Analysis of Kallikrein 6, Acetyl-α-Tubulin, and Aquaporin 1 and 2 Expression Patterns During Normal Human Nephrogenesis and in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT)
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement and Processing of Tissues
2.2. Immunofluorescence Staining
2.3. Data Acquisition
2.4. Image Analysis of the Area Percentage
2.5. Statistical Analysis of the Area Percentage
3. Results
3.1. Normal Human Kidney Development
3.1.1. Double Immunofluorescence Staining with Kallikrein 6 (KLK6) and Acetyl-α-Tubulin During Normal Human Kidney Development
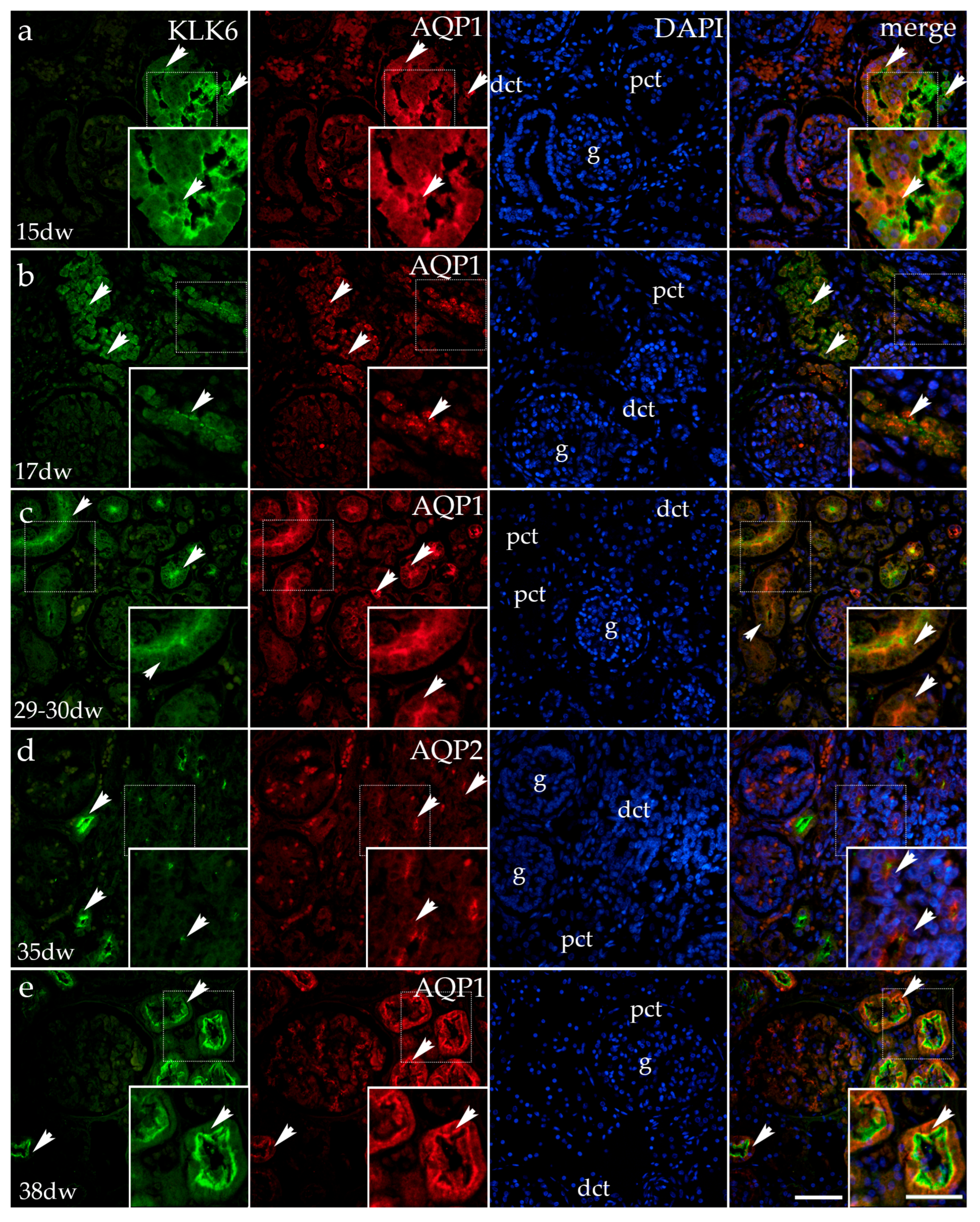
3.1.2. Double Immunofluorescence Staining of KLK6, Aquaporin 1 (AQP1), and Aquaporin 2 (AQP2) in Developing Normal Human Kidneys
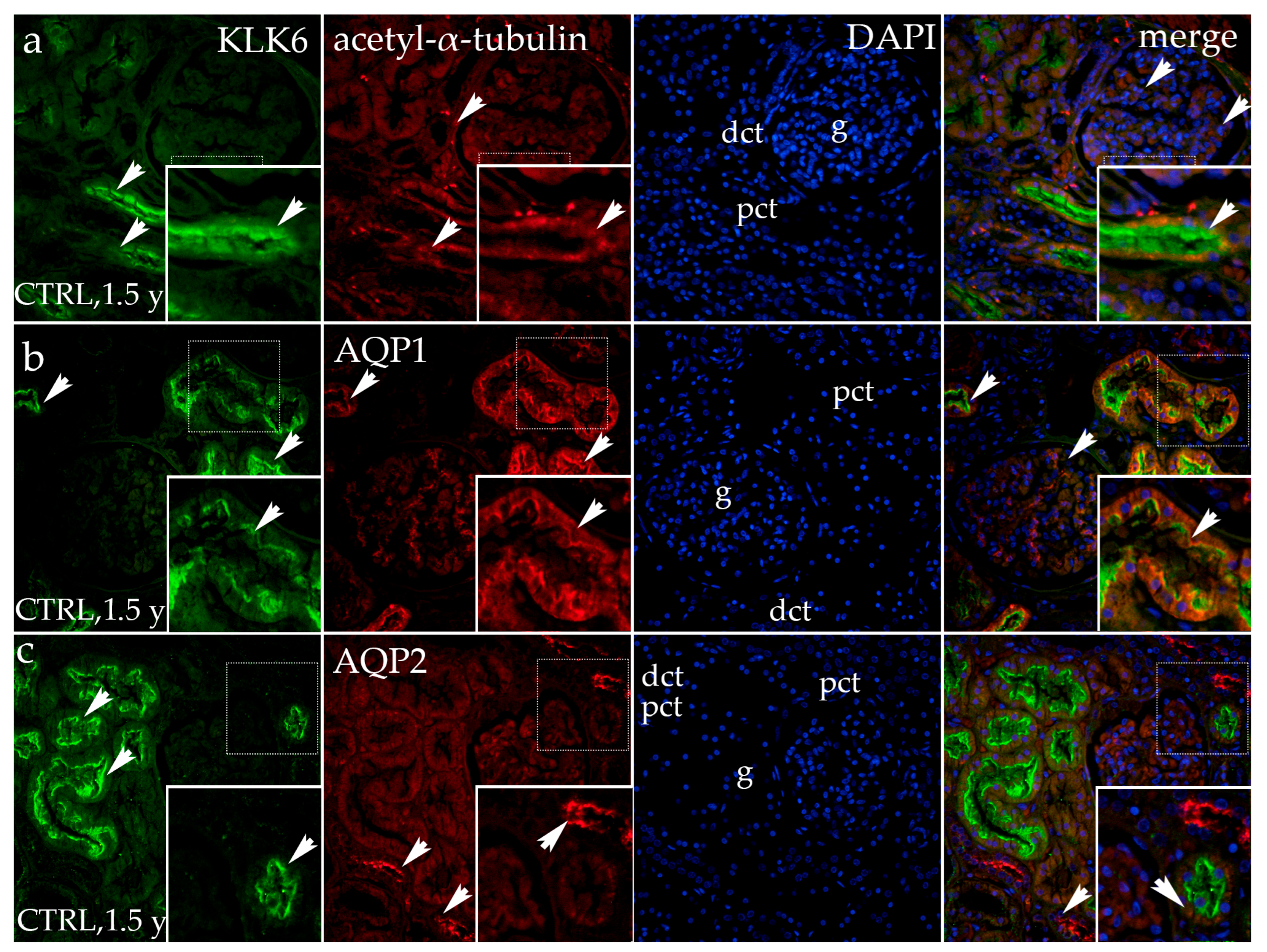
3.1.3. Double Fluorescence Staining of KLK6, Acetyl-α-Tubulin, AQP1, and AQP2 in Postnatal Normal Human Kidneys
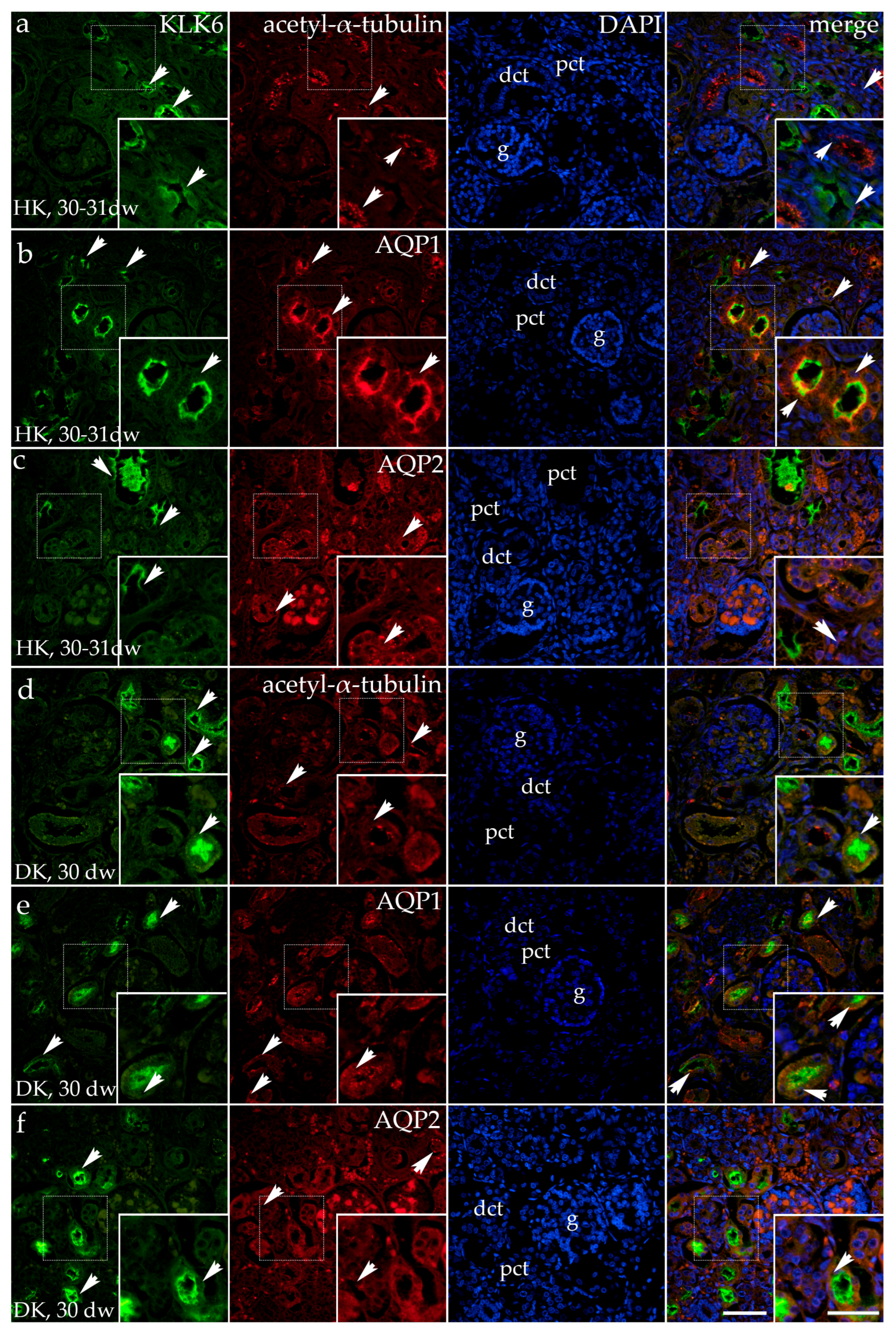
3.2. Double Fluorescence Staining of KLK6 with Acetyl-α-Tubulin, AQP1, and AQP2 Antibodies in the Horseshoe and Duplex Human Kidneys
3.3. Dysplastic Kidneys
Double Fluorescence Staining of KLK6, Acetyl-α-Tubulin, and AQP1 in the Dysplastic and Hypoplastic Human Fetal Kidneys
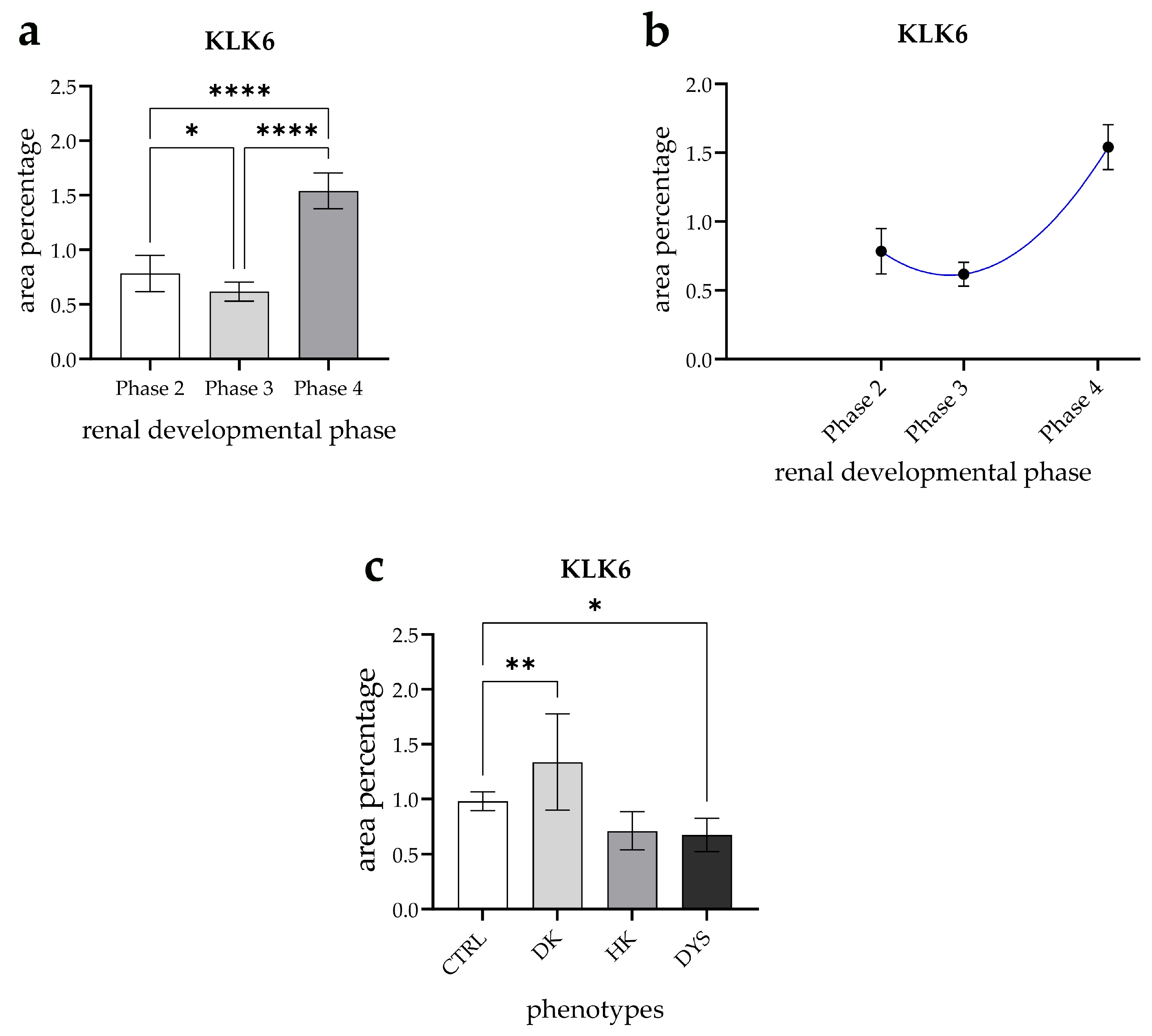
3.4. Expression Dynamics of KLK6 in Normal Fetal Kidney Tissues and Comparison of the Area Percentage of KLK6-Positive Cells Between Control and Kidneys Affected with Congenital Anomalies of the Kidney and Urinary Tract (CAKUT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barasch, J.; Qiao, J.; McWilliams, G.; Chen, D.; Oliver, J.A.; Herzlinger, D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am. J. Physiol.-Ren. Physiol. 1997, 273, F757–F767. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel Rad, N.; Aghdami, N.; Moghadasali, R. Cellular and Molecular Mechanisms of Kidney Development: From the Embryo to the Kidney Organoid. Front. Cell Dev. Biol. 2020, 8, 183. [Google Scholar] [CrossRef]
- Nagalakshmi, V.K.; Yu, J. The ureteric bud epithelium: Morphogenesis and roles in metanephric kidney patterning. Mol. Reprod. Dev. 2015, 82, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.C.; Pazour, G.J.; Lo, C.W. Congenital Heart Defects and Ciliopathies Associated With Renal Phenotypes. Front. Pediatr. 2018, 6, 175. [Google Scholar] [CrossRef]
- Pala, R.; Alomari, N.; Nauli, S.M. Primary Cilium-Dependent Signaling Mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Rao Damerla, R.; Gabriel, G.C.; Li, Y.; Klena, N.T.; Liu, X.; Chen, Y.; Cui, C.; Pazour, G.J.; Lo, C.W. Role of cilia in structural birth defects: Insights from ciliopathy mutant mouse models. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 115–125. [Google Scholar] [CrossRef]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Riedhammer, K.M.; Ćomić, J.; Tasic, V.; Putnik, J.; Abazi-Emini, N.; Paripovic, A.; Stajic, N.; Meitinger, T.; Nushi-Stavileci, V.; Berutti, R.; et al. Exome sequencing in individuals with congenital anomalies of the kidney and urinary tract (CAKUT): A single-center experience. Eur. J. Hum. Genet. 2023, 31, 674–680. [Google Scholar] [CrossRef]
