The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing
Abstract
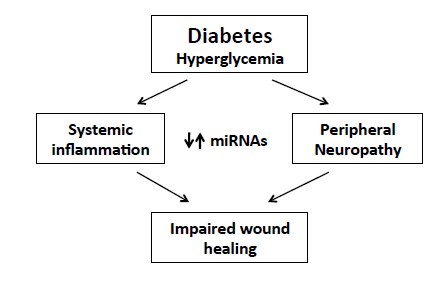
:1. Diabetes and Its Complications
2. MiRNAs in Diabetes Complications
2.1. Macrovascular Complications
2.1.1. Cardiomyopathy
2.1.2. Atherosclerosis
2.2. Microvascular Complications
2.2.1. Diabetic Retinopathy
| microRNAs | Diabetic Retinopathy—miRNA Functions |
|---|---|
| miR-132, miR-155, miR-146, miR-21 | Upregulated with increased NF-kB, ICAM-1 and MCP-1, in diabetic retinal endothelial cells and retinas [69]. |
| miR-34 family | Upregulated in diabetic rats upon VEGF and p53 responses, including in retinas [69]. |
| miR-34a | Downregulated in subconfluent retinal pigment epithelial cells. It can inhibit their proliferation and migration [70]. |
| miR-29b | Upregulation at the early stages of diabetes with potential target the cellular activator of x cellular activator of PKR, RAX (PKR activator X), in retinal ganglion cells [73]. |
| miR-195 | Upregulated in retinas of diabetic rats. Regulates sirtuin 1 mediated tissue damage, in human retinal and dermal microvascular endothelial cells [76]. |
| miR-195 | Upregulated in retinas of diabetic rats. Regulates sirtuin 1 mediated tissue damage, in human retinal and dermal microvascular endothelial cells [76]. |
| miR-200b | Downregulated upon high glycemia with VEGF as a direct target, in diabetic retinas and endothelial cells [81]. Upregulated in Akita mouse retinas. Regulates the expression of oxidation resistance-1 [80]. |
| miR-126 | Downregulated by hypoxia and reduced in the retinal tissue of streptozotocin-induced diabetic rats. VEGF and MMP-9 are possible targets [79]. |
2.2.2. Diabetic Nephropathy
2.2.3. Diabetic Neuropathy
3. MiRNA in Diabetic Wound-Healing Impairment

3.1. Inflammatory Phase
3.2. Proliferation Phase
3.3. Maturation Phase
| Phases of Wound Healing | miRNA Involved | Functions |
|---|---|---|
| Inflammation | miR-16 | Inhibits COX-2 expression in monocytes [147]. |
| miR-126 | Decreases leukocyte adherence to endothelial cells [141]. | |
| miR-146a | Key role as a molecular brake on inflammation [155]. | |
| miR-203 | Inhibits TNF-α and IL24 expression [152]. | |
| Proliferation | miR-21 | Promotes keratinocyte migration and re-epithelialization [185,186], increases the rate of fibroblasts migration towards the wound [164] and delays epithelialization [177]. |
| miR-27b | Rescues impaired BMAC angiogenesis via TSP-1 suppression [124]. | |
| miR-99 family | Reduces re-epithelialization of dermal wounds [189]. | |
| mir-126 | Promotes endothelial cell proliferation, migration and angiogenesis [175,176]. | |
| miR-143/145 | Inhibits angiotensin II formation [174]. | |
| miR-155 | Inhibits KGF expression in fibroblasts [161]. | |
| miR-198 | Inhibits keratinocyte migration [178]. | |
| miR-200 family | Controls epithelial-mesenchymal transition [190]. | |
| miR-203 | Inhibits keratinocyte proliferation and migration [180] but promotes keratinocyte differentiation [182]. | |
| miR-210 | Promotes keratinocyte differentiation [182] and silences Activin A receptor type 1B [201]. | |
| miR-328 | Inhibits the formation of capillary structures [169]. | |
| miR-483-3p | Inhibits keratinocyte proliferation and migration [179]. | |
| miR-503 | Impairs angiogenesis [171]. | |
| Maturation | miR-29b | In vivo topical application to mouse wounds improves collagen type III/I ratios and generates a higher matrix metalloproteinase 8 activity [204]. |
| miR-143 | Inhibits insulin action in cardiomyocytes from T2DM patients [200]. | |
| miR-196a | Decreases expression of type I and III collagens in fibroblasts [196] and its overexpression renders mice resistant to obesity and diabetes. | |
| miR-210 | Silences activin A receptor type 1B [201]. |
4. Potential of MiRNAs as Early Biomarkers for Detection and Treatment of Diabetic Foot Ulceration
4.1. MiRNAs as Biomarkers for the Development of Chronic Diabetic Ulceration
4.2. MiRNAs as Therapeutic Targets for Chronic Diabetic Ulceration
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. Idf diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [PubMed]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [PubMed]
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995, 75, 473–486. [Google Scholar] [PubMed]
- DeFronzo, R.A.; Bonadonna, R.C.; Ferrannini, E. Pathogenesis of niddm. A balanced overview. Diabetes Care 1992, 15, 318–368. [Google Scholar] [PubMed]
- Fontbonne, A.; Eschwege, E.; Cambien, F.; Richard, J.L.; Ducimetiere, P.; Thibult, N.; Warnet, J.M.; Claude, J.R.; Rosselin, G.E. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the paris prospective study. Diabetologia 1989, 32, 300–304. [Google Scholar] [PubMed]
- Srikanth, S.; Deedwania, P. Primary and secondary prevention strategy for cardiovascular disease in diabetes mellitus. Cardiol. Clin. 2011, 29, 47–70. [Google Scholar] [PubMed]
- Yun, J.S.; Ko, S.H.; Kim, J.H.; Moon, K.W.; Park, Y.M.; Yoo, K.D.; Ahn, Y.B. Diabetic retinopathy and endothelial dysfunction in patients with type 2 diabetes mellitus. Diabetes Metab. J. 2013, 37, 262–269. [Google Scholar] [PubMed]
- Klein, R.; Moss, S.E.; Klein, B.E.; Davis, M.D.; DeMets, D.L. Wisconsin epidemiologic study of diabetic retinopathy. XII. Relationship of c-peptide and diabetic retinopathy. Diabetes 1990, 39, 1445–1450. [Google Scholar] [PubMed]
- Andersen, A.R.; Christiansen, J.S.; Andersen, J.K.; Kreiner, S.; Deckert, T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: An epidemiological study. Diabetologia 1983, 25, 496–501. [Google Scholar] [PubMed]
- Higgins, G.C.; Coughlan, M.T. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br. J. Pharmacol. 2014, 171, 1917–1942. [Google Scholar]
- Kim, S.K.; Lee, K.J.; Hahm, J.R.; Lee, S.M.; Jung, T.S.; Jung, J.H.; Kim, S.; Kim, D.R.; Ahn, S.K.; Choi, W.H.; et al. Clinical significance of the presence of autonomic and vestibular dysfunction in diabetic patients with peripheral neuropathy. Diabetes Metab. J. 2012, 36, 64–69. [Google Scholar] [PubMed]
- Da Silva, L.; Carvalho, E.; Cruz, M.T. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert Opin. Biol. Ther. 2010, 10, 1427–1439. [Google Scholar] [PubMed]
- Hata, J.; Arima, H.; Zoungas, S.; Fulcher, G.; Pollock, C.; Adams, M.; Watson, J.; Joshi, R.; Kengne, A.P.; Ninomiya, T.; et al. Effects of the endpoint adjudication process on the results of a randomised controlled trial: The advance trial. PLoS ONE 2013, 8, e55807. [Google Scholar] [PubMed]
