RNA-Seq of Chicken Embryo Liver Reveals Transcriptional Pathways Influenced by Egg Formaldehyde Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chicken Embryo Incubation and Tissue Collection
2.3. Constructing cDNA Library and RNA Sequencing
2.4. Preprocessing, Alignment, and Differential Expression Analysis
2.5. Gene Ontology (GO) and Pathway Enrichment Analysis
2.6. Transcription Factor (TF) Analysis
2.7. Positional Mapping of DEGs to QTL Regions
2.8. Validation of RNA-Seq with qRT-PCR
3. Results
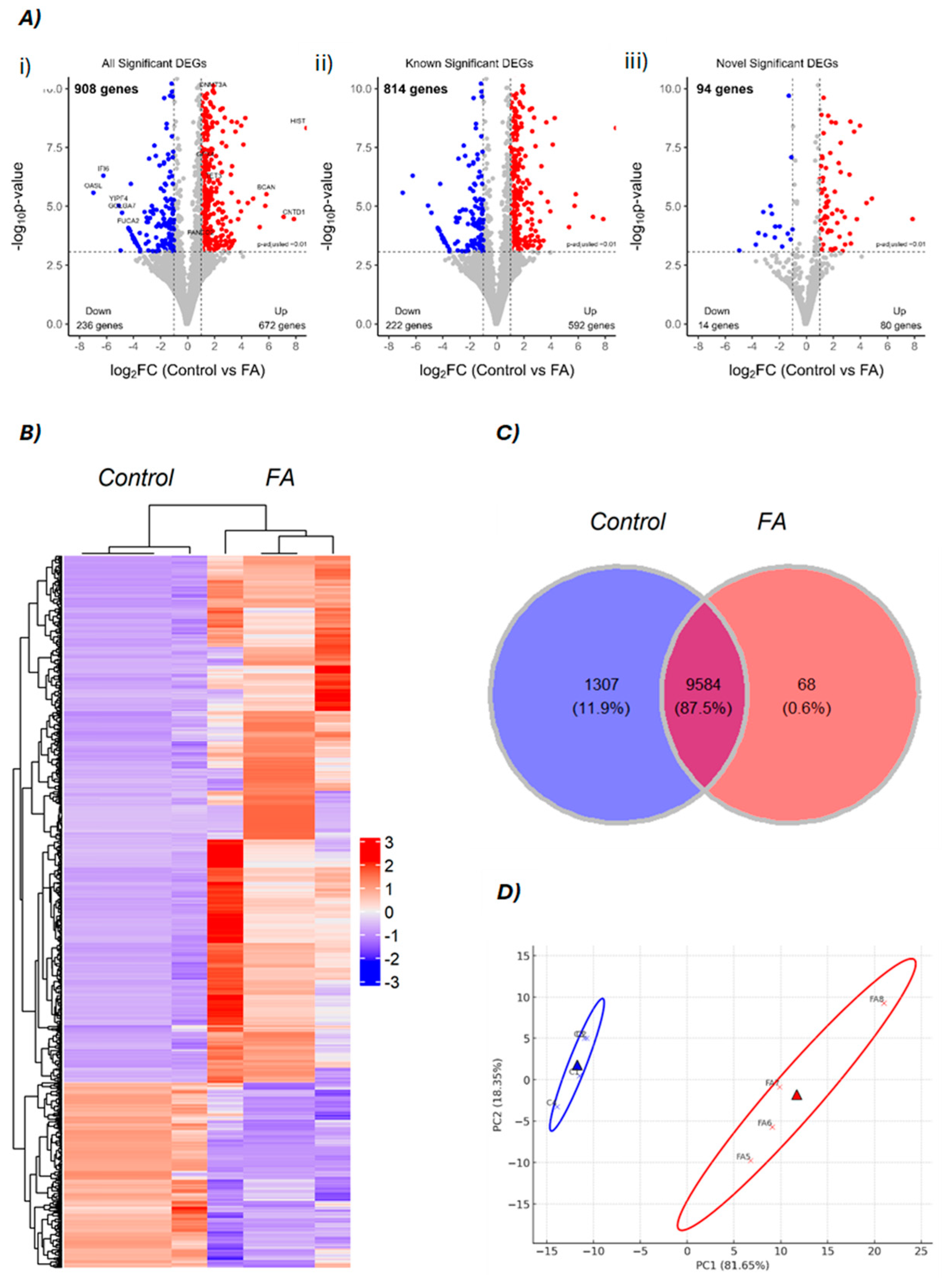
3.1. Sequencing Data and Differential Expression Analysis
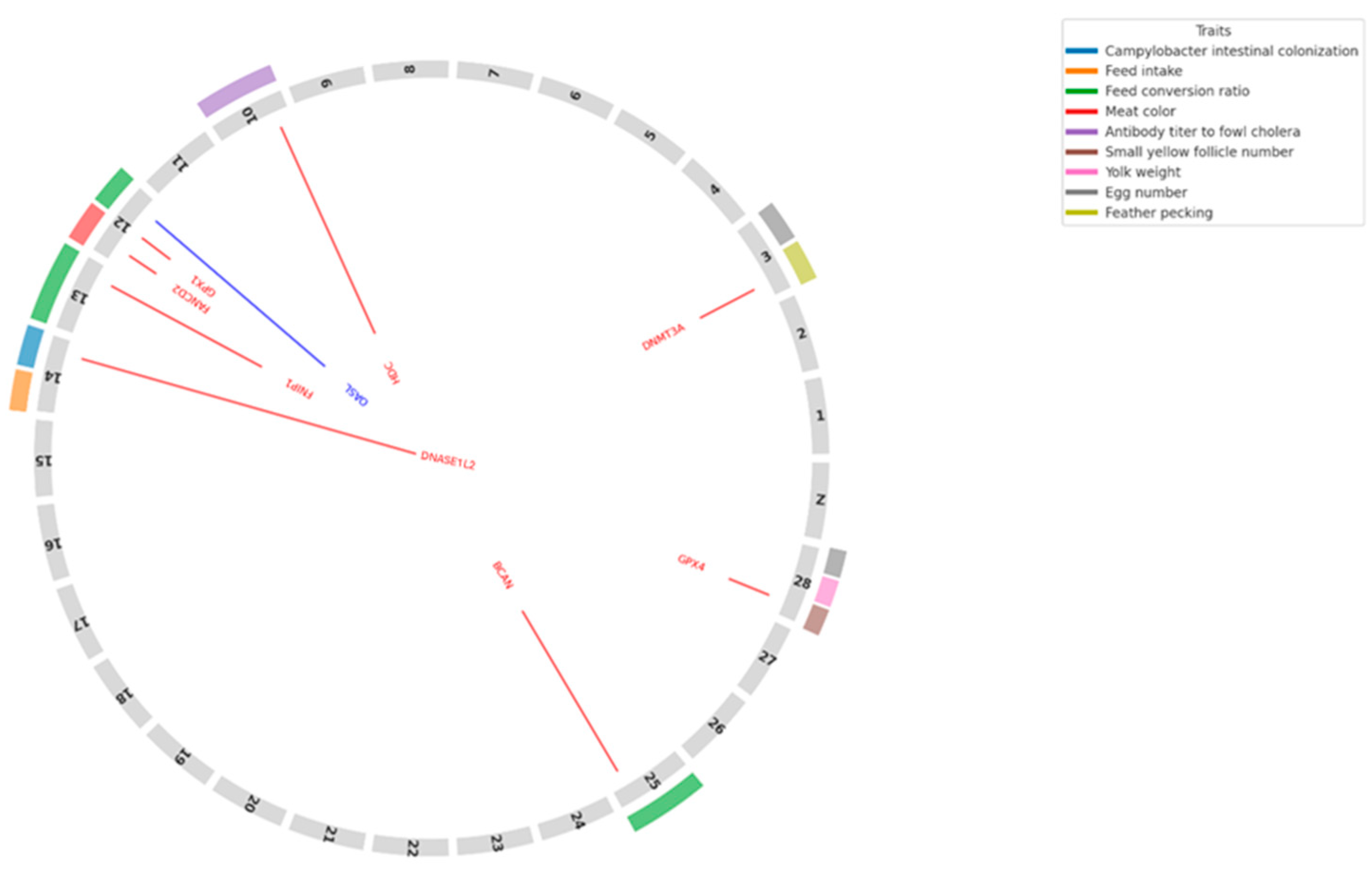
3.2. Identification of Candidate Genes in Quantitative Trait Loci (QTL) Regions
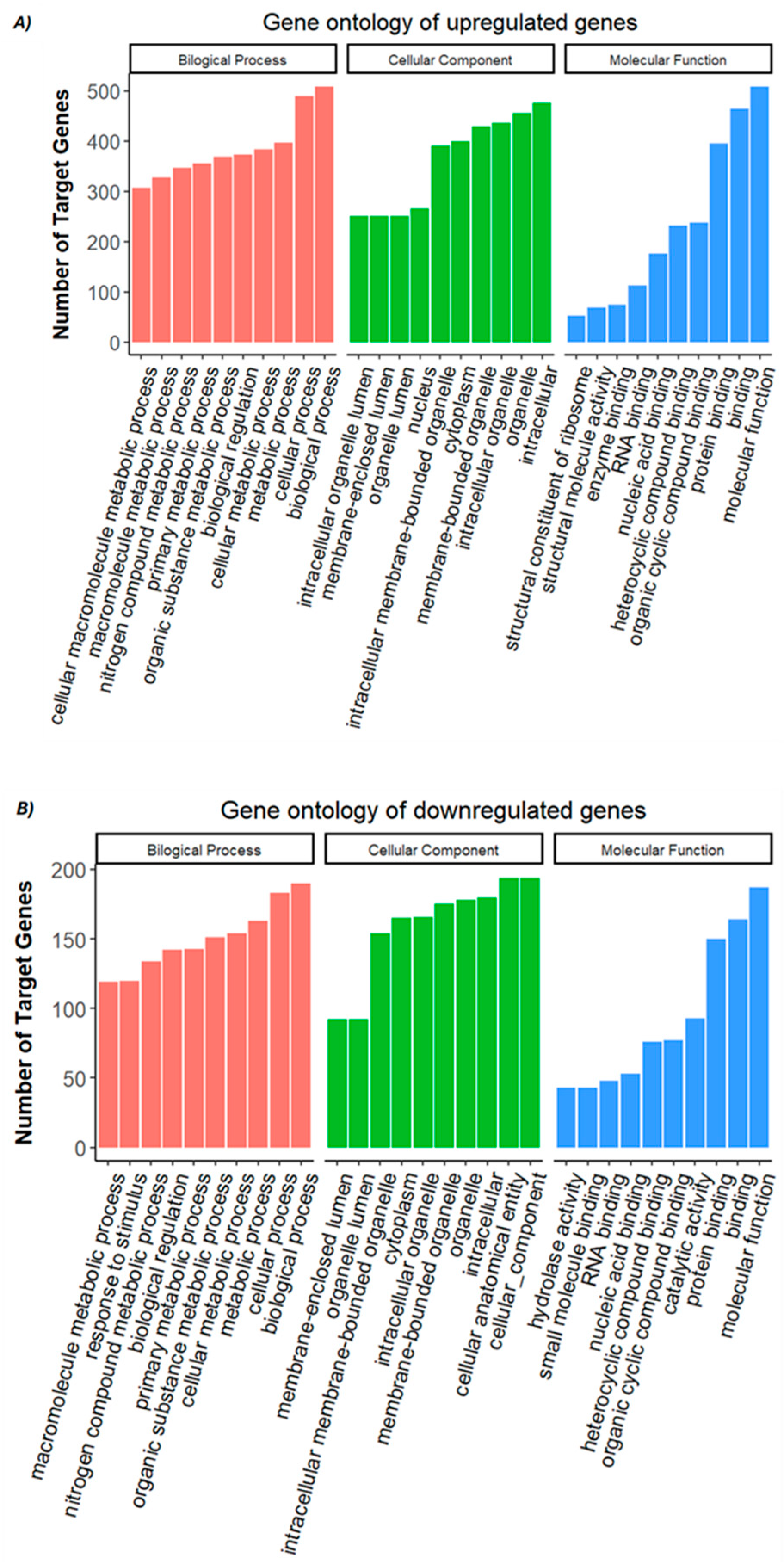
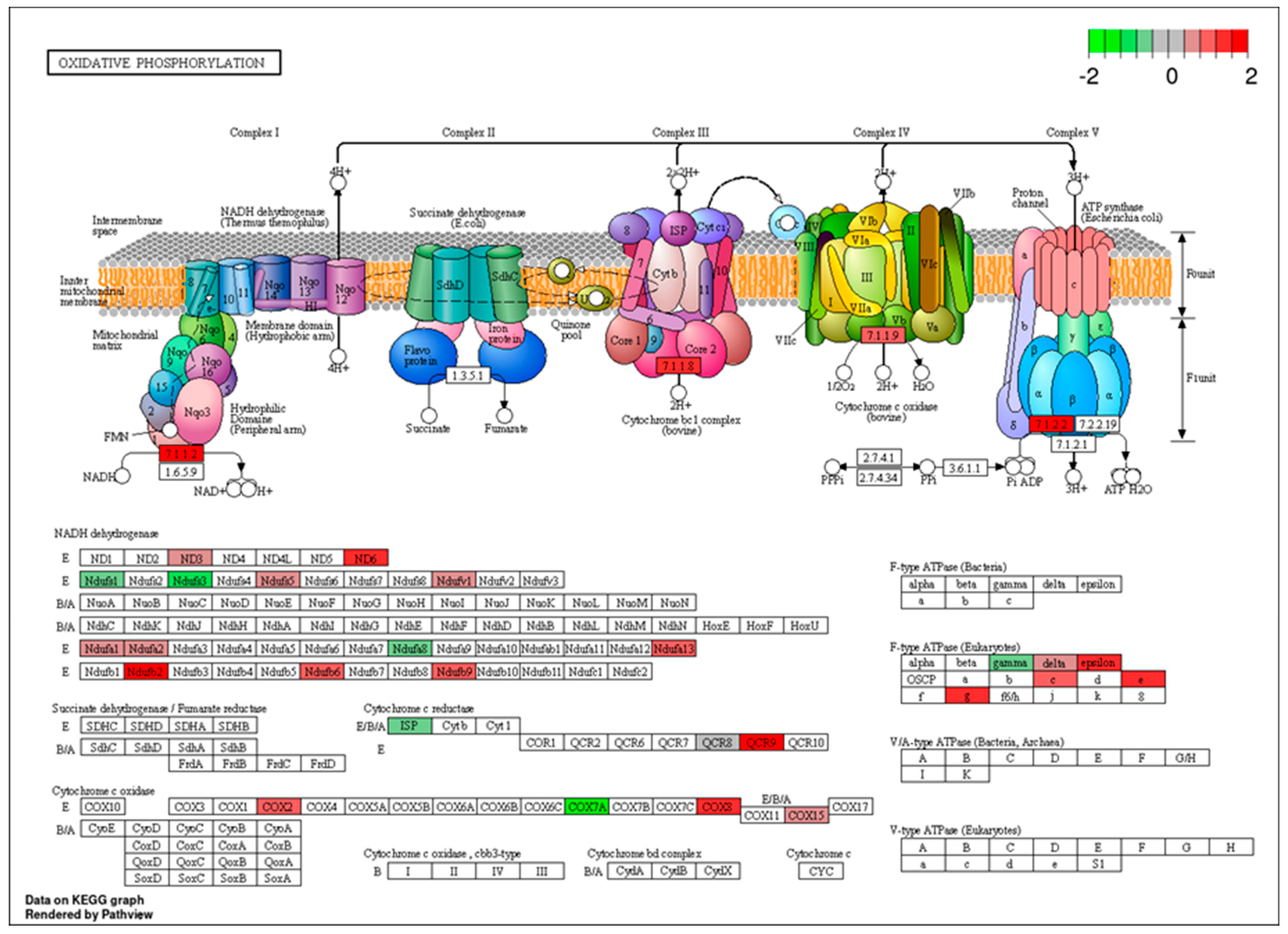
3.3. Functional Enrichment Analysis for Differential Expression Between Control vs. FA
3.4. Transcription Factor (TF) Analysis
3.5. Validation of the Results by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pontel, L.B.; Rosado, I.V.; Burgos-Barragan, G.; Garaycoechea, J.I.; Yu, R.; Arends, M.J.; Chandrasekaran, G.; Broecker, V.; Wei, W.; Liu, L.; et al. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol. Cell 2015, 60, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Pocino, R.N.; Marotta, D.; Protano, C.; Sinibaldi, F.; Simonazzi, S.; Petyx, M.; Iavicoli, S.; Vitali, M. Occupational scenarios and exposure assessment to formaldehyde: A systematic review. Indoor Air 2022, 32, e12949. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ou, J.; Huang, C.; Liu, F.; Ou, S.; Zheng, J. Chemistry of formation and elimination of formaldehyde in foods. Trends Food Sci. Technol. 2023, 139, 104134. [Google Scholar] [CrossRef]
- Cadirci, S. Disinfection of hatching eggs by formaldehyde fumigation—A review. Arch. Geflugelkunde 2009, 73, 116–123. [Google Scholar] [CrossRef]
- Avila, L.P.; Sweeney, K.M.; Schaeffer, C.; Holcombe, N.; Selby, C.; Montiel, E.; Wilson, J.L. Broiler breeder feed treatment with a formaldehyde-based sanitizer and its consequences on reproduction, feed and egg contamination, and offspring livability. J. Appl. Poult. Res. 2023, 32, 100330. [Google Scholar] [CrossRef]
- Ricke, S.C.; Richardson, K.; Dittoe, D.K. Formaldehydes in feed and their potential interaction with the poultry gastrointestinal tract microbial community—A review. Front. Vet. Sci. 2019, 6, 188. [Google Scholar] [CrossRef]
- Berrang, M.E.; Cox, N.A.; Frank, J.F.; Buhr, R.J. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: A review. J. Appl. Poult. Res. 1999, 8, 499–504. [Google Scholar] [CrossRef]
- Gole, V.C.; Chousalkar, K.K.; Roberts, J.R.; Sexton, M.; May, D.; Tan, J.; Kiermeier, A. Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella Typhimurium strains. PLoS ONE 2014, 9, e90987. [Google Scholar] [CrossRef]
