Genetic Editing of Tomato Golgi-Localized Nucleotide Sugar Transporter 1.1 Promotes Immunity Against Phytophthora infestans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Plasmid Constructs
2.3. Phylogenetic Analysis
2.4. Subcellular Localization
2.5. Quantitative RT-PCR
2.6. P. infestans Infection Assays
2.7. Phytohormone Quantification
2.8. ROS Measurements
2.9. RNA-Seq Analysis
2.10. Data Analysis
3. Results
3.1. Expansion and Molecular Divergence of SlGONSTs
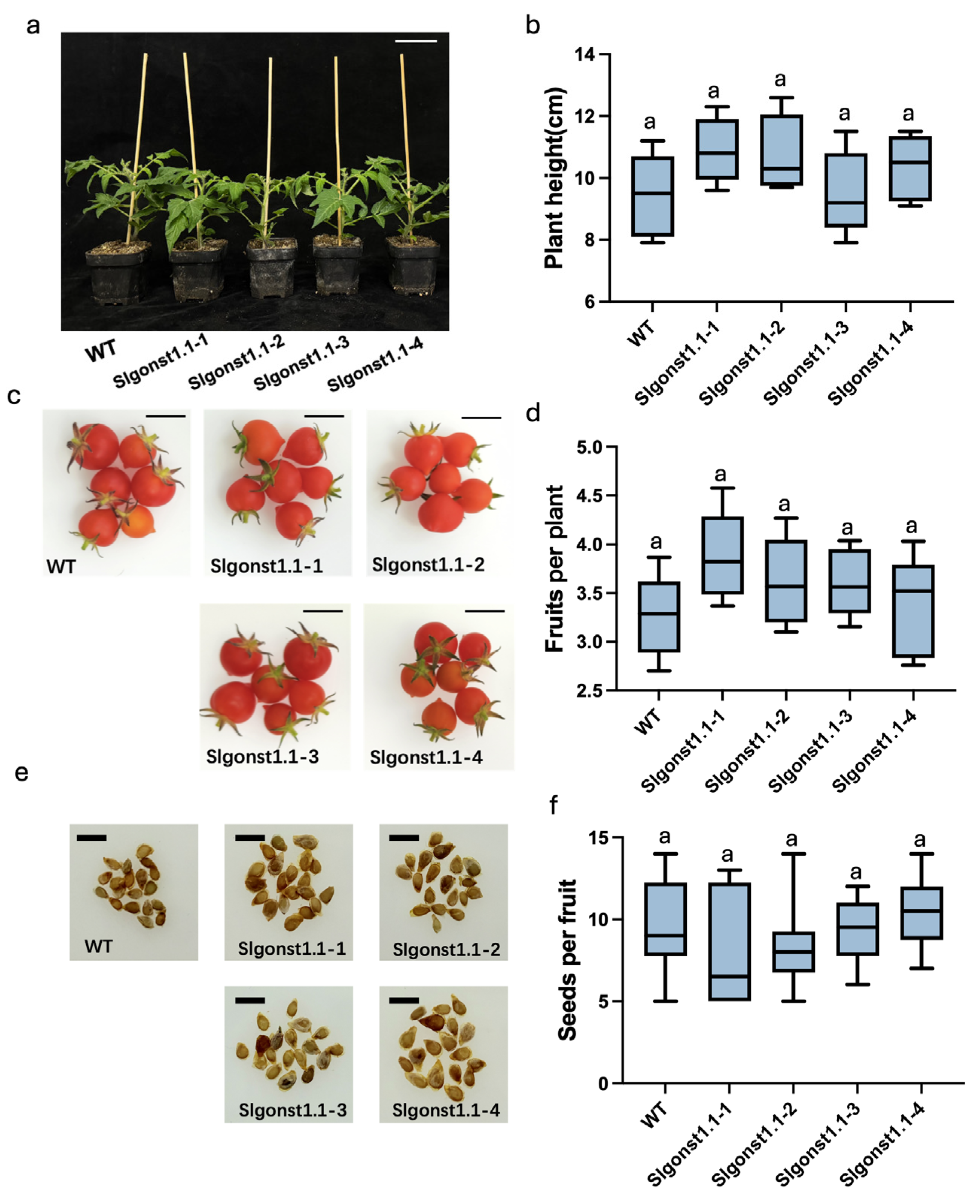
3.2. SlGONST1.1 Lowers S. lycopersicum Resistance Against Late Blight Without Affecting Plant Growth and Development
3.3. SlGONST1.1 Maintains Constitutive Abscisic Acid Levels and Suppresses PAMP-Induced ROS Burst
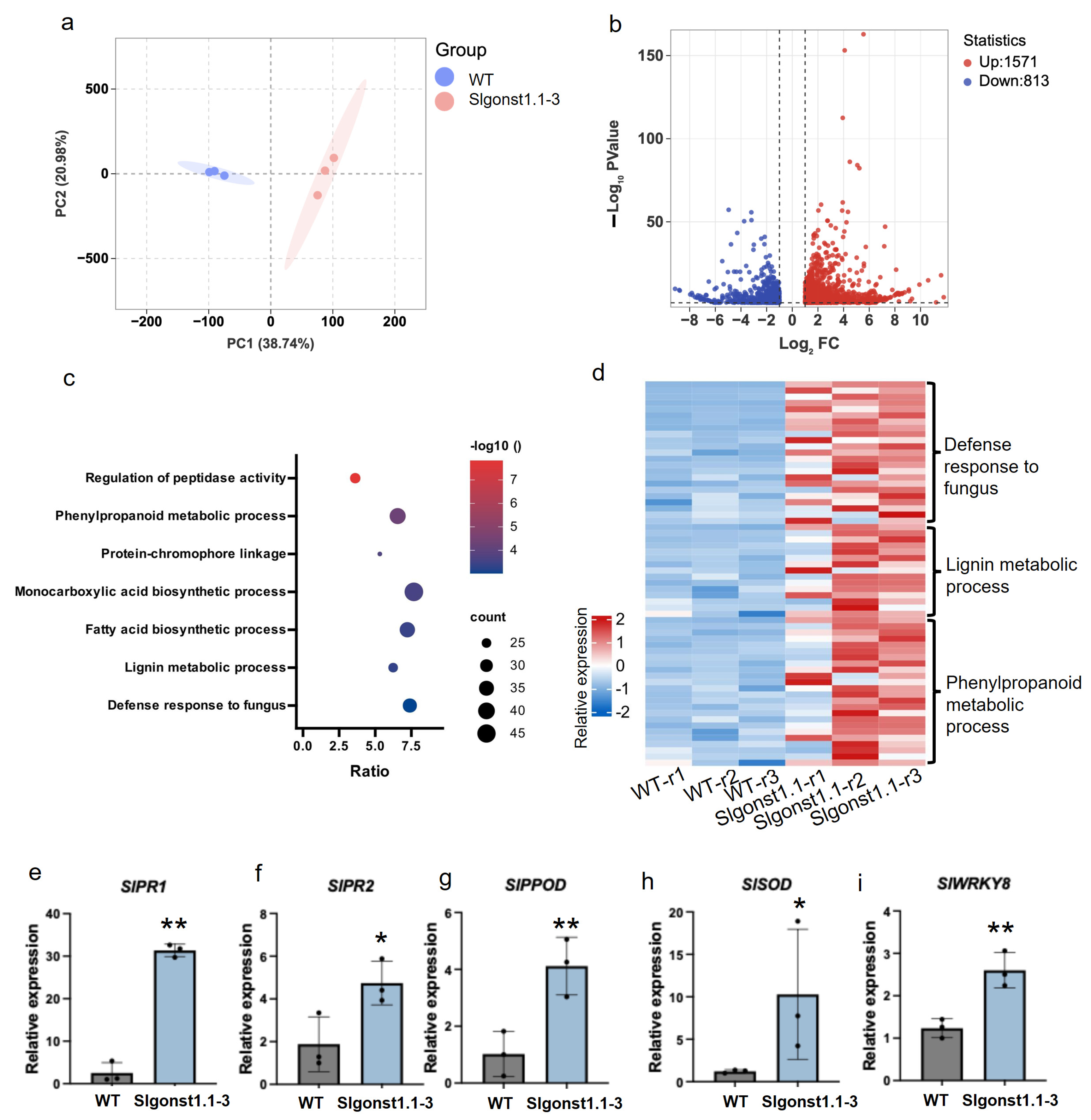
3.4. SlGONST1.1 Suppresses the Expression of Defense-Related Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.-M.; Zhou, S.-Q. Potato late blight caused by Phytophthora infestans: From molecular interactions to integrated management strategies. J. Integr. Agric. 2022, 21, 3456–3466. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Cano, L.M.; Raffaele, S.; Bain, R.A.; Cooke, L.R.; Etherington, G.J.; Deahl, K.L.; Farrer, R.A.; Gilroy, E.M.; Goss, E.M.; et al. Genome Analyses of an Aggressive and Invasive Lineage of the Irish Potato Famine Pathogen. PLoS Pathog. 2012, 8, e1002940. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, C.C.N.; Takken, F.L.W. Susceptibility Genes 101: How to Be a Good Host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef]
- Thomazella, D.P.d.T.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y.; et al. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef]
- Kieu, N.P.; Lenman, M.; Wang, E.S.; Petersen, B.L.; Andreasson, E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021, 11, 4487. [Google Scholar] [CrossRef]
- Sun, K.; Wolters, A.-M.A.; Vossen, J.H.; Rouwet, M.E.; Loonen, A.E.H.M.; Jacobsen, E.; Visser, R.G.F.; Bai, Y. Silencing of six susceptibility genes results in potato late blight resistance. Transgenic Res. 2016, 25, 731–742. [Google Scholar] [CrossRef]