- Mahmoud, A.H.; Talaat, I.M.; Tlili, A.; Hamoudi, R. Congenital anomalies of the kidney and urinary tract. Front. Med. 2024, 11, 1384676. [Google Scholar] [CrossRef]
- Kaijansinkko, H.; Bonthuis, M.; Jahnukainen, K.; Harambat, J.; Vidal, E.; Bakkaloglu, S.A.; Inward, C.; Sinha, M.D.; Roperto, R.M.; Kuehni, C.E.; et al. Clinical outcomes of pediatric kidney replacement therapy after childhood cancer—An ESPN/ERA Registry study. Am. J. Transplant. 2025, 25, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Uy, N.; Reidy, K. Developmental Genetics and Congenital Anomalies of the Kidney and Urinary Tract. J. Pediatr. Genet. 2016, 5, 51–60. [Google Scholar] [CrossRef]
- Mohamed, T.H.; Slaughter, J.L. Congenital Anomalies of the Kidney and Urinary Tract in Preterm Infants—Active Drivers of Increased Disease or Associated Bystanders? JAMA Netw. Open 2022, 5, e2234474. [Google Scholar] [CrossRef] [PubMed]
- Bayani, J.; Diamandis, E.P. The physiology and pathobiology of human kallikrein-related peptidase 6 (KLK6). Clin. Chem. Lab. Med. 2011, 50, 211–233. [Google Scholar] [CrossRef]
- Anisowicz, A.; Sotiropoulou, G.; Stenman, G.; Mok, S.C.; Sager, R. A novel protease homolog differentially expressed in breast and ovarian cancer. Mol. Med. 1996, 2, 624–636. [Google Scholar] [CrossRef]
- Harvey, T.J.; Hooper, J.D.; Myers, S.A.; Stephenson, S.A.; Ashworth, L.K.; Clements, J.A. Tissue-specific expression patterns and fine mapping of the human kallikrein (KLK) locus on proximal 19q13.4. J. Biol. Chem. 2000, 275, 37397–37406. [Google Scholar] [CrossRef] [PubMed]
- Little, S.P.; Dixon, E.P.; Norris, F.; Buckley, W.; Becker, G.W.; Johnson, M.; Dobbins, J.R.; Wyrick, T.; Miller, J.R.; MacKellar, W.; et al. Zyme, a novel and potentially amyloidogenic enzyme cDNA isolated from Alzheimer’s disease brain. J. Biol. Chem. 1997, 272, 25135–25142. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Goettig, P.; Preis, S.; Felber, J.; Bronger, H.; Clements, J.A.; Dorn, J.; Magdolen, V. Kallikrein-related peptidases represent attractive therapeutic targets for ovarian cancer. Expert Opin. Ther. Targets 2018, 22, 745–763. [Google Scholar] [CrossRef]
- Lenga Ma Bonda, W.; Iochmann, S.; Magnen, M.; Courty, Y.; Reverdiau, P. Kallikrein-related peptidases in lung diseases. Biol. Chem. 2018, 399, 959–971. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Diamandis, E.P.; Hollenberg, M.D. Kallikrein-related peptidases: Proteolysis and signaling in cancer, the new frontier. Biol. Chem. 2010, 391, 299–310. [Google Scholar] [CrossRef]