- Motola, G.; Hafez, H.M.; Brüggemann-Schwarze, S. Assessment of three alternative methods for bacterial disinfection of hatching eggs in comparison with conventional approach in commercial broiler hatcheries. PLoS ONE 2023, 18, e0283699. [Google Scholar] [CrossRef]
- Graham, D.B.; Vuong, C.N.; Graham, L.E.; Tellez-Isaias, G.; Hargis, B.M. Value and limitations of formaldehyde for hatch cabinet applications: The search for alternatives. In Broiler Industry; Tellez-Isaias, G., Latorre, J.D., Martínez-Aguilar, Y., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; dos Santos, V.M. Garlic as active principle of sanitiser for hatching eggs. World’s Poult. Sci. J. 2022, 78, 1037–1052. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Lima Dallago, B.S. Spraying hatching eggs with clove essential oil does not compromise the quality of embryos and one-day-old chicks or broiler performance. Animals 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Shu, Q.; Li, Z.; Song, X.; Xu, H. Formaldehyde aggravates allergic contact dermatitis by facilitating NLRP3 inflammasome activation in macrophages. Int. Immunopharmacol. 2023, 117, 109904. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lin, J.; Li, L.; Ding, Z.; Huang, P.; Song, X.; Lou, K.; Wang, W.; Xu, H. Formaldehyde reinforces pro-inflammatory responses of macrophages through induction of glycolysis. Chemosphere 2021, 282, 131149. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Ghosal, G. Mechanisms and regulation of DNA–protein crosslink repair during DNA replication by SPRTN protease. Front. Mol. Biosci. 2022, 9, 916697. [Google Scholar] [CrossRef]
- Ruggiano, A.; Ramadan, K. DNA–protein crosslink proteases in genome stability. Commun. Biol. 2021, 4, 11. [Google Scholar] [CrossRef]
- Hwa Yun, B.; Guo, J.; Bellamri, M.; Turesky, R.J. DNA adducts: Formation, biological effects, and new biospecimens for mass spectrometric measurements in humans. Mass Spectrom. Rev. 2020, 39, 55–82. [Google Scholar] [CrossRef]
- Liu, B.; Xue, Q.; Tang, Y.; Cao, J.; Guengerich, F.P.; Zhang, H. Mechanisms of mutagenesis: DNA replication in the presence of DNA damage. Mutat. Res. Rev. Mutat. Res. 2016, 768, 53–67. [Google Scholar] [CrossRef]
- Bernardini, L.; Barbosa, E.; Charão, M.F.; Goethel, G.; Muller, D.; Bau, C.; Steffens, N.A.; Santos Stein, C.; Moresco, R.N.; Garcia, S.C.; et al. Oxidative damage, inflammation, genotoxic effect, and global DNA methylation caused by inhalation of formaldehyde and the purpose of melatonin. Toxicol. Res. 2021, 9, 778–789. [Google Scholar] [CrossRef]
- Gohil, D.; Sarker, A.H.; Roy, R. Base excision repair: Mechanisms and impact in biology, disease, and medicine. Int. J. Mol. Sci. 2023, 24, 14186. [Google Scholar] [CrossRef]
- Long, M.J.C.; Aye, Y. Formaldehyde regulates one-carbon metabolism and epigenetics. Trends Genet. 2024, 40, 381–382. [Google Scholar] [CrossRef]