- OʼConnor, P.J.; Ismail-Beigi, F. Near-normalization of glucose and microvascular diabetes complications: Data from accord and advance. Ther. Adv. Endocrinol. Metab. 2011, 2, 17–26. [Google Scholar]
- Bianchi, C.; Del Prato, S. Metabolic memory and individual treatment aims in type 2 diabetes-outcome-lessons learned from large clinical trials. Rev. Diabet. Stud. 2011, 8, 432–440. [Google Scholar] [PubMed]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [PubMed]
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [PubMed]
- Maqbool, R.; Hussain, M.U. MicroRNAs and human diseases: Diagnostic and therapeutic potential. Cell Tissue Res. 2014. [Google Scholar] [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The role of microRNAs in human diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar]
- Pillai, R.S.; Bhattacharyya, S.N.; Filipowicz, W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007, 17, 118–126. [Google Scholar]
- Li, L.C.; Okino, S.T.; Zhao, H.; Pookot, D.; Place, R.F.; Urakami, S.; Enokida, H.; Dahiya, R. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. USA 2006, 103, 17337–17342. [Google Scholar] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [PubMed]
- Chew, B.H.; Ghazali, S.S.; Ismail, M.; Haniff, J.; Bujang, M.A. Age ≥60 years was an independent risk factor for diabetes-related complications despite good control of cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp. Gerontol. 2013, 48, 485–491. [Google Scholar] [PubMed]
- Djaberi, R.; Beishuizen, E.D.; Pereira, A.M.; Rabelink, T.J.; Smit, J.W.; Tamsma, J.T.; Huisman, M.V.; Jukema, J.W. Non-invasive cardiac imaging techniques and vascular tools for the assessment of cardiovascular disease in type 2 diabetes mellitus. Diabetologia 2008, 51, 1581–1593. [Google Scholar] [PubMed]
- Rawal, S.; Manning, P.; Katare, R. Cardiovascular microRNAs: As modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc. Diabetol. 2014, 13, 44. [Google Scholar]
- Cheng, J.; Zhang, W.; Zhang, X.; Han, F.; Li, X.; He, X.; Li, Q.; Chen, J. Effect of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: A meta-analysis. JAMA Intern. Med. 2014, 174, 773–785. [Google Scholar] [PubMed]
- Davis, T.M.; Coleman, R.L.; Holman, R.R.; Group, U. Ethnicity and long-term vascular outcomes in type 2 diabetes: A prospective observational study (UKPDS 83). Diabetic Med. 2014, 31, 200–207. [Google Scholar]
- McClelland, A.D.; Kantharidis, P. MicroRNA in the development of diabetic complications. Clin. Sci. 2014, 126, 95–110. [Google Scholar] [PubMed]
- Kantharidis, P.; Wang, B.; Carew, R.M.; Lan, H.Y. Diabetes complications: The microRNA perspective. Diabetes 2011, 60, 1832–1837. [Google Scholar]
- Sayed, A.S.; Xia, K.; Salma, U.; Yang, T.; Peng, J. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ. 2014, 23, 503–510. [Google Scholar] [PubMed]
- De Rosa, S.; Curcio, A.; Indolfi, C. Emerging role of microRNAs in cardiovascular diseases. Circ. J. 2014, 78, 567–575. [Google Scholar] [PubMed]
- Briasoulis, A.; Tousoulis, D.; Vogiatzi, G.; Siasos, G.; Papageorgiou, N.; Oikonomou, E.; Genimata, V.; Konsola, T.; Stefanadis, C. MicroRNAs: Biomarkers for cardiovascular disease in patients with diabetes mellitus. Curr. Top. Med. Chem. 2013, 13, 1533–1539. [Google Scholar] [PubMed]
- Shantikumar, S.; Caporali, A.; Emanueli, C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc. Res. 2012, 93, 583–593. [Google Scholar] [PubMed]
- Ruiz, M.A.; Chakrabarti, S. MicroRNAs: The underlying mediators of pathogenetic processes in vascular complications of diabetes. Can. J. Diabetes 2013, 37, 339–344. [Google Scholar] [PubMed]
- Vickers, K.C.; Rye, K.A.; Tabet, F. MicroRNAs in the onset and development of cardiovascular disease. Clin. Sci. 2014, 126, 183–194. [Google Scholar] [PubMed]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [PubMed]
- Gupta, M.K.; Halley, C.; Duan, Z.H.; Lappe, J.; ViteRNA, J.; Jana, S.; Augoff, K.; Mohan, M.L.; Vasudevan, N.T.; Na, J.; et al. MiRNA-548c: A specific signature in circulating pbmcs from dilated cardiomyopathy patients. J. Mol. Cell Cardiol. 2013, 62, 131–141. [Google Scholar]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang, D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 2013, 112, 1234–1243. [Google Scholar] [PubMed]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 2014, 124, 2136–2146. [Google Scholar] [PubMed]
- Reaven, G.M. Multiple chd risk factors in type 2 diabetes: Beyond hyperglycaemia. Diabetes Obes. Metab. 2002, 4, S13–S18. [Google Scholar] [PubMed]
- Kuwahata, S.; Fujita, S.; Orihara, K.; Hamasaki, S.; Oba, R.; Hirai, H.; Nagata, K.; Ishida, S.; Kataoka, T.; Oketani, N.; et al. High expression level of toll-like receptor 2 on monocytes is an important risk factor for arteriosclerotic disease. Atherosclerosis 2010, 209, 248–254. [Google Scholar] [PubMed]
- Weiner, S.D.; Ahmed, H.N.; Jin, Z.; Cushman, M.; Herrington, D.M.; Nelson, J.C.; di Tullio, M.R.; Homma, S. Systemic inflammation and brachial artery endothelial function in the multi-ethnic study of atherosclerosis (mesa). Heart 2014. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [PubMed]
- Wang, L.; Yang, Y.; Hong, B. Advances in the role of microRNAs in lipid metabolism-related anti-atherosclerotic drug discovery. Expert Opin. Drug Discov. 2013, 8, 977–990. [Google Scholar] [PubMed]
- Zampetaki, A.; Dudek, K.; Mayr, M. Oxidative stress in atherosclerosis: The role of microRNAs in arterial remodeling. Free Radic. Biol. Med. 2013, 64, 69–77. [Google Scholar] [PubMed]
- Magenta, A.; Greco, S.; Gaetano, C.; Martelli, F. Oxidative stress and microRNAs in vascular diseases. Int. J. Mol. Sci. 2013, 14, 17319–17346. [Google Scholar] [PubMed]
- Zernecke, A. MicroRNAs in the regulation of immune cell functions—Implications for atherosclerotic vascular disease. Thromb. Haemost. 2012, 107, 626–633. [Google Scholar] [PubMed]
- Busch, M.; Zernecke, A. MicroRNAs in the regulation of dendritic cell functions in inflammation and atherosclerosis. J. Mol. Med. 2012, 90, 877–885. [Google Scholar] [PubMed]
- Raitoharju, E.; Oksala, N.; Lehtimaki, T. MicroRNAs in the atherosclerotic plaque. Clin. Chem. 2013, 59, 1708–1721. [Google Scholar] [PubMed]