- Azari, A.; Goodarzi, A.; Jafarkhani, B.; Eghbali, M.; Karimi, Z.; Hosseini Balef, S.S.; Irannejad, H. Novel Molecular Targets and Mechanisms for Neuroprotective Modulation in Neurodegenerative Disorders. Cent. Nerv. Syst. Agents Med. Chem. 2022, 22, 88–107. [Google Scholar] [CrossRef]
- Kishibe, M. Physiological and pathological roles of kallikrein-related peptidases in the epidermis. J. Dermatol. Sci. 2019, 95, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yamada, T.; Tsujioka, Y.; Taguchi, J.; Takahashi, M.; Tsuboi, Y.; Fujino, Y.; Nakajima, M.; Yamamoto, T.; Akatsu, H.; et al. Localization of a novel type trypsin-like serine protease, neurosin, in brain tissues of Alzheimer’s disease and Parkinson’s disease. Psychiatry Clin. Neurosci. 2000, 54, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Gabril, M.; White, N.M.; Moussa, M.; Chow, T.-f.F.; Metias, S.M.; Fatoohi, E.; Yousef, G.M. Immunohistochemical analysis of kallikrein-related peptidases in the normal kidney and renal tumors: Potential clinical implications. Biol. Chem. 2010, 391, 403–409. [Google Scholar] [CrossRef]
- Petraki, C.D.; Gregorakis, A.K.; Vaslamatzis, M.M.; Papanastasiou, P.A.; Yousef, G.M.; Levesque, M.A.; Diamandis, E.P. Prognostic implications of the immunohistochemical expression of human kallikreins 5, 6, 10 and 11 in renal cell carcinoma. Tumor. Biol. 2006, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chambrey, R.; Picard, N. Role of tissue kallikrein in regulation of tubule function. Curr. Opin. Nephrol. Hypertens. 2011, 20, 523–528. [Google Scholar] [CrossRef]
- Kakoki, M.; Smithies, O. The kallikrein-kinin system in health and in diseases of the kidney. Kidney Int. 2009, 75, 1019–1030. [Google Scholar] [CrossRef]
- Rhaleb, N.E.; Yang, X.P.; Carretero, O.A. The kallikrein-kinin system as a regulator of cardiovascular and renal function. Compr. Physiol. 2011, 1, 971–993. [Google Scholar] [CrossRef]
- El Moghrabi, S.; Houillier, P.; Picard, N.; Sohet, F.; Wootla, B.; Bloch-Faure, M.; Leviel, F.; Cheval, L.; Frische, S.; Meneton, P.; et al. Tissue kallikrein permits early renal adaptation to potassium load. Proc. Natl. Acad. Sci. USA 2010, 107, 13526–13531. [Google Scholar] [CrossRef]
- Rohrwasser, A.; Ishigami, T.; Gociman, B.; Lantelme, P.; Morgan, T.; Cheng, T.; Hillas, E.; Zhang, S.; Ward, K.; Bloch-Faure, M.; et al. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003, 64, 2155–2162. [Google Scholar] [CrossRef]
- Bera, A.; Gupta, M.L. Microtubules in Microorganisms: How Tubulin Isotypes Contribute to Diverse Cytoskeletal Functions. Front. Cell Dev. Biol. 2022, 10, 913809. [Google Scholar] [CrossRef] [PubMed]
- Al-Bassam, J.; Corbett, K.D. α-Tubulin acetylation from the inside out. Proc. Natl. Acad. Sci. USA 2012, 109, 19515–19516. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed]