- Pham, V.N.; Bruemmer, K.J.; Toh, J.D.W.; Ge, E.J.; Tenney, L.; Ward, C.C.; Dingler, F.A.; Millington, C.L.; Garcia-Prieto, C.A.; Pulos-Holmes, M.C.; et al. Formaldehyde regulates S-adenosylmethionine biosynthesis and one-carbon metabolism. Science 2023, 382, eabp9201. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.S.; Lee, N.; Shin, D.Y.; Jang, Y.J.; Lee, S.H.; Lim, K.M.; Yeon-Soon, A.; Lee, C.M.; Seo, Y.R. Network-based integrated analysis for toxic effects of high-concentration formaldehyde inhalation exposure through the toxicogenomic approach. Sci. Rep. 2022, 12, 5645. [Google Scholar] [CrossRef]
- Leso, V.; Macrini, M.C.; Russo, F.; Iavicoli, I. Formaldehyde exposure and epigenetic effects: A systematic review. Appl. Sci. 2020, 10, 2319. [Google Scholar] [CrossRef]
- Calne, R. Immunological tolerance: The liver effect. J. Gastroenterol. Hepatol. 2002, 17 (Suppl. S4), 280–282. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Physiol. Behav. 2014, 176, 139–148. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Zhang, J.; Zhao, H.; Cui, J.; Wu, J.; Shi, A. Dual effects of endogenous formaldehyde on the organism and drugs for its removal. J. Appl. Toxicol. 2024, 44, 798–817. [Google Scholar] [CrossRef]
- Teng, S.; Beard, K.; Pourahmad, J.; Moridani, M.; Easson, E.; Poon, R.; O’Brien, P.J. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem. Biol. Interact. 2001, 130–132, 285–296. [Google Scholar] [CrossRef]
- Adetuyi, B.O.; Okeowo, T.O.; Adetuyi, O.A.; Abraham Adebisi, O.; Ogunlana, O.O.; Janet Oretade, O.; Marraiki, N.; Beshbishy, A.M.; N Welson, N.; Batiha, G.E. Ganoderma lucidum from red mushroom attenuates formaldehyde-induced liver damage in experimental male rat model. Biology 2020, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.M.; Beer, L.C.; Forga, A.J.; Coles, M.E.; Graham, L.E.; Teague, K.D.; Tellez-Isaias, G.; Hargis, B.M.; Vuong, C.N.; Graham, B.D. Evaluation of the impact of formaldehyde fumigation during the hatching phase on contamination in the hatch cabinet and early performance in broiler chickens. Poult. Sci. 2023, 102, 102584. [Google Scholar] [CrossRef]
- Coulibaly, F.; Onbaşılar, E.E.; Bakır, B.; Sarıçam İnce, S. The effects of using UV light instead of formaldehyde in disinfection of hatching eggs on shell microbial load, embryo development, hatchability, and chick characteristics. Int. J. Environ. Health Res. 2024, 34, 2852–2862. [Google Scholar] [CrossRef]
- Taşdemir, A.N.; Onbaşılar, E.E.; Yalçın, S.; Boyalı, B.; Aygören, H.; Tülek, E.; Sarıçam, S.; Akan, M. Effects of oregano juice on eggshell microbial load, layer embryo development, hatching results, and growth at the first 2 weeks after hatch. Trop. Anim. Health Prod. 2021, 53, 404. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2022, 50, D956–D961. