Abstract
The ability of all organisms to copy their genetic information via DNA replication is a prerequisite for cell division and a biological imperative of life. In multicellular organisms, however, mutations arising from DNA replication errors in the germline and somatic cells are the basis of genetic diseases and cancer, respectively. Within human tumors, replication errors additionally contribute to mutator phenotypes and tumor heterogeneity, which are major confounding factors for cancer therapeutics. Successful DNA replication involves the coordination of many large-scale, complex cellular processes. In this review, we focus on the roles that defects in enzymes that normally act at the replication fork and dysregulation of enzymes that inappropriately damage single-stranded DNA at the fork play in causing mutations that contribute to carcinogenesis. We focus on tumor data and experimental evidence that error-prone variants of replicative polymerases promote carcinogenesis and on research indicating that the primary target mutated by APOBEC (apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like) cytidine deaminases is ssDNA present at the replication fork. Furthermore, we discuss evidence from model systems that indicate replication stress and other cancer-associated metabolic changes may modulate mutagenic enzymatic activities at the replication fork.
1. Introduction
The important task of copying genetic information during each cell division is accomplished through DNA replication. Normal DNA replication is phenomenally accurate. Estimates of the mutation rate per base pair during each replication cycle range from 10−9 (based on exome sequencing of somatic cells and estimation of cell division based on telomere length) [1] to 10−10 (based on mutations accumulated in individual loci) [2]. The fidelity of DNA replication is contingent upon the very high base selectivity of replicative polymerases delta (Polδ) and epsilon (Polε) during dNTP incorporation, the ability of these polymerases to proofread errors using their exonuclease domains, and error-correction by mismatch repair (MMR). In addition, maintenance of proper dNTP pools and an undamaged template are instrumental in minimizing polymerase errors during replication.
Genetic and epigenetic changes within cells that increase the number of errors that occur during DNA replication have many consequences. Mutations introduced during DNA replication provide the genetic basis for phenotypic variation upon which natural selection acts during the process of evolution. However, most mutations that affect protein function are deleterious in nature. Therefore, mutations that reduce replication fidelity in unicellular organisms and in germline cells of multicellular organisms tend to reduce fitness. Extremely inaccurate DNA replication can lead to a rapid accumulation of mutations that disrupts cellular processes needed for viability and extinguish clonal populations of cells within several generations [3,4].
Mutations or dysregulated enzymatic activities that decrease replication fidelity to non-lethal levels increase the likelihood by which loss- and gain-of-function mutations occur and thereby have the potential to indirectly alter many cellular processes. In somatic cells, the establishment of an elevated mutation rate (termed a mutator phenotype) has been proposed to be a key step in the progression of many cancers [5]. This hypothesis is supported by observations that genomic instability is both a common and defining characteristic of cancer. Cells with elevated levels of genomic instability have an increased likelihood to acquire genetic changes that result in the loss of tumor suppressors and/or activation of oncogenes. Both chromosomal instability (loss and gain of entire chromosomes, translocations, and large deletions and duplications) and point mutation instability (deletions, insertions, and base substitutions that typically involve one to three base pairs) contribute to key driver mutations leading to carcinogenic transformation. While it is becoming increasingly clear that cancer cells of many tumor types have elevated rates of mutation [5,6], the molecular basis for the mutator phenotype in many tumors is not fully understood. Here, we review literature indicating that a subset of tumors contains an elevated number of base pair substitutions caused by loss of proofreading capacity and DNA repair activities as well as increased DNA damage at the replication fork.
1.1. An Overview of the Eukaryotic DNA Replication Fork
The basic unit of DNA replication is the replication fork, at which DNA is denatured and copied. Two replication forks commence DNA replication at most origins of replication. In Saccharomyces cerevisiae, replication origins are defined by specific autonomous replicating sequences (ARS) [7,8]. The total number of S. cerevisiae replication origins is in the range of 300 to 400 with a slightly smaller number being utilized for each genome replication event [9]. Larger mammalian genomes employ approximately 40,000 origins [10]. The elements that represent human origins of replication and pathways that determine usage and timing are still poorly understood (reviewed in [11,12,13]). DNA replication is initiated by the action of the origin recognition complex (ORC), which binds to replication origins and serves as the cornerstone from which the pre-replication complex (pre-RC) is assembled. The pre-RC is assembled in G1 and includes the ORC, Cdc6, Ctd1, and the replicative DNA helicase, Mcm2–7. Early during S-phase, the pre-RC is phosphorylated by cyclin-dependent kinases. This event results in the formation of active replication fork(s) by the recruitment of Cdc45, Mcm10, and GINs complex, which constitute the CMG helicase (reviewed in [14]). Next, the DNA polymerase alpha (Polα) containing complex, Polα-primase, synthesizes short RNA-DNA primers on both the leading and lagging strand [15,16] to establish an actively synthesizing replication fork, Figure 1.
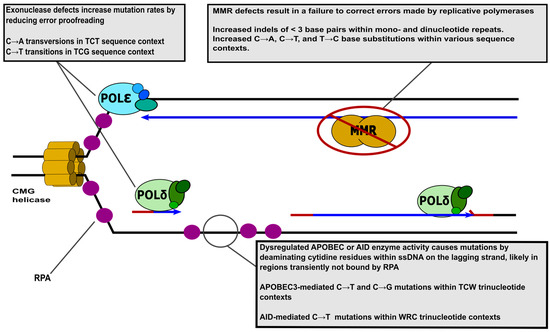
Figure 1.
Replication fork structure and mutagenic changes in enzyme activity. Replicative DNA polymerases Polδ (green) and Polε (blue) are shown on the lagging and leading strands, respectively. ssDNA binding protein RPA is depicted as purple circles. The template DNA stands, RNA primers, and newly synthesized daughter stands are represented by black, red, and blue lines, respectively. Please note that simplified depictions of proteins do not convey structural information and are not to scale. The grey call-out boxes describe mutagenic activities at the replication fork and associated mutation signatures from human tumors. Several important proteins present at the replication fork, the Replication factor C (RFC) complex, proliferating cell nuclear antigen (PCNA), and Polα have been omitted for the sake of simplicity. W (either A or T), R (either A or G).
