma, C.; Feng, Y.; Qian, J.; Li, H.; Bi, H.; Liu, W. The SPL transcription factor TaSPL6 negatively regulates drought stress response in wheat. Plant Physiol. Biochem. 2024, 206, 108264. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, M.; Feng, M.; Liu, G.; Torregrosa, L.; Tao, X.; Ren, R.; Fang, Y.; Zhang, Z.; Meng, J.; et al. MiR156b-targeted VvSBP8/13 functions downstream of the abscisic acid signal to regulate anthocyanins biosynthesis in grapevine fruit under drought. Hortic. Res. 2024, 11, uhad293. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhang, B. Pepper SBP-box transcription factor, CaSBP13, plays a negatively role in drought response. Front. Plant Sci. 2024, 15, 1412685. [Google Scholar] [CrossRef]

| Gene Name | Number of Amino Acids | Molecular Weight (Da) | Theoretical Isoelectric Point (pI) | Instability Index | Aliphatic Index | Grand Average of Hydropathicity |
|---|---|---|---|---|---|---|
| NcSBP1 | 160 | 18,390.48 | 8.99 | 74.72 | 42.12 | −1.209 |
| NcSBP2 | 374 | 41,811.45 | 8.62 | 64.18 | 63.16 | −0.686 |
| NcSBP3 | 338 | 35,693.41 | 9.17 | 53.40 | 54.05 | −0.661 |
| NcSBP4 | 366 | 39,024.07 | 9.19 | 55.27 | 52.27 | −0.716 |
| NcSBP5 | 365 | 40,141.66 | 7.16 | 64.17 | 64.16 | −0.626 |
| NcSBP6 | 227 | 25,268.04 | 6.48 | 35.66 | 52.47 | −0.681 |
| NcSBP7 | 467 | 50,966.04 | 8.66 | 52.99 | 68.37 | −0.479 |
| NcSBP8 | 207 | 23,523.51 | 8.47 | 70.94 | 66.91 | −0.597 |
| NcSBP9 | 148 | 16,714.34 | 9.47 | 75.61 | 42.97 | −1.120 |
| NcSBP10 | 437 | 47,421.41 | 7.96 | 56.03 | 59.89 | −0.635 |
| NcSBP11 | 520 | 57,913.78 | 6.86 | 51.09 | 72.02 | −0.592 |
| NcSBP12 | 458 | 504,469.66 | 8.92 | 50.94 | 63.67 | −0.599 |
| NcSBP13 | 279 | 31,079.96 | 9.76 | 57.12 | 67.78 | −0.617 |
| NcSBP14 | 205 | 22,683.08 | 9.24 | 48.41 | 42.39 | −1.141 |
| NcSBP15 | 200 | 23,186.48 | 5.64 | 97.12 | 40.0 | −1.400 |
| NcSBP16 | 807 | 89,792.49 | 6.55 | 52.81 | 77.41 | −0.399 |
| NcSBP17 | 1007 | 112,571.72 | 7.72 | 53.35 | 82.6 | −0.440 |
| NcSBP18 | 184 | 20,945.47 | 9.07 | 45.69 | 52.01 | −1.048 |
| NcSBP19 | 1038 | 114,331.61 | 6.09 | 49.96 | 85.13 | −0.318 |
| NcSBP20 | 259 | 29,184.32 | 9.59 | 67.50 | 38.46 | −1.140 |
| NcSBP21 | 1007 | 112,572.40 | 7.18 | 55.98 | 80.63 | −0.440 |
| NcSBP22 | 324 | 36,282.26 | 6.70 | 62.34 | 60.43 | −0.694 |
| NcSBP23 | 464 | 50,676.55 | 8.81 | 52.36 | 60.75 | −0.630 |
| NcSBP24 | 464 | 51,786.02 | 8.68 | 49.90 | 66.47 | −0.642 |
| NcSBP25 | 552 | 60,659.47 | 7.94 | 72.92 | 61.54 | −0.808 |
| NcSBP26 | 530 | 58,574.96 | 8.60 | 56.71 | 58.51 | −0.677 |
| NcSBP27 | 218 | 24,525.01 | 6.68 | 83.17 | 40.69 | −1.258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Li, K.; Cai, C.; Wu, W.; Jian, G.; Lei, Z.; Peng, C.; Long, J. Genome-Wide Identification and Expression Pattern Analysis of SBP Gene Family in Neolamarckia cadamba. Genes 2025, 16, 460. https://doi.org/10.3390/genes16040460
Tang L, Li K, Cai C, Wu W, Jian G, Lei Z, Peng C, Long J. Genome-Wide Identification and Expression Pattern Analysis of SBP Gene Family in Neolamarckia cadamba. Genes. 2025; 16(4):460. https://doi.org/10.3390/genes16040460
Chicago/Turabian StyleTang, Linhan, Keying Li, Chuqing Cai, Wenjun Wu, Guichen Jian, Ziming Lei, Changcao Peng, and Jianmei Long. 2025. "Genome-Wide Identification and Expression Pattern Analysis of SBP Gene Family in Neolamarckia cadamba" Genes 16, no. 4: 460. https://doi.org/10.3390/genes16040460
APA StyleTang, L., Li, K., Cai, C., Wu, W., Jian, G., Lei, Z., Peng, C., & Long, J. (2025). Genome-Wide Identification and Expression Pattern Analysis of SBP Gene Family in Neolamarckia cadamba. Genes, 16(4), 460. https://doi.org/10.3390/genes16040460