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Shah, T.M.; Patel, N.V.; Patel, A.B.; Upadhyay, M.R.; Mohapatra, A.; Singh, K.M.; Deshpande, S.D.; Joshi, C.G. A genome-wide approach to screen for genetic variants in broilers (Gallus gallus) with divergent feed conversion ratio. Mol. Genet. Genom. 2016, 291, 1715–1725. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Z.T.; Zheng, R.; Diao, S.; Teng, J.; Yuan, X.; Zhang, H.; Chen, Z.; Zhang, X.; Li, J.; et al. New insights from imputed whole-genome sequence-based genome-wide association analysis and transcriptome analysis: The genetic mechanisms underlying residual feed intake in chickens. Front. Genet. 2020, 11, 243. [Google Scholar] [CrossRef]
- Jie, Y.; Wen, C.; Huang, Q.; Gu, S.; Sun, C.; Li, G.; Yan, Y.; Wu, G.; Yang, N. Distinct patterns of feed intake and their association with growth performance in broilers. Poult. Sci. 2024, 103, 103974. [Google Scholar] [CrossRef]
- Li, Y.; Ma, R.; Qi, R.; Li, H.; Li, J.; Liu, W.; Wan, Y.; Li, S.; Sun, Z.; Xu, J.; et al. Novel insight into the feed conversion ratio in laying hens and construction of its prediction model. Poult. Sci. 2024, 103, 104013. [Google Scholar] [CrossRef]
- Fontaine, J.-M.; Rest, J.S.; Welsh, M.J.; Benndorf, R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones 2003, 8, 62–69. [Google Scholar] [CrossRef]
- Adam-Vizi, V.; Tretter, L. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochem. Int. 2013, 62, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Gui, Z.; Yuan, Y.; Li, Y.; Du, D. Expression analysis of the extensive regulation of mitogen-activated protein kinase (MAPK) family genes in buckwheat (Fagopyrum tataricum) during organ differentiation and stress response. Agronomy 2024, 14, 2613. [Google Scholar] [CrossRef]
- Delfino, D.; Mori, G.; Rivetti, C.; Grigoletto, A.; Bizzotto, G.; Cavozzi, C.; Malatesta, M.; Cavazzini, D.; Pasut, G.; Percudani, R. Actin-Resistant DNase1L2 as a Potential Therapeutics for CF Lung Disease. Biomolecules 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Buchberger, M.; Napirei, M.; Tschachler, E.; Eckhart, L. Inactivation of DNase1L2 and DNase2 in keratinocytes suppresses DNA degradation during epidermal cornification and results in constitutive parakeratosis. Sci. Rep. 2017, 7, 6433. [Google Scholar] [CrossRef]
- Barcik, W.; Pugin, B.; Brescó, M.S.; Westermann, P.; Rinaldi, A.; Groeger, D.; Van Elst, D.; Sokolowska, M.; Krawczyk, K.; Frei, R.; et al. Bacterial secretion of histamine within the gut influences immune responses within the lung. Allergy 2019, 74, 899–909. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Ghosh, A.; Cuevas, R.A.; Forero, A.; Dhar, J.; Ibsen, M.S.; Schmid-Burgk, J.L.; Schmidt, T.; Ganapathiraju, M.K.; et al. Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor. Immunity 2014, 40, 936–948. [Google Scholar] [CrossRef]