The movement of the replication fork is driven by the CMG helicase complex, which unwinds the DNA double helix. Single-stranded DNA binding protein, replication protein A (RPA) [17,18,19,20], coats and stabilizes single-stranded DNA (ssDNA) formed at the replication fork (structural and functional studies are reviewed in [21]). After a single priming event close to the origin, leading strand synthesis occurs in a continuous fashion by Polε. Discontinuous synthesis of the lagging strand is initiated at intervals of approximately 150 nucleotides by the Polα-primase complex which synthesizes short RNA-DNA primers [22]. These primers are subsequently extended by Polδ. The processivity of both Polδ and Polε are increased by proliferating cell nuclear antigen (PCNA), which encircles the DNA template and tethers replicative DNA polymerases to the template DNA (PCNA functions reviewed in [23]). Additional details about the structure and subunits of Polδ and Polε can be found in references [24,25,26,27,28,29,30]. Replication factor C (RFC) acts to load PCNA onto DNA at the replication fork [19,31]. Once Polδ finishes synthesis of each Okazaki fragment and begins strand displacement synthesis into the downstream RNA/DNA primer, flap endonuclease Rad27 (human FEN1) and nuclease/helicase Dna2 (human DNA2) act to remove flaps created by Polδ (the roles of nucleases during Okazaki fragment maturation are reviewed in [32]). The nicks created by flap removal are repaired by DNA ligase (reviewed in [33]) resulting in a continuous lagging strand. In addition to their primary roles at the replication fork described here, many of these proteins have additional functions in replication and repair, which are often regulated by post-translational modifications.
The assignment of polymerases to opposite strands was first supported by evidence that Polδ and Polε proofread errors on opposing strands [34]. Additionally, yeast strains lacking Polδ exonuclease function are not viable in combination with loss of Rad27 [35], and Polδ is capable of using its exonuclease function to maintain a ligatable nick during strand displacement reactions [36], which indicates Polδ has a role in processing Okazaki fragments on the lagging strand. Furthermore, biochemical studies have shown that the CMG helicase interacts with and stabilizes Polε, but not Polδ, on leading strand-like templates in vitro [37]. Recently, Polδ variants [38] and Polε variants [39] that produce biased error rates have been used in conjunction with whole-genome sequencing (WGS) to demonstrate Polε and Polδ synthesis results in errors on the leading and lagging strand, respectively [40]. In contrast to the commonly accepted model, a number of observations reviewed in [41] support a model in which Polδ takes over synthesis on the leading strand after Polε synthesis is impeded. Although the current consensus is that Polδ and Polε are equally responsible for synthesis of nearly the entire genome, some evidence indicates that approximately 1.5% of the mature genome results from Polα synthesis [42]. Several mutations affecting the catalytic subunit of Polα increase the mutation rate in yeast lacking MMR or Polδ proofreading, which further indicates that the mature genome contains DNA synthesized by Polα [43,44]. Although most knowledge pertaining to the roles of replicative polymerases at the replication fork is the result of studies utilizing yeast models and in vitro biochemical studies, recent next-generation sequencing of human tumors with Polε exonuclease domain mutations indicates that the organization of the human replication fork may be similar [45]. These studies have found that Polε-induced mutations occur asymmetrically with respect to direction of replication in a pattern consistent with Polε primarily synthesizing on the leading strand. Additional work using defined experimental systems are needed to determine if current models of the replication fork based on yeast studies accurately depict the architecture of the human replication fork and strand-specific roles of DNA polymerases.
Error-prone translesion synthesis (TLS) polymerases can also synthesize DNA during DNA replication, although their roles are limited to rare circumstances. In yeast models, DNA polymerase zeta (Polζ) can carry out synthesis at the replication fork to bypass lesions that stall Polδ and Polε (reviewed in [46]) and participates in DNA replication under circumstances of replication stress or defective replication [47,48]. In human cells, TLS polymerase eta (Polη) participates in immunoglobulin hypermutation [49], and recent evidence indicates that Polη may contribute to synthesis of regions of the genome that are difficult to replicate [50]. The contribution of TLS enzymes to DNA synthesis at the replication fork in the absence of exogenous DNA damage has not been studied in detail in human cells. Based on the error-prone nature of these polymerases, they may contribute to replication-associated mutagenesis in difficult to replicate genomic regions and under conditions known to commonly cause replication stress in tumor cells.
Upon encountering obstacles to replication (e.g., DNA lesions, DNA secondary structures, and elongating transcription complexes), additional protein factors are recruited to stalled forks to help maintain their integrity. Such factors include the RecQ helicases, BLM (Bloom’s Syndrome helicase), WRN (Werner’s Syndrome helicase), RECQ5 (RecQ-like protein 5), and RECQ1 (RecQ-like protein 1) and DNA translocases, SMARCAL1 (SWI/SNF Related, Matrix Associated, Actin Dependent Regulator of Chromatin, Subfamily A Like 1), ZRANB3 (Zinc Finger RANBP2-Type Containing 3), and HLTF (helicase-like transcription factor) that are thought to limit undesirable recombination at stalled forks and facilitate replication restart (reviewed in [51,52,53,54,55,56,57,58]). Deficiency in these factors results in increases in genome instability as indicated by persistent DNA breakage, RAD51 foci, and in many cases sister chromatid exchanges [59,60,61]. Individuals inflicted with Werner’s Syndrome (deficiency in WRN helicase), Bloom’s Syndrome (deficiency in BLM helicase), Schimke immuno-osseous dysplasia (deficiency in SMARCAL1), or germline mutations in the RECQL gene display elevated incidence of cancer [62,63,64,65], suggesting that the genome instability associated with these defects can lead to cancer-promoting genetic alterations. In contrast, these proteins also appear to support continued replication in rapidly proliferating cancer cells. RecQ helicases are often over-expressed within sporadic human tumors where they likely relieve some oncogene-induced replication stress (reviewed in [66]). Accordingly, depletion of these factors or SMARCAL1 sensitizes cancer cells to chemotherapeutics and can inhibit cancer cell growth [63,67], indicating that targeting these factors may be a powerful cancer therapy.