- Sun, K.; Schipper, D.; Jacobsen, E.; Visser, R.G.F.; Govers, F.; Bouwmeester, K.; Bai, Y. Silencing susceptibility genes in potato hinders primary infection with Phytophthora infestans at different stages. Hortic. Res. 2022, 9, uhab058. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-B.; Park, S.-J.; Park, J.-S.; Lee, H.-J.; Shin, S.Y.; Lee, S.M.; Choi, G.J.; Kim, S.-G.; Cho, H.S.; Jeon, J.-H.; et al. Editing of StSR4 by Cas9-RNPs confers resistance to Phytophthora infestans in potato. Front. Plant Sci. 2022, 13, 997888. [Google Scholar] [CrossRef]
- Baldwin, T.C.; Handford, M.G.; Yuseff, M.-I.; Orellana, A.; Dupree, P. Identification and Characterization of GONST1, a Golgi-Localized GDP-Mannose Transporter in Arabidopsis. Plant Cell 2001, 13, 2283–2295. [Google Scholar] [CrossRef]
- Mortimer, J.C.; Yu, X.; Albrecht, S.; Sicilia, F.; Huichalaf, M.; Ampuero, D.; Michaelson, L.V.; Murphy, A.M.; Matsunaga, T.; Kurz, S.; et al. Abnormal Glycosphingolipid Mannosylation Triggers Salicylic Acid–Mediated Responses in Arabidopsis. Plant Cell 2013, 25, 1881–1894. [Google Scholar] [CrossRef]
- Fang, L.; Ishikawa, T.; Rennie, E.A.; Murawska, G.M.; Lao, J.; Yan, J.; Tsai, A.Y.-L.; Baidoo, E.E.K.; Xu, J.; Keasling, J.D.; et al. Loss of Inositol Phosphorylceramide Sphingolipid Mannosylation Induces Plant Immune Responses and Reduces Cellulose Content in Arabidopsis. Plant Cell 2016, 28, 2991–3004. [Google Scholar] [CrossRef]
- Handford, M.; Sicilia, F.; Brandizzi, F.; Chung, J.H.; Dupree, P. Arabidopsis thaliana expresses multiple Golgi-localised nucleotide-sugar transporters related to GONST1. Mol. Genet. Genom. MGG 2004, 272, 397–410. [Google Scholar] [CrossRef]
- Rautengarten, C.; Ebert, B.; Liu, L.; Stonebloom, S.; Smith-Moritz, A.M.; Pauly, M.; Orellana, A.; Vibe Scheller, H.; Heazlewood, J.L. The Arabidopsis Golgi-localized GDP-L-fucose transporter is required for plant development. Nat. Commun. 2016, 7, 12119. [Google Scholar] [CrossRef]
- Jing, B.; Ishikawa, T.; Soltis, N.; Inada, N.; Liang, Y.; Murawska, G.; Fang, L.; Andeberhan, F.; Pidatala, R.; Yu, X.; et al. The Arabidopsis thaliana nucleotide sugar transporter GONST2 is a functional homolog of GONST1. Plant Direct 2021, 5, e00309. [Google Scholar] [CrossRef]