- Siasos, G.; Kollia, C.; Tsigkou, V.; Basdra, E.K.; Lymperi, M.; Oikonomou, E.; Kokkou, E.; Korompelis, P.; Papavassiliou, A.G. MicroRNAs: Novel diagnostic and prognostic biomarkers in atherosclerosis. Curr. Top. Med. Chem. 2013, 13, 1503–1517. [Google Scholar] [PubMed]
- Leung, A.; Natarajan, R. Noncoding RNAs in vascular disease. Curr. Opin. Cardiol. 2014, 29, 199–206. [Google Scholar] [PubMed]
- Menghini, R.; Stohr, R.; Federici, M. MicroRNAs in vascular aging and atherosclerosis. Ageing Res. Rev. 2014. [Google Scholar] [CrossRef]
- Weber, M.; Kim, S.; Patterson, N.; Rooney, K.; Searles, C.D. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1192–H1203. [Google Scholar] [PubMed]
- Tian, F.J.; An, L.N.; Wang, G.K.; Zhu, J.Q.; Li, Q.; Zhang, Y.Y.; Zeng, A.; Zou, J.; Zhu, R.F.; Han, X.S.; et al. Elevated microRNA-155 promotes foam cell formation by targeting hbp1 in atherogenesis. Cardiovasc. Res. 2014. [Google Scholar] [CrossRef]
- Hu, Y.W.; Hu, Y.R.; Zhao, J.Y.; Li, S.F.; Ma, X.; Wu, S.G.; Lu, J.B.; Qiu, Y.R.; Sha, Y.H.; Wang, Y.C.; et al. An agomir of mir-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS One 2014, 9, e94997. [Google Scholar] [PubMed]
- Yang, Q.; Yang, K.; Li, A. MicroRNA21 protects against ischemiareperfusion and hypoxiareperfusioninduced cardiocyte apoptosis via the phosphatase and tensin homolog/aktdependent mechanism. Mol. Med. Rep. 2014, 9, 2213–2220. [Google Scholar] [PubMed]
- Pan, Y.; Liang, H.; Liu, H.; Li, D.; Chen, X.; Li, L.; Zhang, C.Y.; Zen, K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J. Immunol. 2014, 192, 437–446. [Google Scholar] [PubMed]
- Vinod, M.; Chennamsetty, I.; Colin, S.; Belloy, L.; de Paoli, F.; Schaider, H.; Graier, W.F.; Frank, S.; Kratky, D.; Staels, B.; et al. Mir-206 controls lxralpha expression and promotes lxr-mediated cholesterol efflux in macrophages. Biochim. Biophys. Acta 2014, 1841, 827–835. [Google Scholar] [PubMed]
- Ying, C.; Sui-Xin, L.; Kang-Ling, X.; Wen-Liang, Z.; Lei, D.; Yuan, L.; Fan, Z.; Chen, Z. MicroRNA-492 reverses high glucose-induced insulin resistance in huvec cells through targeting resistin. Mol. Cell. Biochem. 2014, 391, 117–125. [Google Scholar] [PubMed]
- Stephenson, J.; Fuller, J.H. Microvascular and acute complications in iddm patients: The eurodiab iddm complications study. Diabetologia 1994, 37, 278–285. [Google Scholar] [PubMed]
- Scanlon, P.H.; Aldington, S.J.; Stratton, I.M. Epidemiological issues in diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2013, 20, 293–300. [Google Scholar] [PubMed]
- Papavasileiou, E.; Dereklis, D.; Oikonomidis, P.; Grixti, A.; Vineeth Kumar, B.; Prasad, S. An effective programme to systematic diabetic retinopathy screening in order to reduce diabetic retinopathy blindness. Hellenic J. Nucl. Med. 2014, 17, S30–S34. [Google Scholar]
- Kato, M.; Castro, N.E.; Natarajan, R. MicroRNAs: Potential mediators and biomarkers of diabetic complications. Free Radic. Biol. Med. 2013, 64, 85–94. [Google Scholar] [PubMed]
- Xiong, F.; Du, X.; Hu, J.; Li, T.; Du, S.; Wu, Q. Altered retinal microRNA expression profiles in early diabetic retinopathy: An in silico analysis. Curr. Eye Res. 2014, 39, 720–729. [Google Scholar] [PubMed]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [PubMed]
- Hou, Q.; Tang, J.; Wang, Z.; Wang, C.; Chen, X.; Hou, L.; Dong, X.D.; Tu, L. Inhibitory effect of microRNA-34a on retinal pigment epithelial cell proliferation and migration. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6481–6488. [Google Scholar]
- Jindal, V. Neurodegeneration as a primary change and role of neuroprotection in diabetic retinopathy. Mol. Neurobiol. 2014. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Zhou, H. The morphological features and mitochondrial oxidative stress mechanism of the retinal neurons apoptosis in early diabetic rats. J. Diabetes Res. 2014, 2014, 678123. [Google Scholar] [PubMed]
- Silva, V.A.; Polesskaya, A.; Sousa, T.A.; Correa, V.M.; Andre, N.D.; Reis, R.I.; Kettelhut, I.C.; Harel-Bellan, A.; de Lucca, F.L. Expression and cellular localization of microRNA-29b and rax, an activator of the RNA-dependent protein kinase (pkr), in the retina of streptozotocin-induced diabetic rats. Mol. Vis. 2011, 17, 2228–2240. [Google Scholar] [PubMed]
- Chen, X.; Ye, S.; Xiao, W.; Luo, L.; Liu, Y. Differentially expressed microRNAs in tgfbeta2-induced epithelial-mesenchymal transition in retinal pigment epithelium cells. Int. J. Mol. Med. 2014, 33, 1195–1200. [Google Scholar] [PubMed]
- Cheng, H.C.; Ho, T.C.; Chen, S.L.; Lai, H.Y.; Hong, K.F.; Tsao, Y.P. Troglitazone suppresses transforming growth factor beta-mediated fibrogenesis in retinal pigment epithelial cells. Mol. Vis. 2008, 14, 95–104. [Google Scholar] [PubMed]
- Mortuza, R.; Feng, B.; Chakrabarti, S. Mir-195 regulates sirt1-mediated changes in diabetic retinopathy. Diabetologia 2014, 57, 1037–1046. [Google Scholar] [PubMed]
- Bento, C.F.; Fernandes, R.; Matafome, P.; Sena, C.; Seica, R.; Pereira, P. Methylglyoxal-induced imbalance in the ratio of vascular endothelial growth factor to angiopoietin 2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Exp. Physiol. 2010, 95, 955–970. [Google Scholar] [PubMed]
- Ling, S.; Birnbaum, Y.; Nanhwan, M.K.; Thomas, B.; Bajaj, M.; Ye, Y. MicroRNA-dependent cross-talk between vegf and hif1alpha in the diabetic retina. Cell. Signal. 2013, 25, 2840–2847. [Google Scholar] [PubMed]
- Ye, P.; Liu, J.; He, F.; Xu, W.; Yao, K. Hypoxia-induced deregulation of mir-126 and its regulative effect on vegf and mmp-9 expression. Int. J. Med. Sci. 2014, 11, 17–23. [Google Scholar] [PubMed]
- Murray, A.R.; Chen, Q.; Takahashi, Y.; Zhou, K.K.; Park, K.; Ma, J.X. MicroRNA-200b downregulates oxidation resistance 1 (oxr1) expression in the retina of type 1 diabetes model. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1689–1697. [Google Scholar] [PubMed]
- McArthur, K.; Feng, B.; Wu, Y.; Chen, S.; Chakrabarti, S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 2011, 60, 1314–1323. [Google Scholar] [PubMed]
- Gohda, T.; Mima, A.; Moon, J.Y.; Kanasaki, K. Combat diabetic nephropathy: From pathogenesis to treatment. J. Diabetes Res. 2014, 2014, 207140. [Google Scholar] [PubMed]
- Bichu, P.; Nistala, R.; Khan, A.; Sowers, J.R.; Whaley-Connell, A. Angiotensin receptor blockers for the reduction of proteinuria in diabetic patients with overt nephropathy: Results from the amadeo study. Vasc. Health Risk Manag. 2009, 5, 129–140. [Google Scholar] [PubMed]