1.2. Mismatch Repair Deficiencies Promote Cancer
Before the genetic nature of cancer was fully appreciated, Lawrence Loeb authored an article entitled “Errors in DNA Replication as a Basis for Malignant Change” in which the authors predicted that cancer might result from altered DNA polymerases that cause more errors during DNA replication and repair [68]. Numerous observations since then have supported this theory. Most significantly, decades of research examining mismatch repair defects have made it clear that errors originating from DNA synthesis contribute to carcinogenesis.
MMR is a highly-conserved pathway that acts to fix errors made during DNA replication. Eukaryotic MMR begins when a mismatch or insertion/deletion mispair is recognized by MutSα or MutSβ. MutSα is composed of Msh2 and Msh6 and recognizes base-base and small (one or two base) insertion/deletion mispairs. MutSβ is composed of Msh2 and Msh3 and recognizes small and large insertion/deletion mispairs, but not base-base mispairs. Once MutSα or MutSβ is bound to a mismatch, it recruits MutLα, composed of Mlh1 and Pms1 in S. cerevisiae and MLH1 and PMS2 in humans. MutLα acts as an endonuclease, which nicks the strand to be excised and directs the activities of other proteins in subsequent steps. The DNA strand containing the mismatch is excised by the action of Exo1 and the resulting gap is filled by the actions of RPA, RFC, PCNA, and Polδ [69,70]. In yeast, deletion of genes encoding MMR proteins increase forward (CAN1) mutation rates 18- to 40-fold and the rate of frameshifts in homopolymeric runs measured by reversion reporters as much as 662-fold [71]. Elevated spontaneous mutagenesis caused by MMR defects has been observed in many model systems (reviewed in [72]). Defects in MMR also drastically increase the frequency of cancer in mice, reviewed in [73]. In humans, inherited mutations in MMR genes predispose to colorectal cancer (CRC) in Lynch syndrome [74,75]. Additionally, MMR genes are inactivated via hypermethylation in approximately 15% of sporadic CRC, endometrial (EC), and gastric cancers (reviewed in [76]). The use of next-generation sequencing has shown that tumors with MMR defects are commonly hypermutated. For example, in colorectal cancer a distinct set of hypermutated tumors have on average 12-fold more non-silent mutations within sequenced exomes, compared to non-hypermutated CRC tumors. The majority of these hypermutated tumors had either silencing of MLH1 or somatic mutations in MMR genes and displayed microsatellite instability [77]. Tumors with MMR deficiencies have high numbers of short (<3 base pair) insertions and deletions at mono- and polynucleotide repeats and cancer-associated mutational signatures 6, 15, 20 and 26 [78,79]. Common to these MMR mutation signatures are a high probability of C-to-T, C-to-A, and/or T-to-C base substitutions. Each MMR defect-associated mutation signature has multiple preferred trinucleotide sequences in which specific mutations tend to occur [78,79].
1.3. Mutagenic Human Replicative Polymerase Variants Give Rise to Cancer
The base selectivity and proofreading activities of replicative DNA polymerases act in series with MMR to avoid replication errors and reduce the likelihood of mutation [80]. The combination of MMR defects and mutations that lower replicative polymerase fidelity cause a synergistic increase in mutagenesis that often results in lethality due to a rapid accumulation of mutations [3,4,80,81,82]. Recently, multiple studies have provided three lines of evidence that indicate defects in replicative polymerases promote carcinogenesis by increasing mutation rates: (1) mutations in genes encoding the enzymatic subunits of human Polδ and Polε, POLD1 and POLE respectively, predispose to hereditary CRC; (2) a significant number of Polε variants have been found in sporadic, MMR-proficient, hypermutated human tumors; and (3) studies of Polδ and Polε variants found in both hereditary and sporadic CRC using genetic model systems and biochemical approaches indicate that these polymerase variants elevate the spontaneous mutation rate.
Efforts to find novel causes of hereditary CRC using next-generation sequencing found that rare germline POLD1 and POLE mutations predispose individuals to CRC [83]. This study found a perfect linkage between the POLD1-S478N and POLE-L424V mutations and CRC among multiple members of affected families and identified POLD1-P327L as an additional variant likely to be pathogenic [83]. In addition, 39 tumors from individuals with the germline POLE mutation, POLE-L424V, were screened for mutations in six proto-oncogenes and tumor suppressors. All the driver mutations found were base substitutions, many of which were concentrated at atypical hotspots [83]. Because error-prone replicative polymerase variants produce mutational spectra dominated by base substitutions, the previous observation indicates that the Polδ and Polε variants encoded by the germline POLD1 and POLE alleles generate driver mutations in these patients. Since this seminal discovery, several publications have found evidence supporting roles for additional germline POLD1 and POLE mutations in cancer predisposition, in which carriers typically develop multiple adenomas, polyposis, CRC, and/or EC. These pathogenic germline mutations in POLE and POLD1 mutations are summarized in Table 1. Several recent publications indicate that some inherited Polε variants may give rise to significantly different diseases. A 14-year-old boy with polyposis and rectosigmoid carcinoma was found to have inherited a POLE-V411L mutation [84]. Because this case clinically resembled inherited bi-allelic mismatch repair deficiency in its early onset and severity, it appears that different polymerase variants may have more severe phenotypes. Unlike the aforementioned POLD1 and POLE mutations, POLE-W347C may predispose to cutaneous melanoma and affected patients do not have CRC or EC [85].

Table 1.
Pathogenic replicative polymerase mutations.