- Solic, I.; Racetin, A.; Filipovic, N.; Mardesic, S.; Bocina, I.; Galesic-Ljubanovic, D.; Glavina Durdov, M.; Saraga-Babić, M.; Vukojevic, K. Expression Pattern of α-Tubulin, Inversin and Its Target Dishevelled-1 and Morphology of Primary Cilia in Normal Human Kidney Development and Diseases. Int. J. Mol. Sci. 2021, 22, 3500. [Google Scholar] [CrossRef]
- Perutina, I.; Kelam, N.; Maglica, M.; Racetin, A.; Rizikalo, A.; Filipović, N.; Prusac, I.K.; Bošnjak, M.; Mišković, J.; Kablar, B.; et al. Spatiotemporal distribution of Wnt signaling pathway markers in human congenital anomalies of kidney and urinary tract. Acta Histochem. 2025, 127, 152235. [Google Scholar] [CrossRef]
- Kolb, R.J.; Nauli, S.M. Ciliary dysfunction in polycystic kidney disease: An emerging model with polarizing potential. Front. Biosci. 2008, 13, 4451–4466. [Google Scholar] [CrossRef] [PubMed]
- Lina, F.; Satlinb, L.M. Polycystic kidney disease: The cilium as a common pathway in cystogenesis. Curr. Opin. Pediatr. 2004, 16, 171–176. [Google Scholar] [CrossRef]
- Manissorn, J.; Khamchun, S.; Vinaiphat, A.; Thongboonkerd, V. Alpha-tubulin enhanced renal tubular cell proliferation and tissue repair but reduced cell death and cell-crystal adhesion. Sci. Rep. 2016, 6, 28808. [Google Scholar] [CrossRef]
- Devuyst, O.; Burrow, C.R.; Smith, B.L.; Agre, P.; Knepper, M.A.; Wilson, P.D. Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am. J. Physiol. 1996, 271, F169–F183. [Google Scholar] [CrossRef]
- Nielsen, S.; Frøkiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef]
- An, F.; Zhu, S. The Distribution and Function of Aquaporin 1 and 2 Along the Kidney in Different Species. Int. J. Morphol. 2021, 39, 890–895. [Google Scholar] [CrossRef]
- Mutter, C.M.; Smith, T.; Menze, O.; Zakharia, M.; Nguyen, H. Diabetes Insipidus: Pathogenesis, Diagnosis, and Clinical Management. Cureus 2021, 13, 13523. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Chen, E.; Zhang, W. Aqp2(+) Progenitor Cells Maintain and Repair Distal Renal Segments. J. Am. Soc. Nephrol. 2022, 33, 1357–1376. [Google Scholar] [CrossRef]
- Kwon, T.H.; Frøkiær, J.; Nielsen, S. Regulation of aquaporin-2 in the kidney: A molecular mechanism of body-water homeostasis. Kidney Res. Clin. Pract. 2013, 32, 96–102. [Google Scholar] [CrossRef]
- Vukićević, T.; Schulz, M.; Faust, D.; Klussmann, E. The Trafficking of the Water Channel Aquaporin-2 in Renal Principal Cells-a Potential Target for Pharmacological Intervention in Cardiovascular Diseases. Front. Pharmacol. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Kelam, J.; Kelam, N.; Filipović, N.; Komić, L.; Racetin, A.; Komić, D.; Kostić, S.; Kuzmić Prusac, I.; Vukojević, K. Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT. Genes 2024, 15, 702. [Google Scholar] [CrossRef] [PubMed]