- Collins, A.J.; Foley, R.N.; Herzog, C.; Chavers, B.; Gilbertson, D.; Ishani, A.; Kasiske, B.; Liu, J.; Mau, L.W.; McBean, M.; et al. United states renal data system 2008 annual data report. Am. J. Kidney Dis. 2009, 53, S1–S374. [Google Scholar] [PubMed]
- Alvarez, M.L.; Distefano, J.K. The role of non-coding RNAs in diabetic nephropathy: Potential applications as biomarkers for disease development and progression. Diabetes Res. Clin. Pract. 2013, 99, 1–11. [Google Scholar] [PubMed]
- Macisaac, R.J.; Ekinci, E.I.; Jerums, G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am. J. Kidney Dis. 2014, 63, S39–S62. [Google Scholar] [PubMed]
- Jalal, D.I.; Rivard, C.J.; Johnson, R.J.; Maahs, D.M.; McFann, K.; Rewers, M.; Snell-Bergeon, J.K. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: Findings from the coronary artery calcification in type 1 diabetes study. Nephrol. Dial. Transplant. 2010, 25, 1865–1869. [Google Scholar] [PubMed]
- Acosta, J.B.; del Barco, D.G.; Vera, D.C.; Savigne, W.; Lopez-Saura, P.; Guillen Nieto, G.; Schultz, G.S. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int. Wound J. 2008, 5, 530–539. [Google Scholar] [PubMed]
- Lin, C.L.; Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Wang, J.Y.; Huang, Y.T.; Wang, S.Y.; Wang, F.S. Cannabinoid receptor 1 disturbance of PPARγ2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J. Mol. Med. 2014, 92, 779–792. [Google Scholar] [PubMed]
- Bocci, V.; Zanardi, I.; Huijberts, M.S.; Travagli, V. An integrated medical treatment for type-2 diabetes. Diabetes Metab. Syndr. 2014, 8, 57–61. [Google Scholar] [PubMed]
- Tan, S.M.; Sharma, A.; Stefanovic, N.; Yuen, D.Y.; Karagiannis, T.C.; Meyer, C.; Ward, K.W.; Cooper, M.E.; de Haan, J.B. A derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner, lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes 2014, 63, 3091–3103. [Google Scholar] [PubMed]
- Acikgoz, Y.; Can, B.; Bek, K.; Acikgoz, A.; Ozkaya, O.; Genc, G.; Sarikaya, S. The effect of simvastatin and erythropoietin on renal fibrosis in rats with unilateral ureteral obstruction. Renal Fail. 2014, 36, 252–257. [Google Scholar]
- Brennan, E.; McEvoy, C.; Sadlier, D.; Godson, C.; Martin, F. The genetics of diabetic nephropathy. Genes (Basel) 2013, 4, 596–619. [Google Scholar]
- McClelland, A.; Hagiwara, S.; Kantharidis, P. Where are we in diabetic nephropathy: MicroRNAs and biomarkers? Curr. Opin. Nephrol. Hypertens. 2014, 23, 80–86. [Google Scholar] [CrossRef]
- Khella, H.W.; Bakhet, M.; Lichner, Z.; Romaschin, A.D.; Jewett, M.A.; Yousef, G.M. MicroRNAs in kidney disease: An emerging understanding. Am. J. Kidney Dis. 2013, 61, 798–808. [Google Scholar] [PubMed]
- Chandrasekaran, K.; Karolina, D.S.; Sepramaniam, S.; Armugam, A.; Wintour, E.M.; Bertram, J.F.; Jeyaseelan, K. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012, 81, 617–627. [Google Scholar] [PubMed]
- Srivastava, S.P.; Koya, D.; Kanasaki, K. MicroRNAs in kidney fibrosis and diabetic nephropathy: Roles on emt and endmt. Biomed. Res. Int. 2013, 2013, 125469. [Google Scholar] [PubMed]
- Kato, M.; Park, J.T.; Natarajan, R. MicroRNAs and the glomerulus. Exp. Cell Res. 2012, 318, 993–1000. [Google Scholar] [PubMed]
- Liang, M.; Liu, Y.; Mladinov, D.; Cowley, A.W., Jr.; Trivedi, H.; Fang, Y.; Xu, X.; Ding, X.; Tian, Z. MicroRNA: A new frontier in kidney and blood pressure research. Am. J. Physiol. Renal. Physiol. 2009, 297, F553–F558. [Google Scholar] [PubMed]
- Hagiwara, S.; McClelland, A.; Kantharidis, P. MicroRNA in diabetic nephropathy: Renin angiotensin, age/rage, and oxidative stress pathway. J. Diabetes Res. 2013, 2013, 173783. [Google Scholar] [PubMed]
- Lan, H.Y. Transforming growth factor-beta/smad signalling in diabetic nephropathy. Clin. Exp. Pharmacol. Physiol. 2012, 39, 731–738. [Google Scholar] [PubMed]
- Zhang, L.; He, S.; Guo, S.; Xie, W.; Xin, R.; Yu, H.; Yang, F.; Qiu, J.; Zhang, D.; Zhou, S.; et al. Down-regulation of mir-34a alleviates mesangial proliferation in vitro and glomerular hypertrophy in early diabetic nephropathy mice by targeting gas1. J. Diabetes Complicat. 2014, 28, 259–264. [Google Scholar] [PubMed]
- Lin, C.L.; Lee, P.H.; Hsu, Y.C.; Lei, C.C.; Ko, J.Y.; Chuang, P.C.; Huang, Y.T.; Wang, S.Y.; Wu, S.L.; Chen, Y.S.; et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. 2014. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Taila, M.; Alvarez, M.L. Emerging roles for miRNAs in the development, diagnosis, and treatment of diabetic nephropathy. Curr. Diab. Rep. 2013, 13, 582–591. [Google Scholar]
- Cachia, M.J.; Peakman, M.; Zanone, M.; Watkins, P.J.; Vergani, D. Reproducibility and persistence of neural and adrenal autoantibodies in diabetic autonomic neuropathy. Diabetic Med. 1997, 14, 461–465. [Google Scholar] [PubMed]
- Kostev, K.; Jockwig, A.; Hallwachs, A.; Rathmann, W. Prevalence and risk factors of neuropathy in newly diagnosed type 2 diabetes in primary care practices: A retrospective database analysis in germany and uk. Prim. Care Diabetes 2014, 8, 250–255. [Google Scholar] [PubMed]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [PubMed]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [PubMed]
- Tellechea, A.; Kafanas, A.; Leal, E.C.; Tecilazich, F.; Kuchibhotla, S.; Auster, M.E.; Kontoes, I.; Paolino, J.; Carvalho, E.; Nabzdyk, L.P.; et al. Increased skin inflammation and blood vessel density in human and experimental diabetes. Int. J. Low Extrem. Wounds 2013, 12, 4–11. [Google Scholar] [PubMed]
- Deli, G.; Bosnyak, E.; Pusch, G.; Komoly, S.; Feher, G. Diabetic neuropathies: Diagnosis and management. Neuroendocrinology 2013, 98, 267–280. [Google Scholar] [PubMed]
- Bansal, V.; Kalita, J.; Misra, U.K. Diabetic neuropathy. Postgrad. Med. J. 2006, 82, 95–100. [Google Scholar] [PubMed]
- Malik, R.; Veves, A.; Tesfaye, S.; Smith, G.; Cameron, N.; Zochodne, D.; Lauria, G.; The Toronto Consensus Panel on Diabetic Neuropathy. Small fiber neuropathy: Role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab. Res. Rev. 2011. [Google Scholar] [CrossRef]
- Daemen, M.A.; Kurvers, H.A.; Kitslaar, P.J.; Slaaf, D.W.; Bullens, P.H.; van den Wildenberg, F.A. Neurogenic inflammation in an animal model of neuropathic pain. Neurol. Res. 1998, 20, 41–45. [Google Scholar] [PubMed]
- Pradhan Nabzdyk, L.; Kuchibhotla, S.; Guthrie, P.; Chun, M.; Auster, M.E.; Nabzdyk, C.; Deso, S.; Andersen, N.; Gnardellis, C.; LoGerfo, F.W.; et al. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J. Vasc. Surg. 2013, 58, 766–775. [Google Scholar] [PubMed]