Cancer genome sequencing projects have also identified somatic changes in the exonuclease domain of Polε in approximately 3% of sporadic CRC tumors and 7% of sporadic EC tumors [77,96,97,98]. Because these POLE exonulease domain mutations are found primarily in tumors that do not have microsatellite instability and are hypermutated, the current consensus is that the encoded Polε variants are responsible for the high number of mutations found in these tumors and are pathogenic, Table 1, and reviewed in [99,100]. Tumors with known pathogenic POLE mutations represent a separate class of tumors due to the number of mutations present. The density of mutations in hypermutated CRC cancers with MMR deficiencies is approximately 12 to 55 mutations per 106 base pairs. In contrast, hypermutated tumors with POLE variants have mutation densities ranging from approximately 60 to 380 mutations per 106 base pairs, and are thus termed “ultra-mutated” [77]. Because next-generation sequencing methods employed in these studies only detect near clonal mutations and not mutations present in individual tumor cells, these mutation densities likely grossly under-estimate the total number of mutations caused by POLE variants within tumors.
Several lines of evidence indicate that germline and somatic POLE and PODL1 variants increase cancer predisposition by elevating mutation rates. For POLD1 variants that predispose to CRC, mutations affecting residues homologous to D316 and L474 [87] and S478 [83] were previously shown to increase mutagenesis in yeast models. The most common POLE mutation found in sporadic CRC and EC, P286R, was found to increase the mutation rate when modeled yeast [89]. Inexplicably, the increase in the mutation rate caused by the analogous mutation in diploid yeast was approximately 300-fold greater than that caused by a mutation eliminating Polε proofreading [86,91,92,94]. In contrast, four human single nucleotide polymorphisms (SNPs) modeled in yeast, pol3-K855H, pol3-K1084Q, pol2-F709I, and pol2-E1582A did not change the rate of spontaneous mutagenesis [101]. In addition, the cancer-associated human Polε variants (P286R, P286H, F367F, S459F, and L424V) have been shown to have reduced exonuclease activity and higher error rates in vitro using LacZ gap-filling assays [45]. Together these studies indicate that a subset of replicative polymerase variants found in human cancers promote carcinogenesis by increasing mutation rates in vivo.
Much work remains to be done before a comprehensive understanding of the role that replicative polymerase variants play in promoting cancer can be realized. Recent efforts to sequence cancer genomes have led to the discovery of least 346 unique mutations in POLE alone (cataloged within the cBioPortal data sets, http://www.cbioportal.org, [102,103]). Additionally, the number of POLD1 and POLE mutations in human cancers will likely increase substantially as more cancer genomes are sequenced. A major challenge going forward will be to differentiate the few polymerase variants that reduce replication fidelity and promote cancer from the large number of randomly occurring passenger mutations within POLE and POLD1. Next-generation sequencing of sporadic endometrial and colorectal tumors have made it clear that POLE exonuclease domain mutations (EDMs) are causative in a subset of hypermutated, microsatellite stable (MSS) tumors (reviewed in [99,100]). Based on these findings, it would seem prudent to study those somatic POLE mutations that fall within the exonuclease domain and are found in MSS hypermutated tumors. However, compelling results from [101,104] suggest that less frequent somatically occurring, cancer-associated POLD1 mutations outside of the exonuclease domain found in MMR deficient tumors have the potential to elevate mutation rates and promote cancer. Therefore, solely focusing upon POLE and/or EDMs may fail to identify all the replicative polymerase variants that contribute to cancer etiology. Consequently, most current efforts to identify pathogenic germline POLD1 and POLE mutations have focused solely on the exonuclease domain [86,90,91,92,93,94]. The most direct and definitive method to assess the pathogenicity of cancer-associated polymerase variants is to determine if they elevate mutation rates in human cell lines. Surprisingly, no studies have been published that show any cancer-associated polymerase variant increases mutation rates in cultured human cells.
Several interesting conundrums exist in respect to mutagenic polymerase variants and cancer. First, it is unclear why hypermutated tumors with POLE exonuclease domain mutations have better survival than other tumors of the same cancer type. Although it is easy to imagine that hypermutated tumors would be more resistant to chemotherapies due to increased tumor heterogeneity, in fact the opposite appears to be true. Results from a recent study indicate that tumors hypermutated by mutant Polε may invoke a stronger immune response [105], which may explain this contradiction. Alternatively, the extremely high mutation load within these tumors may place a fitness burden on these tumors. Second, it is unclear why error-prone replicative polymerase variants tend to give rise to a limited number of tumor types. Third, it is unknown why almost all sporadic polymerase variants that give rise to hypermutated tumors are within the exonuclease domain of Polε. Mutations that decrease or eliminate exonuclease function might be more prevalent than specific mutations that decrease base selectivity. Finally, given that the exonuclease domains of Polδ and Polε are a similar size, share a great deal of homology, and that both polymerases synthesize similar amounts of DNA during replication, why POLE mutations are almost exclusively found as promoters of sporadic cancer is unclear. It has been speculated by others that proofreading-deficient Polδ variants might lead to a more severe phenotype due to their propensity to elevate frameshift mutations in addition to base substitutions and are therefore selected against in cells. However, because germline POLD1 mutations that likely decrease, or eliminate exonuclease function give rise to hereditary CRC, it is unlikely that similar somatic mutations would be selected against. One possibility is that during sporadic tumorigenesis, human cancer cells require Polδ exonuclease for functions needed to cope with DNA damage resulting from replication stress and elevated levels of reactive oxygen species and Polε exonuclease function is dispensable for these functions.
1.4. Damage to Single-Stranded DNA on the Lagging Strand Template Causes Mutation in Cancer
In addition to deficiencies in mismatch repair and polymerase exonuclease activity generating mutations during replication, recent evidence has highlighted increased damage in ssDNA formed on the lagging strand template as an important source of replication-associated mutagenesis. In human cancers, this is exemplified by the mutagenic activity of APOBEC cytidine deaminases. Eleven AID/APOBEC family members are encoded in the human genome, of which, seven are APOBEC3 members [106] (Table 2). APOBECs are involved in several normal biological processes including roles in lipid metabolism and immune function (e.g., antibody maturation and inhibiting viral propagation) [106]. The APOBEC3 enzymes (A3) mediate their cellular effects by catalyzing the sequence-specific deamination of deoxycytidines to deoxyuridines within single-stranded nucleic acids [107,108,109,110]. Most APOBECs target the trinucleotide sequences TCA and TCT (hereafter referred to jointly as TCW) [106]. Their C-to-U editing functionality can ultimately result in either C-to-T transitions or C-to-G transversions depending on the efficiency by which uracil glycosylase activity converts deamination-induced deoxyuridines to abasic sites and the choice of DNA polymerase inserting nucleotides across from the abasic sites [111].