- Kelam, N.; Racetin, A.; Polović, M.; Benzon, B.; Ogorevc, M.; Vukojević, K.; Glavina Durdov, M.; Dunatov Huljev, A.; Kuzmić Prusac, I.; Čarić, D.; et al. Aberrations in FGFR1, FGFR2, and RIP5 Expression in Human Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Int. J. Mol. Sci. 2022, 23, 15537. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R. The Declaration of Helsinki and public health. Bull. World Health Organ. 2008, 86, 650–652. [Google Scholar] [CrossRef]
- O’Rahilly, R. Guide to the staging of human embryos. Anat. Anz. 1972, 130, 556–559. [Google Scholar]
- Maglica, M.; Kelam, N.; Perutina, I.; Racetin, A.; Rizikalo, A.; Filipović, N.; Kuzmić Prusac, I.; Mišković, J.; Vukojević, K. Immunoexpression Pattern of Autophagy-Related Proteins in Human Congenital Anomalies of the Kidney and Urinary Tract. Int. J. Mol. Sci. 2024, 25, 6829. [Google Scholar] [CrossRef]
- Pavlović, N.; Kelam, N.; Racetin, A.; Filipović, N.; Pogorelić, Z.; Prusac, I.K.; Vukojević, K. Expression Profiles of ITGA8 and VANGL2 Are Altered in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Molecules 2024, 29, 3294. [Google Scholar] [CrossRef] [PubMed]
- Pavic, B.; Ogorevc, M.; Boric, K.; Vukovic, D.; Saraga-Babic, M.; Mardesic, S. Connexin 37, 40, 43 and Pannexin 1 Expression in the Gastric Mucosa of Patients with Systemic Sclerosis. Biomedicines 2023, 11, 2487. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Winyard, P.; Napolitano, R.; Sebire, N. Normal and Abnormal Human Kidney Development. In Pediatric Nephropathology & Childhood Kidney Tumors; Liapis, H., Ed.; Diagnostic Pediatric Pathology; Cambridge University Press: Cambridge, UK, 2023; pp. 1–16. [Google Scholar]
- Osathanondh, V.; Potter, E.L. Development of Human Kidney as Shown by Microdissection. III. Formation and Interrelationship of Collecting Tubules and Nephrons. Arch. Pathol. 1963, 76, 290–302. [Google Scholar]
- Isert, S.; Müller, D.; Thumfart, J. Factors Associated With the Development of Chronic Kidney Disease in Children with Congenital Anomalies of the Kidney and Urinary Tract. Front. Pediatr. 2020, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Molecular basis of renal fibrosis. Pediatr. Nephrol. 2000, 15, 290–301. [Google Scholar] [CrossRef]
- Rodríguez-Iturbe, B.; Vaziri, N.D.; Herrera-Acosta, J.; Johnson, R.J. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: All for one and one for all. Am. J. Physiol. Ren. Physiol. 2004, 286, F606–F616. [Google Scholar] [CrossRef] [PubMed]
- Costa-Neto, C.M.; Dillenburg-Pilla, P.; Heinrich, T.A.; Parreiras-e-Silva, L.T.; Pereira, M.G.; Reis, R.I.; Souza, P.P. Participation of kallikrein-kinin system in different pathologies. Int. Immunopharmacol. 2008, 8, 135–142. [Google Scholar] [CrossRef]