- Moura, L.I.; Dias, A.M.; Leal, E.C.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin—An efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [PubMed]
- Bali, K.K.; Hackenberg, M.; Lubin, A.; Kuner, R.; Devor, M. Sources of individual variability: MiRNAs that predispose to neuropathic pain identified using genome-wide sequencing. Mol. Pain 2014, 10, 22. [Google Scholar] [PubMed]
- Li, H.; Huang, Y.; Ma, C.; Yu, X.; Zhang, Z.; Shen, L. Mir-203 involves in neuropathic pain development and represses rap1a expression in nerve growth factor differentiated neuronal PC12 cells. Clin. J. Pain 2014. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhou, W.; Kang, L.M.; Yan, H.; Zhang, L.; Xu, B.H.; Cai, W.H. Intrathecal mir-96 inhibits nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem. Res. 2014, 39, 76–83. [Google Scholar] [PubMed]
- Sakai, A.; Saitow, F.; Miyake, N.; Miyake, K.; Shimada, T.; Suzuki, H. Mir-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 2013, 136, 2738–2750. [Google Scholar] [PubMed]
- Ciccacci, C.; Morganti, R.; di Fusco, D.; DʼAmato, C.; Cacciotti, L.; Greco, C.; Rufini, S.; Novelli, G.; Sangiuolo, F.; Marfia, G.A.; et al. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol. 2014, 51, 673–671. [Google Scholar] [PubMed]
- Zhou, S.; Gao, R.; Hu, W.; Qian, T.; Wang, N.; Ding, G.; Ding, F.; Yu, B.; Gu, X. Mir-9 inhibits schwann cell migration by targeting cthrc1 following sciatic nerve injury. J. Cell Sci. 2014, 127, 967–976. [Google Scholar] [PubMed]
- Gupta, S.K.; Singh, S.K. Diabetic foot: A continuing challenge. Adv. Exp. Med. Biol. 2012, 771, 123–138. [Google Scholar] [PubMed]
- Lu, M.H.; Hu, C.J.; Chen, L.; Peng, X.; Chen, J.; Hu, J.Y.; Teng, M.; Liang, G.P. Mir-27b represses migration of mouse mscs to burned margins and prolongs wound repair through silencing SDF-1a. PLoS ONE 2013, 8, e68972. [Google Scholar] [PubMed]
- Wang, J.M.; Tao, J.; Chen, D.D.; Cai, J.J.; Irani, K.; Wang, Q.; Yuan, H.; Chen, A.F. MicroRNA mir-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arterioscler Thromb Vasc. Biol. 2014, 34, 99–109. [Google Scholar] [PubMed]
- Meisgen, F.; Xu Landen, N.; Bouez, C.; Zuccolo, M.; Gueniche, A.; Stahle, M.; Sonkoly, E.; Breton, L.; Pivarcsi, A. Activation of toll-like receptors alters the microRNA expression profile of keratinocytes. Exp. Dermatol. 2014, 23, 281–283. [Google Scholar] [PubMed]
- Ning, M.S.; Andl, T. Control by a hairʼs breadth: The role of microRNAs in the skin. Cell Mol. Life Sci. 2013, 70, 1149–1169. [Google Scholar]
- Schneider, M.R. MicroRNAs as novel players in skin development, homeostasis and disease. Br. J. Dermatol. 2012, 166, 22–28. [Google Scholar] [PubMed]
- Moura, L.I.; Cruz, M.T.; Carvalho, E. The effect of neurotensin in human keratinocytes—Implication on impaired wound healing in diabetes. Exp. Biol. Med. (Maywood) 2014, 239, 6–12. [Google Scholar]
- Moura, L.I.; Silva, L.; Leal, E.C.; Tellechea, A.; Cruz, M.T.; Carvalho, E. Neurotensin modulates the migratory and inflammatory response of macrophages under hyperglycemic conditions. Biomed. Res. Int. 2013, 2013, 941764. [Google Scholar] [PubMed]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar] [PubMed]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Ann. Rev. Immunol. 2004, 22, 891–928. [Google Scholar]
- Le, Y.; Yang, Y.; Cui, Y.; Yazawa, H.; Gong, W.; Qiu, C.; Wang, J.M. Receptors for chemotactic formyl peptides as pharmacological targets. Int. Immunopharmacol. 2002, 2, 1–13. [Google Scholar] [PubMed]
- Zhai, Y.; Zhong, Z.; Chen, C.Y.; Xia, Z.; Song, L.; Blackburn, M.R.; Shyu, A.B. Coordinated changes in mRNA turnover, translation, and RNA processing bodies in bronchial epithelial cells following inflammatory stimulation. Mol. Cell Biol. 2008, 28, 7414–7426. [Google Scholar] [PubMed]
- Nakamachi, Y.; Kawano, S.; Takenokuchi, M.; Nishimura, K.; Sakai, Y.; Chin, T.; Saura, R.; Kurosaka, M.; Kumagai, S. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheumatol. 2009, 60, 1294–1304. [Google Scholar]
- Dorhoi, A.; Iannaccone, M.; Farinacci, M.; Fae, K.C.; Schreiber, J.; Moura-Alves, P.; Nouailles, G.; Mollenkopf, H.J.; Oberbeck-Muller, D.; Jorg, S.; et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J. Clin. Invest. 2013, 123, 4836–4848. [Google Scholar] [PubMed]
- Pradhan, L.; Cai, X.; Wu, S.; Andersen, N.D.; Martin, M.; Malek, J.; Guthrie, P.; Veves, A.; Logerfo, F.W. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J. Surg. Res. 2011, 167, 336–342. [Google Scholar] [PubMed]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regener. 2006, 14, 558–565. [Google Scholar]
- Min, M.; Peng, L.; Yang, Y.; Guo, M.; Wang, W.; Sun, G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflammatory Bowel Dis. 2014, 20, 652–659. [Google Scholar]
- Fabbri, E.; Borgatti, M.; Montagner, G.; Bianchi, N.; Finotti, A.; Lampronti, I.; Bezzerri, V.; Dechecchi, M.C.; Cabrini, G.; Gambari, R. Expression of microRNA-93 and Interleukin-8 during pseudomonas aeruginosa mediated induction of pro-inflammatory responses. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1144–1155. [Google Scholar] [PubMed]
- Zykova, S.N.; Jenssen, T.G.; Berdal, M.; Olsen, R.; Myklebust, R.; Seljelid, R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 2000, 49, 1451–1458. [Google Scholar] [PubMed]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [PubMed]
- Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Rovira, O.; Guerra, E.; Esteve, E.; Xifra, G.; Martinez, C.; Ricart, W.; Rieusset, J.; et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 2014, 67, 51375–51383. [Google Scholar]
- Zhang, T.; Lv, C.; Li, L.; Chen, S.; Liu, S.; Wang, C.; Su, B. Plasma mir-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. Biomed. Res. Int. 2013, 2013, 761617. [Google Scholar] [PubMed]
- Dianzani, U.; Funaro, A.; DiFranco, D.; Garbarino, G.; Bragardo, M.; Redoglia, V.; Buonfiglio, D.; de Monte, L.B.; Pileri, A.; Malavasi, F. Interaction between endothelium and CD4+CD45RA+ lymphocytes. Role of the human CD38 molecule. J. Immunol. 1994, 153, 952–959. [Google Scholar] [PubMed]
- Jude, J.A.; Dileepan, M.; Subramanian, S.; Solway, J.; Panettieri, R.A., Jr.; Walseth, T.F.; Kannan, M.S. Mir-140-3p regulation of tnf-alpha-induced cd38 expression in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L460–L468. [Google Scholar] [PubMed]