- Ranaware, P.B.; Mishra, A.; Vijayakumar, P.; Gandhale, P.N.; Kumar, H.; Kulkarni, D.D.; Raut, A.A. Genome-wide host gene expression analysis in chicken lungs infected with avian influenza viruses. PLoS ONE 2016, 11, e0153671. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Jang, H.J.; Monson, M.S.; Lamont, S.J. Role of the chicken oligoadenylate synthase-like gene during in vitro Newcastle disease virus infection. Poult. Sci. 2021, 100, 101067. [Google Scholar] [CrossRef]
- Park, J.-W.; Ndimukaga, M.; So, J.; So, J.; Kim, S.; Truong, A.D.; Tran, H.T.T.; Dang, H.V.; Song, K.D. Molecular analysis of chicken interferon-alpha inducible protein 6 gene and transcriptional regulation. J. Anim. Sci. Technol. 2023, 65, 183–196. [Google Scholar] [CrossRef]
- Wan, L.; Wang, S.; Xie, Z.; Ren, H.; Xie, L.; Luo, S.; Li, M.; Xie, Z.; Fan, Q.; Zeng, T.; et al. Chicken IFI6 inhibits avian reovirus replication and affects related innate immune signaling pathways. Front. Microbiol. 2023, 14, 1237438. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Liu, Y.; Zhang, H.; Ou, C.; Zhang, Y.; Liu, T.; Wang, Q.; Ma, J. Effects of infectious bursal disease virus infection on interferon and antiviral gene expression in layer chicken bursa. Microb. Pathog. 2020, 144, 104182. [Google Scholar] [CrossRef] [PubMed]
- Visinoni, S.; Khalid, N.F.I.; Joannides, C.N.; Shulkes, A.; Yim, M.; Whitehead, J.; Tiganis, T.; Lamont, B.J.; Favaloro, J.M.; Proietto, J.; et al. The role of liver fructose-1,6-bisphosphatase in regulating appetite and adiposity. Diabetes 2012, 61, 1122–1132. [Google Scholar] [CrossRef]
- Fam, B.C.; Joannides, C.N.; Andrikopoulos, S. The liver: Key in regulating appetite and body weight. Adipocyte 2012, 1, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Reingruber, H.; Pontel, L.B. Formaldehyde metabolism and its impact on human health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, F.; Yang, Z.; Zhang, Y.; Guo, D.; Zhang, Q. Formaldehyde induced the cardiac damage by regulating the NO/cGMP signaling pathway and L-Ca2+ channels. Toxicol. Res. 2023, 12, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, G.; Bellisario, V.; Ghelli, F.; Bono, R. Occupational exposure to formaldehyde and oxidative stress in Italian workers. Eur. J. Public Health 2021, 31 (Suppl. S3), ckab165.649. [Google Scholar] [CrossRef]
- Kövesi, B.; Cserháti, M.; Erdélyi, M.; Zándoki, E.; Mézes, M.; Balogh, K. Long-term effects of ochratoxin A on the glutathione redox system and its regulation in chicken. Antioxidants 2019, 8, 178. [Google Scholar] [CrossRef]
- Ihsan, A.U.; Khan, F.U.; Khongorzul, P.; Ahmad, K.A.; Naveed, M.; Yasmeen, S.; Cao, Y.; Taleb, A.; Maiti, R.; Akhter, F.; et al. Role of oxidative stress in pathology of chronic prostatitis/chronic pelvic pain syndrome and male infertility and antioxidants function in ameliorating oxidative stress. Biomed. Pharmacother. 2018, 106, 714–723. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.B.; Park, J.K.; Yoo, Y.D. TNF-α-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-XL. Cell Death Differ. 2010, 17, 1420–1434. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive oxygen species induce COX-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 2015, 5, 492–501. [Google Scholar] [CrossRef]