- Pathak, M.; Wong, S.; Dreveny, I.; Emsley, J. Structure of plasma and tissue kallikreins. Thromb. Haemost. 2013, 110, 423–433. [Google Scholar] [CrossRef]
- Emanueli, C.; Madeddu, P. Role of the kallikrein–kinin system in the maturation of cardiovascular phenotype. Am. J. Hypertens. 1999, 12, 988–999. [Google Scholar] [CrossRef]
- Pizard, A.; Richer, C.; Bouby, N.; Picard, N.; Meneton, P.; Azizi, M.; Alhenc-Gelas, F. Genetic deficiency in tissue kallikrein activity in mouse and man: Effect on arteries, heart and kidney. Biol. Chem. 2008, 389, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Rückert, F.; Hennig, M.; Petraki, C.D.; Wehrum, D.; Distler, M.; Denz, A.; Schröder, M.; Dawelbait, G.; Kalthoff, H.; Saeger, H.D.; et al. Co-expression of KLK6 and KLK10 as prognostic factors for survival in pancreatic ductal adenocarcinoma. Br. J. Cancer 2008, 99, 1484–1492. [Google Scholar] [CrossRef]
- Ahmed, N.; Dorn, J.; Napieralski, R.; Drecoll, E.; Kotzsch, M.; Goettig, P.; Zein, E.; Avril, S.; Kiechle, M.; Diamandis, E.P.; et al. Clinical relevance of kallikrein-related peptidase 6 (KLK6) and 8 (KLK8) mRNA expression in advanced serous ovarian cancer. Biol. Chem. 2016, 397, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- White, N.M.A.; Mathews, M.; Yousef, G.M.; Prizada, A.; Popadiuk, C.; Doré, J.J.E. KLK6 and KLK13 predict tumor recurrence in epithelial ovarian carcinoma. Br. J. Cancer 2009, 101, 1107–1113. [Google Scholar] [CrossRef]
- Kountourakis, P.; Psyrri, A.; Scorilas, A.; Camp, R.; Markakis, S.; Kowalski, D.; Diamandis, E.P.; Dimopoulos, M.A. Prognostic value of kallikrein-related peptidase 6 protein expression levels in advanced ovarian cancer evaluated by automated quantitative analysis (AQUA). Cancer Sci. 2008, 99, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Pampalakis, G.; Zingkou, E.; Sidiropoulos, K.G.; Diamandis, E.P.; Zoumpourlis, V.; Yousef, G.M.; Sotiropoulou, G. Biochemical pathways mediated by KLK6 protease in breast cancer. Mol. Oncol. 2019, 13, 2329–2343. [Google Scholar] [CrossRef]
- Pandey, R.; Zhou, M.; Chen, Y.; Darmoul, D.; Kisiel, C.C.; Nfonsam, V.N.; Ignatenko, N.A. Molecular Pathways Associated with Kallikrein 6 Overexpression in Colorectal Cancer. Genes 2021, 12, 749. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, M.; Lin, M. KLK6 Functions as an Oncogene and Unfavorable Prognostic Factor in Bladder Urothelial Carcinoma. Dis. Markers 2022, 2022, 3373851. [Google Scholar] [CrossRef]
- Martínez-Morillo, E.; Diamandis, A.; Diamandis, E.P. Reference intervals and biological variation for kallikrein 6: Influence of age and renal failure. Clin. Chem. Lab. Med. 2012, 50, 931–934. [Google Scholar] [CrossRef]
- Vio, C.P.; Loyola, S.; Velarde, V. Localization of components of the kallikrein-kinin system in the kidney: Relation to renal function. State of the art lecture. Hypertension 1992, 19, II10. [Google Scholar] [CrossRef]