- Collares, C.V.; Evangelista, A.F.; Xavier, D.J.; Rassi, D.M.; Arns, T.; Foss-Freitas, M.C.; Foss, M.C.; Puthier, D.; Sakamoto-Hojo, E.T.; Passos, G.A.; et al. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res. Notes 2013, 6, 491. [Google Scholar] [PubMed]
- Shanmugam, N.; Reddy, M.A.; Natarajan, R. Distinct roles of heterogeneous nuclear ribonuclear protein k and microRNA-16 in cyclooxygenase-2 RNA stability induced by s100b, a ligand of the receptor for advanced glycation end products. J. Biol. Chem. 2008, 283, 36221–36233. [Google Scholar] [PubMed]
- Mokhtar, S.S.; Vanhoutte, P.M.; Leung, S.W.S.; Yusof, M.I.; Sulaiman, W.A.W.; Saad, A.Z.M.; Suppian, R.; Rasoo, A.H.G. Reduced expression of prostacyclin synthase and nitric oxide synthase in subcutaneous arteries of type 2 diabetic patients. Tohoku J. Exp. Med. 2013, 231, 217–222. [Google Scholar] [PubMed]
- Matouskova, P.; Bartikova, H.; Bousova, I.; Hanusova, V.; Szotakova, B.; Skalova, L. Reference genes for real-time PCR quantification of messenger RNAs and microRNAs in mouse model of obesity. PLoS ONE 2014, 9, e86033. [Google Scholar] [PubMed]
- Ortega, F.J.; Mercader, J.M.; Catalan, V.; Moreno-Navarrete, J.M.; Pueyo, N.; Sabater, M.; Gomez-Ambrosi, J.; Anglada, R.; Fernandez-Formoso, J.A.; Ricart, W.; et al. Targeting the circulating microRNA signature of obesity. Clin. Chem. 2013, 59, 781–792. [Google Scholar] [PubMed]
- Nesca, V.; Guay, C.; Jacovetti, C.; Menoud, V.; Peyot, M.L.; Laybutt, D.R.; Prentki, M.; Regazzi, R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia 2013, 56, 2203–2212. [Google Scholar] [PubMed]
- Primo, M.N.; Bak, R.O.; Schibler, B.; Mikkelsen, J.G. Regulation of pro-inflammatory cytokines TNFα and IL24 by microRNA-203 in primary keratinocytes. Cytokine 2012, 60, 741–748. [Google Scholar] [PubMed]
- Mi, Q.; Riviere, B.; Clermont, G.; Steed, D.L.; Vodovotz, Y. Agent-based model of inflammation and wound healing: Insights into diabetic foot ulcer pathology and the role of transforming growth factor-beta1. Wound Repair Regener. 2007, 15, 671–682. [Google Scholar]
- Ennis, W.J.; Sui, A.; Bartholomew, A. Stem cells and healing: Impact on inflammation. Adv. Wound Care (New Rochelle) 2013, 2, 369–378. [Google Scholar]
- Xu, J.; Wu, W.; Zhang, L.; Dorset-Martin, W.; Morris, M.W.; Mitchell, M.E.; Liechty, K.W. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: Correction with mesenchymal stem cell treatment. Diabetes 2012, 61, 2906–2912. [Google Scholar] [PubMed]
- Dickinson, S.; Hancock, D.P.; Petocz, P.; Ceriello, A.; Brand-Miller, J. High-glycemic index carbohydrate increases nuclear factor-κb activation in mononuclear cells of young, lean healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1188–1193. [Google Scholar] [PubMed]
- Stegenga, M.E.; van der Crabben, S.N.; Dessing, M.C.; Pater, J.M.; van den Pangaart, P.S.; de Vos, A.F.; Tanck, M.W.; Roos, D.; Sauerwein, H.P.; van der Poll, T. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabetic Med. 2008, 25, 157–164. [Google Scholar] [PubMed]
- Bogdanski, P.; Pupek-Musialik, D.; Dytfeld, J.; Jagodzinski, P.P.; Jablecka, A.; Kujawa, A.; Musialik, K. Influence of insulin therapy on expression of chemokine receptor CCR5 and selected inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Clin. Pharmacol. Ther. 2007, 45, 563–567. [Google Scholar] [PubMed]
- Balasubramanyam, M.; Aravind, S.; Gokulakrishnan, K.; Prabu, P.; Sathishkumar, C.; Ranjani, H.; Mohan, V. Impaired MIR-146a expression links subclinical inflammation and insulin resistance in type 2 diabetes. Mol. Cell Biochem. 2011, 351, 197–205. [Google Scholar] [PubMed]
- Tellechea, A.; Leal, E.; Veves, A.; Cravalho, E. Inflammatory and angiogenic abnormalities in diabetic wound healing: Role of neuropeptides and therapeutic perspectives. Open Circ. Vasc. J. 2010, 3, 43–55. [Google Scholar]
- Pottier, N.; Maurin, T.; Chevalier, B.; Puissegur, M.P.; Lebrigand, K.; Robbe-Sermesant, K.; Bertero, T.; Lino Cardenas, C.L.; Courcot, E.; Rios, G.; et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: Implication in epithelial-mesenchymal interactions. PLoS ONE 2009, 4, e6718. [Google Scholar] [PubMed]
- Corral-Fernandez, N.E.; Salgado-Bustamante, M.; Martinez-Leija, M.E.; Cortez-Espinosa, N.; Garcia-Hernandez, M.H.; Reynaga-Hernandez, E.; Quezada-Calvillo, R.; Portales-Perez, D.P. Dysregulated MIR-155 expression in peripheral blood mononuclear cells from patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2013, 121, 347–353. [Google Scholar] [PubMed]
- Kishore, R.; Verma, S.K.; Mackie, A.R.; Vaughan, E.E.; Abramova, T.V.; Aiko, I.; Krishnamurthy, P. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS One 2013, 8, e60161. [Google Scholar] [PubMed]
- Madhyastha, R.; Madhyastha, H.; Nakajima, Y.; Omura, S.; Maruyama, M. MicroRNA signature in diabetic wound healing: Promotive role of MIR-21 in fibroblast migration. Int. Wound J. 2012, 9, 355–361. [Google Scholar] [PubMed]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [PubMed]
- Brancato, S.K.; Albina, J.E. Wound macrophages as key regulators of repair: Origin, phenotype, and function. Am. J. Pathol. 2011, 178, 19–25. [Google Scholar] [PubMed]
- Meng, S.; Cao, J.T.; Zhang, B.; Zhou, Q.; Shen, C.X.; Wang, C.Q. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene spred-1. J. Mol. Cell Cardiol. 2012, 53, 64–72. [Google Scholar] [PubMed]
- Meng, S.; Cao, J.; Zhang, X.; Fan, Y.; Fang, L.; Wang, C.; Lv, Z.; Fu, D.; Li, Y. Downregulation of microRNA-130a contributes to endothelial progenitor cell dysfunction in diabetic patients via its target Runx3. PLoS ONE 2013, 8, e68611. [Google Scholar] [PubMed]
- Wang, C.H.; Lee, D.Y.; Deng, Z.; Jeyapalan, Z.; Lee, S.C.; Kahai, S.; Lu, W.Y.; Zhang, Y.; Yang, B.B. MicroRNA mir-328 regulates zonation morphogenesis by targeting CD44 expression. PLoS ONE 2008, 3, e2420. [Google Scholar] [PubMed]
- Collares, C.; Evangelista, A.; Xavier, D.; Macedo, C.; Rassi, D.; Foss-Freitas, M.; Foss, M.; Sakamoto-Hojo, E.; Passos, G.; Donadi, E. Meta-analysis of differentially expressed microRNAs in type 1, type 2 and gestational diabetes mellitus. Endocrine Abstracts 2012, 29, OC17.6. [Google Scholar]
- Caporali, A.; Emanueli, C. MicroRNA-503 and the extended microRNA-16 family in angiogenesis. Trends Cardiovasc. Med. 2011, 21, 162–166. [Google Scholar]