Table 2.
APOBEC characteristics and their involvement in cancer mutagenesis.
While APOBECs are typically tightly regulated by controlled expression [119] and cellular localization to the cytoplasm [120], deleterious consequences can result when off-target editing of the host’s genome occurs. Accordingly, emerging data indicate that APOBECs play a role in the etiology of many human cancers. An overabundance of APOBEC signature mutations (C-to-T and C-to-G substitutions in TCW sequences) have been found in ~15% of sequenced tumor samples [78]. APOBEC-mutagenized tumors frequently display mutation densities up to 50 mutations per 106 bp [121,122], indicating that like MMR and replicative polymerase defects, APOBEC-derived mutagenesis is a process that litters the genomic landscape with somatic point mutations. Cumulative evidence has shown that APOBEC expression causes a mutator phenotype with a positive correlation between increased APOBEC mRNA expression and the extent of APOBEC mutagenesis [121,122,123]. The nucleotide context of mutational signatures, their genomic distribution, and regions of localized hypermutation (termed kataegis) found in studies characterizing APOBEC activity in model systems are extremely similar to those observed in human tumors, suggesting that APOBEC activity potentially contributes to the onset and/or progression of tumor formation by increasing the mutational burden (reviewed in [124]). Additionally, bioinformatics analyses by Henderson et al. revealed that the proto-oncogene, PIK3CA, was frequently mutated in tumor types expressing high APOBEC mRNA levels such as HPV-positive CESC and HNSCC (cervical squamous cell carcinoma and endocervical adenocarcinoma and head and neck squamous cell carcinoma) [125]. Moreover, 88% of these PIK3CA mutations occurred in two hotspot sites occurring at APOBEC-targeted sequences (TCW) in the helical domain of the protein that binds the p85 inhibitory protein, as opposed to the more common activating kinase domain mutation which does not occur at an APOBEC target sequence. This evidence strongly indicates that in some capacity, APOBEC enzymes contribute to the mutations selected for during cancer development. In accord with these observations, over-expression of APOBEC1 and APOBEC2 in mice has been shown to be sufficient to induce tumorigenesis, suggesting that unrestrained activity of this family of enzymes is carcinogenic [126,127]. However, no elevation of mutation was detected in APOBEC2-induced tumors and mutagenesis in mouse tumors induced by APOBEC1 were not evaluated leaving the mechanism of this tumorigenesis unclear.
Determining the identity of the APOBECs that mediate cancer mutagenesis has been a recent focus of the field. APOBEC3A (A3A) and APOBEC3B (A3B) have nuclear localization capabilities, making them likely candidates for genomic DNA editing [120,128]. Experimentally, A3B was shown to be over-expressed, the primary source of cytidine deaminase activity, and a source of mutation in a panel of breast carcinoma cell lines, indicating a role for this enzyme in breast cancer mutagenesis [129]. Similarly, additional bioinformatics analyses found that A3B mRNA expression levels positively correlate with the amount of APOBEC signature mutations in multiple tumor types including breast, bladder, cervix, head and neck, and lung (adenocarcinoma and squamous cell carcinoma) [121,122]. Recently, a human polymorphism upstream of the APOBEC3A gene and linked to bladder cancer risk, was shown to increase A3B expression, suggesting that greater amounts of this enzyme in cells may be carcinogenic [130]. However, seemingly paradoxical, a germline APOBEC3A-APOBEC3B fusion polymorphism causing deletion of A3B is associated with greater risk for breast, ovarian and liver cancer along with an overall increase in mutations present in ΔA3B−/− breast cancers [130,131,132,133,134,135]. One potential explanation for this is that the deletion of A3B results in increased activity of other APOBEC enzymes, perhaps in a compensatory fashion. Caval et al. [131] studied the consequences of the fusion of the A3B-3′UTR to A3A, which occurs in individuals containing the A3B deletion polymorphism. They found that the replacement of the A3A-3′UTR with that of A3B’s resulted in stabilization of A3A mRNA, increased A3A expression, and genomic DNA editing by A3A [131]. Supporting a role for A3A in cancer mutagenesis, Chan et al. [136] determined that when expressed in yeast, A3A and A3B preferred slightly different DNA sequences, targeting YTCA and RTCA, respectively. They further showed that A3A-like (YTCA) mutations were more abundant than A3B-like (RTCA) mutations in many sequenced tumors [136]. In addition to A3A and A3B activity, other APOBECs have been linked to cancer development. AID’s role in promoting cancers of the blood has been long established (reviewed in [137]), while APOBEC1 over-expression has been linked to the onset of esophageal adenocarcinomas [78,138]. Recent work by Reuben Harris and colleagues now suggests that A3H-I haplotype activity may account for some of the APOBEC-induced mutation load based on A3B-null breast tumor analysis [139].
Since APOBEC enzymes are ssDNA specific, determining the source of their substrate in a double-stranded genome has been a matter of great interest. Several candidate metabolic processes expose ssDNA for APOBEC mutagenesis, including transcription, DNA repair and DNA replication. Transcription-associated ssDNA was originally believed to be the main target of APOBEC activity, primarily by extension of AID’s known dependence on transcription to mediate somatic hypermutation and class switch recombination during B-cell maturation [112,113,114,115]. In fact, the expression of lamprey APOBEC, as well as hypermutator forms of AID and APOBEC3G (A3G) in yeast revealed an overabundance of mutations occurring mostly 5′ of transcription start sites, indicating that transcription intermediates can be targets of these enzymes [140,141]. Such damage to transcription bubbles could be very significant to human cancer mutagenesis as oncogene activation can lead to the elevated formation of R-loops as transcription becomes upregulated [142].