- Alcón, P.; Kaczmarczyk, A.P.; Ray, K.K.; Liolios, T.; Guilbaud, G.; Sijacki, T.; Shen, Y.; McLaughlin, S.H.; Sale, J.E.; Knipscheer, P.; et al. FANCD2–FANCI surveys DNA and recognizes double- to single-stranded junctions. Nature 2024, 632, 1165–1173. [Google Scholar] [CrossRef]
- Speit, G.; Schmid, O. Local genotoxic effects of formaldehyde in humans measured by the micronucleus test with exfoliated epithelial cells. Mutat. Res. Rev. Mutat. Res. 2006, 613, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Spinieli, R.L.; Cazuza, R.; Sales, A.J.; Carolino, R.; Franci, J.A.; Tajerian, M.; Leite-Panissi, C.R.A. Acute restraint stress regulates brain DNMT3a and promotes defensive behaviors in male rats. Neurosci. Lett. 2024, 820, 137589. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.; Xu, L.; Xiao, R.; Lu, X.; Chen, L.; Chong, J.; Li, H.; He, C.; Fu, X.D.; et al. Molecular basis for 5-carboxycytosine recognition by RNA polymerase II elongation complex. Nature 2015, 523, 621–625. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yang, M.; Li, S.; Zhang, G.; Ding, Z.; Zhang, L.; Shi, G.; Li, Y. Mechanisms and biotechnological applications of transcription factors. Synth. Syst. Biotechnol. 2023, 8, 565–577. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kim, H.-J.; Kim, E.-J.; Kim, K.-S.; Hong, S.; Park, H.-G.; Lee, M.-O. RORα decreases oxidative stress through the induction of SOD2 and GPx1 expression and thereby protects against nonalcoholic steatohepatitis in mice. Antioxid. Redox Signal. 2014, 21, 2083–2094. [Google Scholar] [CrossRef]
- Li, S.; Yang, Q.; Zhou, Z.; Fu, M.; Yang, X.; Hao, K.; Liu, Y. SNHG3 cooperates with ELAVL2 to modulate cell apoptosis and extracellular matrix accumulation by stabilizing SNAI2 in human trabecular meshwork cells under oxidative stress. Environ. Toxicol. 2021, 36, 1070–1079. [Google Scholar] [CrossRef]
- Miao, W.; Chen, M.; Chen, M.; Cui, C.; Zhu, Y.; Luo, X.; Wu, B. Nr2f2 overexpression aggravates ferroptosis and mitochondrial dysfunction by regulating the PGC-1α signaling in diabetes-induced heart failure mice. Mediat. Inflamm. 2022, 2022, 8373389. [Google Scholar] [CrossRef]
- Castillo, D.S.; Campalans, A.; Belluscio, L.M.; Carcagno, A.L.; Radicella, J.P.; Cánepa, E.T.; Pregi, N. E2F1 and E2F2 induction in response to DNA damage preserves genomic stability in neuronal cells. Cell Cycle 2015, 14, 1300–1314. [Google Scholar] [CrossRef]