- Jordan, S.D.; Kruger, M.; Willmes, D.M.; Redemann, N.; Wunderlich, F.T.; Bronneke, H.S.; Merkwirth, C.; Kashkar, H.; Olkkonen, V.M.; Bottger, T.; et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated akt activation and impairs glucose metabolism. Nat. Cell Biol. 2011, 13, 434–446. [Google Scholar] [PubMed]
- Blumensatt, M.; Greulich, S.; de Wiza, D.H.; Mueller, H.; Maxhera, B.; Rabelink, M.J.; Hoeben, R.C.; Akhyari, P.; Al-Hasani, H.; Ruige, J.B.; et al. Activin a impairs insulin action in cardiomyocytes via up-regulation of mir-143. Cardiovasc. Res. 2013, 100, 201–210. [Google Scholar] [PubMed]
- Kohlstedt, K.; Trouvain, C.; Boettger, T.; Shi, L.; Fisslthaler, B.; Fleming, I. Amp-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ. Res. 2013, 112, 1150–1158. [Google Scholar] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA mir-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [PubMed]
- Van Solingen, C.; Seghers, L.; Bijkerk, R.; Duijs, J.M.; Roeten, M.K.; van Oeveren-Rietdijk, A.M.; Baelde, H.J.; Monge, M.; Vos, J.B.; de Boer, H.C.; et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J. Cell Mol. Med. 2009, 13, 1577–1585. [Google Scholar] [PubMed]
- Pastar, I.; Khan, A.A.; Stojadinovic, O.; Lebrun, E.A.; Medina, M.C.; Brem, H.; Kirsner, R.S.; Jimenez, J.J.; Leslie, C.; Tomic-Canic, M. Induction of specific microRNAs inhibits cutaneous wound healing. J. Biol. Chem. 2012, 287, 29324–29335. [Google Scholar] [PubMed]
- Sundaram, G.M.; Common, J.E.; Gopal, F.E.; Srikanta, S.; Lakshman, K.; Lunny, D.P.; Lim, T.C.; Tanavde, V.; Lane, E.B.; Sampath, P. “See-saw” expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature 2013, 495, 103–106. [Google Scholar] [PubMed]
- Bertero, T.; Gastaldi, C.; Bourget-Ponzio, I.; Imbert, V.; Loubat, A.; Selva, E.; Busca, R.; Mari, B.; Hofman, P.; Barbry, P.; et al. Mir-483-3p controls proliferation in wounded epithelial cells. FASEB J. 2011, 25, 3092–3105. [Google Scholar] [PubMed]
- Viticchie, G.; Lena, A.M.; Cianfarani, F.; Odorisio, T.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. MicroRNA-203 contributes to skin re-epithelialization. Cell Death Dis. 2012, 3, e435. [Google Scholar] [PubMed]
- Ferland-McCollough, D.; Fernandez-Twinn, D.S.; Cannell, I.G.; David, H.; Warner, M.; Vaag, A.A.; Bork-Jensen, J.; Brons, C.; Gant, T.W.; Willis, A.E.; et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2012, 19, 1003–1012. [Google Scholar] [PubMed]
- Hildebrand, J.; Rutze, M.; Walz, N.; Gallinat, S.; Wenck, H.; Deppert, W.; Grundhoff, A.; Knott, A. A comprehensive analysis of microRNA expression during human keratinocyte differentiation in vitro and in vivo. J. Invest. Dermatol. 2011, 131, 20–29. [Google Scholar] [PubMed]
- Nielsen, L.B.; Wang, C.; Sorensen, K.; Bang-Berthelsen, C.H.; Hansen, L.; Andersen, M.L.; Hougaard, P.; Juul, A.; Zhang, C.Y.; Pociot, F.; et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: Evidence that mir-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp. Diabetes Res. 2012, 2012, 896362. [Google Scholar] [PubMed]
- Greco, S.; Fasanaro, P.; Castelvecchio, S.; DʼAlessandra, Y.; Arcelli, D.; di Donato, M.; Malavazos, A.; Capogrossi, M.C.; Menicanti, L.; Martelli, F. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes 2012, 61, 1633–1641. [Google Scholar]
- Yang, X.; Wang, J.; Guo, S.L.; Fan, K.J.; Li, J.; Wang, Y.L.; Teng, Y.; Yang, X. Mir-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int. J. Biol. Sci. 2011, 7, 685–690. [Google Scholar] [PubMed]
- Wang, T.; Feng, Y.; Sun, H.; Zhang, L.; Hao, L.; Shi, C.; Wang, J.; Li, R.; Ran, X.; Su, Y.; et al. Mir-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am. J. Pathol. 2012, 181, 1911–1920. [Google Scholar] [PubMed]
- Zeng, J.; Xiong, Y.; Li, G.; Liu, M.; He, T.; Tang, Y.; Chen, Y.; Cai, L.; Jiang, R.; Tao, J. Mir-21 is overexpressed in response to high glucose and protects endothelial cells from apoptosis. Exp. Clin. Endocrinol. Diabetes 2013, 121, 425–430. [Google Scholar] [PubMed]
- Chakraborty, C.; George Priya Doss, C.; Bandyopadhyay, S. MiRNAs in insulin resistance and diabetes-associated pancreatic cancer: The “minute and miracle” molecule moving as a monitor in the “genomic galaxy”. Curr. Drug Targets 2013, 14, 1110–1117. [Google Scholar]
- Jin, Y.; Tymen, S.D.; Chen, D.; Fang, Z.J.; Zhao, Y.; Dragas, D.; Dai, Y.; Marucha, P.T.; Zhou, X. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS ONE 2013, 8, e64434. [Google Scholar] [PubMed]
- Adam, L.; Zhong, M.; Choi, W.; Qi, W.; Nicoloso, M.; Arora, A.; Calin, G.; Wang, H.; Siefker-Radtke, A.; McConkey, D.; et al. Mir-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. 2009, 15, 5060–5072. [Google Scholar] [PubMed]
- Reddy, M.A.; Jin, W.; Villeneuve, L.; Wang, M.; Lanting, L.; Todorov, I.; Kato, M.; Natarajan, R. Pro-inflammatory role of microRNA-200 in vascular smooth muscle cells from diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 721–729. [Google Scholar] [PubMed]
- Costa, R.; Negrao, R.; Valente, I.; Castela, A.; Duarte, D.; Guardao, L.; Magalhaes, P.J.; Rodrigues, J.A.; Guimaraes, J.T.; Gomes, P.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013, 76, 2047–2053. [Google Scholar]
- Darby, I.A.; Bisucci, T.; Hewitson, T.D.; MacLellan, D.G. Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int. J. Biochem. Cell Biol. 1997, 29, 191–200. [Google Scholar] [PubMed]
- Lin, A.; Hokugo, A.; Nishimura, I. Wound closure and wound management: A new therapeutic molecular target. Cell Adh. Migr. 2010, 4, 396–399. [Google Scholar] [PubMed]
- Honda, N.; Jinnin, M.; Kajihara, I.; Makino, T.; Makino, K.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; et al. TGF-β-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type i collagen expression in scleroderma dermal fibroblasts. J. Immunol. 2012, 188, 3323–3331. [Google Scholar] [PubMed]
- Kashiyama, K.; Mitsutake, N.; Matsuse, M.; Ogi, T.; Saenko, V.A.; Ujifuku, K.; Utani, A.; Hirano, A.; Yamashita, S. Mir-196a downregulation increases the expression of type i and iii collagens in keloid fibroblasts. J. Invest. Dermatol. 2012, 132, 1597–1604. [Google Scholar] [PubMed]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential role for mir-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [PubMed]
- Moura, J.; da Silva, L.; Cruz, M.T.; Carvalho, E. Molecular and cellular mechanisms of bone morphogenetic proteins and activins in the skin: Potential benefits for wound healing. Arch. Dermatol. Res. 2013, 305, 557–569. [Google Scholar] [PubMed]