- Sechet, J.; Htwe, S.; Urbanowicz, B.; Agyeman, A.; Feng, W.; Ishikawa, T.; Colomes, M.; Kumar, K.S.; Kawai-Yamada, M.; Dinneny, J.R.; et al. Suppression of Arabidopsis GGLT1 affects growth by reducing the L-galactose content and borate cross-linking of rhamnogalacturonan-II. Plant J. 2018, 96, 1036–1050. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Z.; Wan, W.; Qi, J.; Liu, X.-F.; Wang, Y.; Lyu, Y.; Li, T.; Dong, S.; Huang, S.; et al. Time-course dual RNA-seq analyses and gene identification during early stages of plant-Phytophthora infestans interactions. Plant Physiol. 2025, 197, kiaf112. [Google Scholar] [CrossRef]
- Van Eck, J.; Keen, P.; Tjahjadi, M. Agrobacterium tumefaciens-Mediated Transformation of Tomato. In Transgenic Plants: Methods and Protocols; Kumar, S., Barone, P., Smith, M., Eds.; Springer: New York, NY, USA, 2019; pp. 225–234. [Google Scholar]
- Sanderfoot, A.A.; Kovaleva, V.; Bassham, D.C.; Raikhel, N.V. Interactions between Syntaxins Identify at Least Five SNARE Complexes within the Golgi/Prevacuolar System of the Arabidopsis Cell. Mol. Biol. Cell 2001, 12, 3733–3743. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.-T.; Maurel, C.; Lin, J. Single-Molecule Analysis of PIP2;1 Dynamics and Partitioning Reveals Multiple Modes of Arabidopsis Plasma Membrane Aquaporin Regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef] [PubMed]
- Karki, H.; Halterman, D.A. Phytophthora infestans (Late blight) Infection Assay in a Detached Leaf of Potato. Bio Protoc. 2021, 11, e3926. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ke, J.; Huang, P.; Fatima, I.; Cheng, T.; Tang, M. Promotion of natural flowers by JcFT depends on JcLFY in the perennial woody species Jatropha curcas. Plant Sci. 2022, 318, 111236. [Google Scholar] [CrossRef]
- Smith, J.M.; Heese, A. Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods 2014, 10, 6. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Monjil, M.S.; Kato, H.; Ota, S.; Matsuda, K.; Suzuki, N.; Tenhiro, S.; Tatsumi, A.; Pring, S.; Miura, A.; Camagna, M.; et al. Two structurally different oomycete lipophilic microbe-associated molecular patterns induce distinctive plant immune responses. Plant Physiol. 2024, 196, 479–494. [Google Scholar] [CrossRef]