Similarly, the formation of kataegic events linked to the ectopic expression of AID, A3A, A3B, and A3G in yeast is dependent on Ung1 activity, indicating that DNA repair intermediates can provide substrates for these enzymes as well [116]. DNA double-strand break (DSB) repair intermediates may provide the greatest amount of substrate for kataegis, as these events are greatly elevated by induction of DSBs. Homology-directed repair of DSBs provides large stretches of ssDNA through 5′ to 3′ double-strand break resection [143,144], which APOBECs likely can mutagenize. Additionally, break-induced replication (BIR), a variant of homologous recombination involving only one end of a DSB, creates a very long ssDNA intermediate during the extended D-loop synthesis used to repair these breaks [145]. This synthesis is a form of conservative replication that has been shown to serve as a source of kataegis induced by alkylating DNA damage and presumably APOBEC enzymes as well [146].
Despite these links describing APOBEC mutagenesis of transcription and DSB repair processes, results from several studies indicate that most APOBEC-induced mutations occur during DNA replication in cancer genomes. Single-stranded DNA formed on the lagging strand template during Okazaki fragment synthesis provides the most abundant source of ssDNA during normal cell division, Figure 1. Moreover, establishment of bi-directional replication forks results in ssDNA in the lagging strand template occurring on different DNA strands dependent on the direction of fork movement. Multiple analyses of the distribution of APOBEC-induced mutations identified by WGS have utilized the asymmetry in the location of lagging strand-associated ssDNA to correlate the substitution patterns of APOBEC mutagenesis with replication-associated ssDNA. Bhagwat et al. [147] expressed the catalytic domain of human A3G in E. coli defective for repair of uracil (ung mutant) and determined that C-to-T substitutions induced by this enzyme preferentially occurred in replichore 1, while G-to-A substitutions occurred more frequently in replichore 2 of the genome. As replichore 1 and replichore 2 are replicated in clockwise and anticlockwise directions respectively, this distribution is consistent with cytidine deamination occurring predominantly in ssDNA on the lagging strand template [147]. No mutational strand bias was observed in relationship to transcriptional direction, indicating that in replicating cells, the primary substrate for A3G mutagenesis is ssDNA at the replication fork. The authors saw a similar phenomenon with spontaneous mutagenesis, indicating that mutagenesis associated with damage to ssDNA at the fork may be a general source of mutation beyond APOBEC activity. In concert with this finding, other APOBECs likewise have been experimentally shown to prefer replication-based substrates. In yeast ectopically expressing A3A or A3B, strand-biased mutations were observed in gene mutation reporters placed on either side of a single autonomously replicating sequence (ARS). Through WGS, the pattern of mutagenesis identified was indicative of replicative asymmetry across the genome as there was a predominance of G-to-A substitutions 5′ of origins and C-to-T substitutions 3′ of origins [117]. As with A3G mutation in E. coli, neither A3A- nor A3B-induced mutations in yeast displayed significant transcriptional strand asymmetries, indicating that both of these APOBECs predominately mutate replication intermediates and that this preference is generally applicable to the entire APOBEC family. Supporting this, even forced S-phase expression of AID, an APOBEC whose mutagenic capacity is undeniably linked in transcription, results in increased cell death, suggesting that this enzyme may also be able to deaminate replication-associated ssDNA if it is available [148]. APOBEC deamination of replication intermediates has also been reported in human cells where it is a source of DSBs produced by S-phase expression of A3A [118]. These experimental analyses have since served as crucial support that during tumor development, APOBECs likely mutagenize cancer genomes by taking advantage of the highly proliferative nature of these cells. WGS of hundreds of samples across multiple tumor types indicate that, as in yeast and E. coli expressing APOBEC enzymes, APOBECs predominantly deaminate the lagging strand template in human tumors. While the locations of origin of replication in human cells are largely unknown, the direction of replication across individual regions of the genome can be inferred from replication timing profiles. Using this information, three groups have profiled the position of APOBEC signature mutations in relationship to replication directions, uncovering a significant elevation of C substitutions in regions replicated with rightward moving forks, while G substitutions occurred predominantly in regions replicated with leftward moving forks. This “replicative asymmetry” (also termed “R-class”) is consistent with mutagenesis of the ssDNA lagging strand template and has been observed for other mutation signatures associated with replication defects (i.e., MMR defects and Polε mutations). Only limited transcriptional asymmetry was observed among APOBEC-induced mutations, in contrast to UV and tobacco smoke-induced mutations whose localization is lessened on the transcribed strand of genes by transcription coupled repair [149,150,151].
Despite the significant advances in understanding the roles of APOBEC enzymes in tumor mutagenesis, multiple questions remain. While it is generally accepted that these enzymes are responsible for the production of large numbers of mutations in cancer, in many cases the initiating events leading to their dysregulation are still unknown. The association of APOBEC mutagenesis with cervical and head and neck cancers [78,121,122,125], which frequently involve HPV infection, suggest that up-regulation of these enzymes by HPV or induction of replication stress by HPV encoded proteins that inhibit RB1 function [152,153] may be a key event in initially establishing an APOBEC mutator phenotype. However, the cellular events that cause mutagenesis resulting from aberrant APOBEC activity in other tumor types is unknown. Understanding the root causes of APOBEC dysregulation is likely to provide key insights into the tumor specificity of these enzymes. Similarly, the biological effects of APOBEC mutagenesis on cancer development, progression, and treatment are largely unclear. The association of APOBEC polymorphisms with cancer risk and the apparent APOBEC-induction of PIK3CA mutations [125] indicate that these enzymes likely play significant roles in promoting cancer onset. However, the large numbers of mutations these enzymes induce suggests that they may additionally contribute to continued evolution of the tumor and ultimately to therapy resistance. This role is supported by experimental evidence indicating that elevated A3B expression in breast cancer cell lines increases the resistance of derived xenografted tumors to the drug tamoxifen [154]. Intriguingly, APOBEC mutagenesis within tumors may not solely provide deleterious effects. Similar to tumors with polymerase defects that also produce high mutation loads, high numbers of APOBEC mutations in bladder cancer associate with longer patient survival times [130]. This suggests that the activity of these enzymes may reduce the overall fitness of cancer cells. This effect may enable the development of future therapeutic strategies that take advantage of liabilities associated with APOBEC activity.