- Munz, B.; Tretter, Y.P.; Hertel, M.; Engelhardt, F.; Alzheimer, C.; Werner, S. The roles of activins in repair processes of the skin and the brain. Mol. Cell Endocrinol. 2001, 180, 169–177. [Google Scholar] [PubMed]
- Wu, H.; Wu, M.; Chen, Y.; Allan, C.A.; Phillips, D.J.; Hedger, M.P. Correlation between blood activin levels and clinical parameters of type 2 diabetes. Exp. Diabetes Res. 2012, 2012, 410579. [Google Scholar] [PubMed]
- Mizuno, Y.; Tokuzawa, Y.; Ninomiya, Y.; Yagi, K.; Yatsuka-Kanesaki, Y.; Suda, T.; Fukuda, T.; Katagiri, T.; Kondoh, Y.; Amemiya, T.; et al. Mir-210 promotes osteoblastic differentiation through inhibition of acvr1b. FEBS Lett 2009, 583, 2263–2268. [Google Scholar] [PubMed]
- Liu, Y.; Taylor, N.E.; Lu, L.; Usa, K.; Cowley, A.W., Jr.; Ferreri, N.R.; Yeo, N.C.; Liang, M. Renal medullary microRNAs in dahl salt-sensitive rats: Mir-29b regulates several collagens and related genes. Hypertension 2010, 55, 974–982. [Google Scholar] [PubMed]
- Cushing, L.; Kuang, P.P.; Qian, J.; Shao, F.; Wu, J.; Little, F.; Thannickal, V.J.; Cardoso, W.V.; Lu, J. Mir-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 287–294. [Google Scholar] [PubMed]
- Monaghan, M.; Browne, S.; Schenke-Layland, K.; Pandit, A. A collagen-based scaffold delivering exogenous microRNA-29b to modulate extracellular matrix remodeling. Mol. Ther. 2014, 22, 786–796. [Google Scholar] [PubMed]
- Shilo, S.; Roy, S.; Khanna, S.; Sen, C.K. MicroRNA in cutaneous wound healing: A new paradigm. DNA Cell Biol. 2007, 26, 227–237. [Google Scholar] [PubMed]
- Banerjee, J.; Sen, C.K. MicroRNAs in skin and wound healing. Methods Mol. Biol. 2013, 936, 343–356. [Google Scholar] [PubMed]
- Sand, M.; Gambichler, T.; Sand, D.; Skrygan, M.; Altmeyer, P.; Bechara, F.G. MicroRNAs and the skin: Tiny players in the bodyʼs largest organ. J. Dermatol. Sci. 2009, 53, 169–175. [Google Scholar] [PubMed]
- Motameny, S.; Wolters, S.; Nurnberg, P.; Schumacher, B. Next generation sequencing of miRNAs—Strategies, resources and methods. Genes (Basel) 2010, 1, 70–84. [Google Scholar]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [PubMed]
- Pelaez, P.; Trejo, M.S.; Iniguez, L.P.; Estrada-Navarrete, G.; Covarrubias, A.A.; Reyes, J.L.; Sanchez, F. Identification and characterization of microRNAs in phaseolus vulgaris by high-throughput sequencing. BMC Genet. 2012, 13, 83. [Google Scholar] [PubMed]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [PubMed]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [PubMed]
- Farr, R.J.; Joglekar, M.V.; Taylor, C.J.; Hardikar, A.A. Circulating non-coding RNAs as biomarkers of beta cell death in diabetes. Pediatr. Endocrinol. Rev. 2013, 11, 14–20. [Google Scholar] [PubMed]
- Hulsmans, M.; Holvoet, P. MicroRNAs as early biomarkers in obesity and related metabolic and cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5704–5717. [Google Scholar]
- Takahashi, P.; Xavier, D.J.; Evangelista, A.F.; Manoel-Caetano, F.S.; Macedo, C.; Collares, C.V.; Foss-Freitas, M.C.; Foss, M.C.; Rassi, D.M.; Donadi, E.A.; et al. MicroRNA expression profiling and functional annotation analysis of their targets in patients with type 1 diabetes mellitus. Gene 2014, 539, 213–223. [Google Scholar] [PubMed]
- Salas-Perez, F.; Codner, E.; Valencia, E.; Pizarro, C.; Carrasco, E.; Perez-Bravo, F. MicroRNAs mir-21a and mir-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. Immunobiology 2013, 218, 733–737. [Google Scholar]
- Baran-Gale, J.; Fannin, E.E.; Kurtz, C.L.; Sethupathy, P. Beta cell 5'-shifted isomirs are candidate regulatory hubs in type 2 diabetes. PLoS ONE 2013, 8, e73240. [Google Scholar] [PubMed]
- Al-Wahbi, A.M. Impact of a diabetic foot care education program on lower limb amputation rate. Vasc. Health Risk Manag. 2010, 6, 923–934. [Google Scholar] [PubMed]
- Game, F.L.; Hinchliffe, R.J.; Apelqvist, J.; Armstrong, D.G.; Bakker, K.; Hartemann, A.; Londahl, M.; Price, P.E.; Jeffcoate, W.J. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab. Res. Rev. 2012, 28, S119–S141. [Google Scholar]
- Sieveking, D.P.; Ng, M.K. Cell therapies for therapeutic angiogenesis: Back to the bench. Vasc. Med. 2009, 14, 153–166. [Google Scholar] [PubMed]
- Kirana, S.; Stratmann, B.; Prante, C.; Prohaska, W.; Koerperich, H.; Lammers, D.; Gastens, M.H.; Quast, T.; Negrean, M.; Stirban, O.A.; et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int. J. Clin. Pract. 2012. [Google Scholar] [CrossRef]
- Shu, Y.; Pi, F.; Sharma, A.; Rajabi, M.; Haque, F.; Shu, D.; Leggas, M.; Evers, B.M.; Guo, P. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 74–89. [Google Scholar] [PubMed]
- Park, C.Y.; Choi, Y.S.; McManus, M.T. Analysis of microRNA knockouts in mice. Hum. Mol. Genet. 2010, 19, R169–R175. [Google Scholar] [PubMed]
- Ruberti, F.; Barbato, C.; Cogoni, C. Targeting microRNAs in neurons: Tools and perspectives. Exp. Neurol. 2012, 235, 419–426. [Google Scholar] [PubMed]
- Van Solingen, C.; Araldi, E.; Chamorro-Jorganes, A.; Fernandez-Hernando, C.; Suarez, Y. Improved repair of dermal wounds in mice lacking microRNA-155. J. Cell Mol. Med. 2014. [Google Scholar] [CrossRef]
- Alipour, M.R.; Khamaneh, A.M.; Yousefzadeh, N.; Mohammad-nejad, D.; Soufi, F.G. Upregulation of microRNA-146a was not accompanied by downregulation of pro-inflammatory markers in diabetic kidney. Mol. Biol. Rep. 2013, 40, 6477–6483. [Google Scholar]
- Guo, Q.; Zhang, J.; Li, J.; Zou, L.; Zhang, J.; Xie, Z.; Fu, X.; Jiang, S.; Chen, G.; Jia, Q.; et al. Forced mir-146a expression causes autoimmune lymphoproliferative syndrome in mice via downregulation of fas in germinal center b cells. Blood 2013, 121, 4875–4883. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, J.; Børsheim, E.; Carvalho, E. The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing. Genes 2014, 5, 926-956. https://doi.org/10.3390/genes5040926
Moura J, Børsheim E, Carvalho E. The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing. Genes. 2014; 5(4):926-956. https://doi.org/10.3390/genes5040926
Chicago/Turabian StyleMoura, João, Elisabet Børsheim, and Eugenia Carvalho. 2014. "The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing" Genes 5, no. 4: 926-956. https://doi.org/10.3390/genes5040926
APA StyleMoura, J., Børsheim, E., & Carvalho, E. (2014). The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing. Genes, 5(4), 926-956. https://doi.org/10.3390/genes5040926