- Zahid, M.A.; Kieu, N.P.; Carlsen, F.M.; Lenman, M.; Konakalla, N.C.; Yang, H.; Jyakhwa, S.; Mravec, J.; Vetukuri, R.; Petersen, B.L.; et al. Enhanced stress resilience in potato by deletion of Parakletos. Nat. Commun. 2024, 15, 5224. [Google Scholar] [CrossRef]
- Liu, H.-F.; Xue, X.-J.; Yu, Y.; Xu, M.-M.; Lu, C.-C.; Meng, X.-L.; Zhang, B.-G.; Ding, X.-H.; Chu, Z.-H. Copper ions suppress abscisic acid biosynthesis to enhance defence against Phytophthora infestans in potato. Mol. Plant Pathol. 2020, 21, 636–651. [Google Scholar] [CrossRef]
- Guo, L.; Qi, Y.; Mu, Y.; Zhou, J.; Lu, W.; Tian, Z. Potato StLecRK-IV.1 negatively regulates late blight resistance by affecting the stability of a positive regulator StTET8. Hortic. Res. 2022, 9, uhac010. [Google Scholar] [CrossRef]
- Lenarčič, T.; Albert, I.; Böhm, H.; Hodnik, V.; Pirc, K.; Zavec, A.B.; Podobnik, M.; Pahovnik, D.; Žagar, E.; Pruitt, R.; et al. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 2017, 358, 1431–1434. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Cai, Y.; Wang, Y.; Wang, Z.; Lyu, Y.; Li, T.; Zhang, X.; Zhou, S. Genetic Editing of Tomato Golgi-Localized Nucleotide Sugar Transporter 1.1 Promotes Immunity Against Phytophthora infestans. Genes 2025, 16, 470. https://doi.org/10.3390/genes16040470
He P, Cai Y, Wang Y, Wang Z, Lyu Y, Li T, Zhang X, Zhou S. Genetic Editing of Tomato Golgi-Localized Nucleotide Sugar Transporter 1.1 Promotes Immunity Against Phytophthora infestans. Genes. 2025; 16(4):470. https://doi.org/10.3390/genes16040470
Chicago/Turabian StyleHe, Peize, Yanling Cai, Yanzi Wang, Zhiqing Wang, Yaqing Lyu, Tao Li, Xingtan Zhang, and Shaoqun Zhou. 2025. "Genetic Editing of Tomato Golgi-Localized Nucleotide Sugar Transporter 1.1 Promotes Immunity Against Phytophthora infestans" Genes 16, no. 4: 470. https://doi.org/10.3390/genes16040470
APA StyleHe, P., Cai, Y., Wang, Y., Wang, Z., Lyu, Y., Li, T., Zhang, X., & Zhou, S. (2025). Genetic Editing of Tomato Golgi-Localized Nucleotide Sugar Transporter 1.1 Promotes Immunity Against Phytophthora infestans. Genes, 16(4), 470. https://doi.org/10.3390/genes16040470