1.5. Future Directions: Tumor Specific Metabolic Changes as Modulators of Replication Fork-Associated Mutagenesis
We speculate that the rate of mutagenesis resulting from activities at the replication fork may be affected by mutations that activate oncogenes and inactivate tumor suppressors. Consequently, mutation rates in tumor cells might fluctuate throughout the process of carcinogenesis. In addition, mutations present in tumor subpopulations as both drivers and passengers that increase the rate of mutation could allow tumors to acquire mutations needed for progression and resistance to therapies while allowing most cells to escape the deleterious effects of an ultra-high mutation rate.
Pathways that regulate dNTP levels are often mutated in human cancers. Mutations that activate the Ras signaling pathway decrease dNTP pools by decreasing levels of RRM2, a subunit of human ribonucleotide reductase (RNR) [155]. Loss of the retinoblastoma tumor suppressor (RB) causes elevated expression of many genes involved in dNTP metabolism and an elevation of dNTP pools [156]. AMP-activated protein kinase (AMPK) activity is often deregulated in cancer. AMPK regulates phosphotransferase nucleoside diphosphate kinase (NDPK), which is the enzyme responsible for converting dNDPs to dNTPs [157]. The proto-oncogene MYC (C-Myc) is overexpressed in most human tumors. Overexpression of C-Myc in normal human cells leads to increased expression of thymidylate synthase (TS), inosine monophosphate dehydrogenase 2 (IMPDH2) and phosphoribosyl pyrophosphate synthetase 2 (PRPS2) and increased dNTP pools [158]. Tumor suppressor p53 restricts human RNR activity by binding to human RNR regulatory subunits RRM2 and p53R2 [159]. Taken together, the results of these studies indicate that dNTP pools likely fluctuate during the process of carcinogenesis.
In respect to polymerases acting at the replication fork, both decreased and increased dNTP levels have been shown to decrease replication fidelity. In yeast, decreasing dNTP pools by exposure to the ribonucleotide reductase inhibitor, hydroxyurea, results in an increase in mutagenesis that is primarily Polζ dependent [48]. Conversely, in vitro experiments have shown that increasing dNTP concentration both improves the likelihood that a replicative polymerase will extend from a mismatched primer terminus [160,161,162], and increases errors during synthesis [163]. Consistent with these findings, proportional increases in dNTP levels in E. coli are also mutagenic [164,165]. Furthermore, several studies in yeast have shown that moderately decreasing dNTP levels in yeast by deletion of DUN1, suppresses the mutator phenotype of both Polδ and Polε variants [4,81,163,166]. Taken together, these results from biochemical experiments and model systems indicate that changes in dNTP levels which occur during carcinogenesis likely substantially modulate mutagenesis caused by polymerase variants in human tumors.
We speculate that several phenomena occurring during carcinogenesis may modulate APOBEC-induced mutagenesis at the replication fork. First, oncogene activation and elevated DNA damage during cancer development can cause replication stress that increases the formation of replication-associated ssDNA [167]. Such increases in ssDNA availability may provide greater opportunities for APOBECs to damage the chromosomes of proliferating tumor cells, resulting in dramatically higher APOBEC-induced mutation densities. Recent evidence suggests that synergistic interactions between APOBEC mutagenesis and replication stress may occur through two mechanisms. First, replication stress appears to increase the expression level of A3B in a variety of cancer cell lines, thereby increasing the cellular pool of this mutator [168]. Secondly, a greater mutagenic response was observed for both A3A and A3B expressed in yeast in the presence of the replication inhibitor hydroxyurea (HU) as well as in strains lacking replication fork stability proteins [117]. Mutation spectra indicate that the observed increase in mutagenesis likely occurred due to more replication-associated ssDNA being available on both the leading and lagging strand during DNA replication. Consequently, cancer-associated mutations that result in replication stress by decreasing ribonucleotide reductase expression [155] could increase APOBEC-induced mutagenesis. Although speculative, in cells in which oncogene activation leads to increased replication stress caused by elevated replication origin firing, dNTPs levels could also become insufficient for efficient replication and result in more ssDNA being available to APOBECs. Genetic and epigenetic differences that influence dNTP levels in tumor cells should be studied as a possible explanation for why tumors with similar APOBEC expression levels have drastically different amounts of APOBEC-signature mutations. The extent to which the synergistic interactions between replication stress and APOBEC activity impact the abundance of mutations in tumors remains unclear.
2. Conclusions
In humans, each cell division requires the replication of approximately 3.3 × 109 base pairs of DNA. Current estimates indicate the number of cells in the human body is around 3.72 × 1013 [169], and approximately 5 × 1010 to 7 × 1010 cells are replaced daily. Taken together the amount of DNA that is replicated over a human lifetime is staggering. Fortunately, genomic stability is typically maintained by a semi-conservative process for DNA replication with multiple mechanisms that increase fidelity such that typically less than one mutation occurs per cell division [2]. Although multiple processes safeguard the fidelity of DNA replication, there are still inherent risks involved in this necessary process. Mutations generated during DNA replication promote carcinogenesis by inactivating tumor suppressors and activating oncogenes. Recent developments have made it apparent that the risks associated with DNA replication are increased by specific mutations in replicative polymerases that promote carcinogenesis. Furthermore, ssDNA produced by the process of DNA replication represents a potential risk for mutagenesis mediated by chemicals and enzymes. Consequently, targeting of replication-associated ssDNA by APOBEC enzymes, whose activity is dysregulated in some cancer cells, results in significant mutagenesis in many tumors. Replication-associated mutagenesis both promotes carcinogenesis and likely affects clinical outcomes by increasing tumor heterogeneity. Further characterizing mutations and pathways that modulate risks associated with DNA replication will provide a better understanding of the etiology of cancer-causing mutations and may provide future opportunities for cancer treatment.
Acknowledgments
This work was supported by grants ES022633 from the National Institute of Environmental Health Sciences; and the Breast Cancer Research Program Breakthrough Award BC141727 from the Department of Defense to Steven A. Roberts.