- Saraga-Babić, M.; Vukojević, K.; Bočina, I.; Drnašin, K.; Saraga, M. Ciliogenesis in normal human kidney development and post-natal life. Pediatr. Nephrol. 2012, 27, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, F.; Sun, Y.; Lei, L.; Zhou, H.; Lei, T.; Xia, Y.; Verkman, A.S.; Yang, B. Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling. Faseb. J. 2015, 29, 1551–1563. [Google Scholar] [CrossRef]
- Noitem, R.; Yuajit, C.; Soodvilai, S.; Muanprasat, C.; Chatsudthipong, V. Steviol slows renal cyst growth by reducing AQP2 expression and promoting AQP2 degradation. Biomed. Pharmacother. 2018, 101, 754–762. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Yousef, G.M. Human tissue kallikrein gene family: A rich source of novel disease biomarkers. Expert Rev. Mol. Diagn. 2001, 1, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, K.; Avgeris, M.; Scorilas, A. Targeting kallikrein-related peptidases in prostate cancer. Expert Opin. Ther. Targets 2014, 18, 365–383. [Google Scholar] [CrossRef]
- Paliouras, M.; Borgono, C.; Diamandis, E.P. Human tissue kallikreins: The cancer biomarker family. Cancer Lett. 2007, 249, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Borgoño, C.A.; Diamandis, E.P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer 2004, 4, 876–890. [Google Scholar] [CrossRef]
- Michael, I.P.; Sotiropoulou, G.; Pampalakis, G.; Magklara, A.; Ghosh, M.; Wasney, G.; Diamandis, E.P. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J. Biol. Chem. 2005, 280, 14628–14635. [Google Scholar] [CrossRef]


| Phenotype | Developmental Phase | Renal and Related Pathology | Gestational Phase | Number of Samples |
|---|---|---|---|---|
| Normal kidneys (CTRL) | Ph2 | N/A | 15 dw | 1 |
| N/A | 16 dw | 2 | ||
| N/A | 17 dw | 2 | ||
| N/A | 18 dw | 2 | ||
| Ph3 | N/A | 23 dw | 1 | |
| N/A | 24 dw | 1 | ||
| N/A | 28 dw | 2 | ||
| N/A | 29–30 dw | 1 | ||
| Ph4 | N/A | 32 dw | 1 | |
| N/A | 35 dw | 1 | ||
| N/A | 37 dw | 1 | ||
| N/A | 38 dw | 2 | ||
| N/A | 1 m | 1 | ||
| N/A | 1.5 y | 1 | ||
| N/A | 7 y | 1 | ||
| Horseshoe kidney (HK) | Ren concreatus arcuatus, Cystae multiplices corticales | 22 dw | 1 | |
| Ren concreatus arcuatus, Tetras Fallot | 26 dw | 1 | ||
| Syndroma Edwards, Ren arcuatus | 30–31 dw | 1 | ||
| Syndroma Edwards, Ren arcuatus | 34 dw | 1 | ||
| Dysplastic kidney (DYS) | Megaureter lateris dextri, Dysplasia renis | 21 dw | 2 | |
| Renis dysplastica cysticus lateralis sinistri, agenesia renis dextri | ||||
| Dysplasia multicystica renis dextri, Cystes parvae focales | 27 dw | 1 | ||
| Cystes multiplices renum praecipue, Dylatatio cystica calycium, Teratoma sacrococcygeale | 28 dw | 2 | ||