- Dunnick, J.K.; Merrick, B.A.; Brix, A.; Morgan, D.L.; Gerrish, K.; Wang, Y.; Flake, G.; Foley, J.; Shockley, K.R. Molecular changes in the nasal cavity after N,N-dimethyl-p-toluidine exposure. Toxicol. Pathol. 2016, 44, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Pulliero, A. The effects of environmental chemical carcinogens on the microRNA machinery. Int. J. Hyg. Environ. Health 2014, 217, 601–627. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Bai, Y.; Chen, Z.; Li, Y.; Luo, L.; Huang, J.; Yang, J.; Liao, H.; Guo, L. Downregulation of HOXA1 gene affects small cell lung cancer cell survival and chemoresistance under the regulation of miR-100. Eur. J. Cancer 2014, 50, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Wang, K.; Li, Q.; Yan, S.; Shi, H.; Liu, L.; Liang, S.; Yang, M.; Su, Z.; et al. RNA-seq reveals pathways responsible for meat quality characteristic differences between two Yunnan indigenous chicken breeds and commercial broilers. Foods 2024, 13, 2008. [Google Scholar] [CrossRef]
- Rehman, M.S.; Rehman, S.-U.; Yousaf, W.; Hassan, F.U.; Ahmad, W.; Liu, Q.; Pan, H. The potential of Toll-like receptors to modulate avian immune system: Exploring the effects of genetic variants and phytonutrients. Front. Genet. 2021, 12, 671235. [Google Scholar] [CrossRef]
- Jagadeesan, K.; Kumar, S.; Rahim, A.; Yadav, A.S.; Das, A.K. Investigating whether microsatellites could influence the relative expression level of TLR4 gene in bursa of Rhode Island Red chicken. Indian J. Anim. Sci. 2022, 92, 1020–1023. [Google Scholar] [CrossRef]
- Volkova, N.A.; Romanov, M.N.; Vetokh, A.N.; Larionova, P.V.; Volkova, L.A.; Abdelmanova, A.S.; Sermyagin, A.A.; Griffin, D.K.; Zinovieva, N.A. Genome-wide association study reveals the genetic architecture of growth and meat production traits in a chicken F2 resource population. Genes 2024, 15, 1246. [Google Scholar] [CrossRef]
- Kang, H.; Zhao, D.; Xiang, H.; Li, J.; Zhao, G.; Li, H. Large-scale transcriptome sequencing in broiler chickens to identify candidate genes for breast muscle weight and intramuscular fat content. Genet. Sel. Evol. 2021, 53, 66. [Google Scholar] [CrossRef]
- Vaccaro, L.A.; Porter, T.E.; Ellestad, L.E. The effect of commercial genetic selection on somatotropic gene expression in broilers: A potential role for insulin-like growth factor binding proteins in regulating broiler growth and body composition. Front. Physiol. 2022, 13, 935311. [Google Scholar] [CrossRef]
- Vinotha, V.; Baskaralingam, V. Significance of vitellogenin in egg yolk production and egg quality. In Vitellogenin in Fishes—Diversification, Biological Properties, and Future Perspectives; Baskaralingam, V., Vanichviriyakit, R., Eds.; Springer: Singapore, 2023; pp. 197–201. [Google Scholar] [CrossRef]
- Lutz, V.; Stratz, P.; Preuß, S.; Tetens, J.; Grashorn, M.A.; Bessei, W.; Bennewitz, J. A genome-wide association study in a large F2-cross of laying hens reveals novel genomic regions associated with feather pecking and aggressive pecking behavior. Genet. Sel. Evol. 2017, 49, 18. [Google Scholar] [CrossRef]