| Renes multicystici dysplastici, Megaureter bilateralis, Stenosis ostia ureteris bilateralis, Syndroma Potter, Syndroma Down | 33–34 dw | 2 | ||
| Renes dysplastici cystici, Syndroma Potter | 35 dw | 1 | ||
| Dysplasia renis multicystica bilateralis | ||||
| Agenesis renis dextri et dysplasia renis sinistri cum ureter duplex | 37 dw | 1 | ||
| Dysplasia hypoplastica, renis bilateralis, Syndroma Down, Syndroma Potter | 38 dw | 1 | ||
| Dysplasia renis, Syndroma Potter | 39 dw | 1 | ||
| Duplex kidneys (DK) | Ureter duplex lateris dextri | 24 dw | 1 | |
| Ureter duplex lateris sinistri | 30 dw | 1 | ||
| Pyelon et ureter duplex bilateralis | 41 dw | 1 | ||
| Antibody | Catalog Number | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-kallikrein 6 antibody | ab191281 | Goat | 1:100 | Abcam (Cambridge, UK) |
| Anti-acetyl-α-tubulin (Lys40) (6-11B-1) | 12152S | Mouse | 1:500 | Cell Signaling Technology (CST), (Danvers, MA, USA) | |
| Aquaporin 1/AQP1 Antibody (B-11) | sc-25287 | Mouse | 1:50 | Santa Cruz Biotechnology (Dallas, TX, USA) | |
| Aquaporin 2/AQP2 Antibody (E-2) | sc-515770 | Mouse | 1:50 | Santa Cruz Biotechnology (Dallas, TX, USA) | |
| Secondary | Alexa Fluor® 488 AffiniPure™ Donkey Anti-Goat IgG (H + L) | 705-545-003 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) |
| Rhodamine Red™-X (RRX) AffiniPure™ Donkey Anti-Mouse IgG (H + L) | 715-295-151 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelam, N.; Ogorevc, M.; Gotovac, I.; Kuzmić Prusac, I.; Vukojević, K.; Saraga-Babić, M.; Mardešić, S. Analysis of Kallikrein 6, Acetyl-α-Tubulin, and Aquaporin 1 and 2 Expression Patterns During Normal Human Nephrogenesis and in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Genes 2025, 16, 499. https://doi.org/10.3390/genes16050499
Kelam N, Ogorevc M, Gotovac I, Kuzmić Prusac I, Vukojević K, Saraga-Babić M, Mardešić S. Analysis of Kallikrein 6, Acetyl-α-Tubulin, and Aquaporin 1 and 2 Expression Patterns During Normal Human Nephrogenesis and in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Genes. 2025; 16(5):499. https://doi.org/10.3390/genes16050499
Chicago/Turabian StyleKelam, Nela, Marin Ogorevc, Ivona Gotovac, Ivana Kuzmić Prusac, Katarina Vukojević, Mirna Saraga-Babić, and Snježana Mardešić. 2025. "Analysis of Kallikrein 6, Acetyl-α-Tubulin, and Aquaporin 1 and 2 Expression Patterns During Normal Human Nephrogenesis and in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT)" Genes 16, no. 5: 499. https://doi.org/10.3390/genes16050499
APA StyleKelam, N., Ogorevc, M., Gotovac, I., Kuzmić Prusac, I., Vukojević, K., Saraga-Babić, M., & Mardešić, S. (2025). Analysis of Kallikrein 6, Acetyl-α-Tubulin, and Aquaporin 1 and 2 Expression Patterns During Normal Human Nephrogenesis and in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Genes, 16(5), 499. https://doi.org/10.3390/genes16050499