- Zhao, X.; Nie, C.; Zhang, J.; Li, X.; Zhu, T.; Guan, Z.; Chen, Y.; Wang, L.; Lv, X.Z.; Yang, W.; et al. Identification of candidate genomic regions for chicken egg number traits based on genome-wide association study. BMC Genomics. 2021, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Psifidi, A.; Banos, G.; Matika, O.; Desta, T.T.; Bettridge, J.; Hume, D.A.; Dessie, T.; Christley, R.; Wigley, P.; Hanotte, O.; et al. Genome-wide association studies of immune, disease and production traits in indigenous chicken ecotypes. Genet. Sel. Evol. 2016, 48, 74. [Google Scholar] [CrossRef] [PubMed]
- Pampouille, E.; Berri, C.; Boitard, S.; Hennequet-Antier, C.; Beauclercq, S.A.; Godet, E.; Praud, C.; Jégo, Y.; Le Bihan-Duval, E. Mapping QTL for white striping in relation to breast muscle yield and meat quality traits in broiler chickens. BMC Genomics. 2018, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Sun, H.; Qu, L.; Ma, M.; Dou, T.; Lu, J.; Guo, J.; Hu, Y.; Wang, X.; Li, Y.; et al. Genetic architecture and candidate genes identified for follicle number in chicken. Sci. Rep. 2017, 7, 16412. [Google Scholar] [CrossRef]
- Sun, C.; Lu, J.; Yi, G.; Yuan, J.; Duan, Z.; Qu, L.; Xu, G.; Wang, K.; Yang, N. Promising loci and genes for yolk and ovary weight in chickens revealed by a genome-wide association study. PLoS ONE. 2015, 10, e0137145. [Google Scholar] [CrossRef][Green Version]
- Tarsani, E.; Kranis, A.; Maniatis, G.; Avendano, S.; Hager-Theodorides, A.L.; Kominakis, A. Deciphering the mode of action and position of genetic variants impacting on egg number in broiler breeders. BMC Genomics. 2020, 21, 512. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, M.; Sajid, G.A.; Büyükkılıç Beyzi, S.; Kızılaslan, M.; Arzık, Y.; Yalçın, S.; White, S.N.; Cinar, M.U. RNA-Seq of Chicken Embryo Liver Reveals Transcriptional Pathways Influenced by Egg Formaldehyde Treatment. Genes 2025, 16, 471. https://doi.org/10.3390/genes16050471
Özdemir M, Sajid GA, Büyükkılıç Beyzi S, Kızılaslan M, Arzık Y, Yalçın S, White SN, Cinar MU. RNA-Seq of Chicken Embryo Liver Reveals Transcriptional Pathways Influenced by Egg Formaldehyde Treatment. Genes. 2025; 16(5):471. https://doi.org/10.3390/genes16050471
Chicago/Turabian StyleÖzdemir, Mustafa, Ghulam Asghar Sajid, Selma Büyükkılıç Beyzi, Mehmet Kızılaslan, Yunus Arzık, Servet Yalçın, Stephen N. White, and Mehmet Ulas Cinar. 2025. "RNA-Seq of Chicken Embryo Liver Reveals Transcriptional Pathways Influenced by Egg Formaldehyde Treatment" Genes 16, no. 5: 471. https://doi.org/10.3390/genes16050471
APA StyleÖzdemir, M., Sajid, G. A., Büyükkılıç Beyzi, S., Kızılaslan, M., Arzık, Y., Yalçın, S., White, S. N., & Cinar, M. U. (2025). RNA-Seq of Chicken Embryo Liver Reveals Transcriptional Pathways Influenced by Egg Formaldehyde Treatment. Genes, 16(5), 471. https://doi.org/10.3390/genes16050471