Author Contributions
Tony M. Mertz and Victoria Harcy contributed equally to the writing of this manuscript. Steven A. Roberts edited draft versions. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saini, N.; Roberts, S.A.; Klimczak, L.J.; Chan, K.; Grimm, S.A.; Dai, S.; Fargo, D.C.; Boyer, J.C.; Kaufmann, W.K.; Taylor, J.A.; et al. The impact of environmental and endogenous damage on somatic mutation load in human skin fibroblasts. PLoS Genet. 2016, 12, e1006385. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.W.; Charlesworth, B.; Charlesworth, D.; Crow, J.F. Rates of spontaneous mutation. Genetics 1998, 148, 1667–1686. [Google Scholar] [PubMed]
- Herr, A.J.; Ogawa, M.; Lawrence, N.A.; Williams, L.N.; Eggington, J.M.; Singh, M.; Smith, R.A.; Preston, B.D. Mutator suppression and escape from replication error-induced extinction in yeast. PLoS Genet. 2011, 7, e1002282. [Google Scholar] [CrossRef]
- Herr, A.J.; Kennedy, S.R.; Knowels, G.M.; Schultz, E.M.; Preston, B.D. DNA replication error-induced extinction of diploid yeast. Genetics 2014, 196, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L.A. Human cancers express a mutator phenotype: Hypothesis, origin, and consequences. Cancer Res. 2016, 76, 2057–2059. [Google Scholar] [CrossRef] [PubMed]
- Bielas, J.H.; Loeb, K.R.; Rubin, B.P.; True, L.D.; Loeb, L.A. Human cancers express a mutator phenotype. Proc. Natl. Acad. Sci. USA 2006, 103, 18238–18242. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.F.; Newlon, C.S. The ARS309 chromosomal replicator of Saccharomyces cerevisiae depends on an exceptional ars consensus sequence. Proc. Natl. Acad. Sci. USA 1997, 94, 10786–10791. [Google Scholar] [CrossRef] [PubMed]
- Stinchcomb, D.; Struhl, K.; Davis, R. Isolation and characterisation of a yeast chromosomal replicator. Nature 1979, 282, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nieduszynski, C.A.; Knox, Y.; Donaldson, A.D. Genome-wide identification of replication origins in yeast by comparative genomics. Genes Dev. 2006, 20, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Huberman, J.A.; Riggs, A.D. Autoradiography of chromosomal DNA fibers from chinese hamster cells. Proc. Natl. Acad. Sci. USA 1966, 55, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Mechali, M. Eukaryotic DNA replication origins: Many choices for appropriate answers. Nat. Rev. Mol. Cell Biol. 2010, 11, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Takisawa, H.; Mimura, S.; Kubota, Y. Eukaryotic DNA replication: From pre-replication complex to initiation complex. Curr. Opin. Cell Biol. 2000, 12, 690–696. [Google Scholar] [CrossRef]
- Bell, S.P. The origin recognition complex: From simple origins to complex functions. Genes Dev. 2002, 16, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Boos, D.; Frigola, J.; Diffley, J.F. Activation of the replicative DNA helicase: Breaking up is hard to do. Curr. Opin. Cell Biol. 2012, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L. The pol α-primase complex. In The Eukaryotic Replisome: A Guide to Protein Structure and Function; Springer: New York, NY, USA, 2012; pp. 157–169. [Google Scholar]
- Muzi-Falconi, M.; Giannattasio, M.; Foiani, M.; Plevani, P. The DNA polymerase alpha-primase complex: Multiple functions and interactions. Sci. World J. 2003, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wold, M.S.; Kelly, T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc. Natl. Acad. Sci. USA 1988, 85, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Wobbe, C.R.; Weissbach, L.; Borowiec, J.A.; Dean, F.B.; Murakami, Y.; Bullock, P.; Hurwitz, J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Fairman, M.P.; Stillman, B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988, 7, 1211–1218. [Google Scholar] [PubMed]
- Brill, S.J.; Stillman, B. Yeast replication factor-A functions in the unwinding of the SV40 origin of DNA replication. Nature 1989, 342, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Fanning, E.; Klimovich, V.; Nager, A.R. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nuclic Acids Res. 2006, 34, 4126–4137. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Whitehouse, I. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature 2012, 483, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Baranovskiy, A.G.; Babayeva, N.D.; Liston, V.G.; Rogozin, I.B.; Koonin, E.V.; Pavlov, Y.I.; Vassylyev, D.G.; Tahirov, T.H. X-ray structure of the complex of regulatory subunits of human DNA polymerase delta. Cell Cycle 2008, 7, 3026–3036. [Google Scholar] [CrossRef] [PubMed]
- Doublie, S.; Zahn, K.E. Structural insights into eukaryotic DNA replication. Front. Microbiol. 2014, 5, 444. [Google Scholar] [CrossRef] [PubMed]
- Hogg, M.; Osterman, P.; Bylund, G.O.; Ganai, R.A.; Lundstrom, E.B.; Sauer-Eriksson, A.E.; Johansson, E. Structural basis for processive DNA synthesis by yeast DNA polymerase varepsilon. Nat. Struct. Mol. Biol. 2014, 21, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Isoz, I.; Persson, U.; Volkov, K.; Johansson, E. The C-terminus of Dpb2 is required for interaction with Pol2 and for cell viability. Nuclic Acids Res. 2012, 40, 11545–11553. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Rajashankar, K.R.; Buku, A.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. Crystal structure of yeast DNA polymerase epsilon catalytic domain. PLoS ONE 2014, 9, e94835. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Wang, X.; Zhang, S.; Zhang, Z.; Lee, E.Y.; Lee, M.Y. Dynamics of enzymatic interactions during short flap human okazaki fragment processing by two forms of human DNA polymerase delta. DNA Repair 2013, 12, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Swan, M.K.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase delta. Nat. Struct. Mol. Biol. 2009, 16, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Tsurimoto, T.; Stillman, B. Purification of a cellular replication factor, RF-C, that is required for coordinated synthesis of leading and lagging strands during simian virus 40 DNA replication in vitro. Mol. Cell. Biol. 1989, 9, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shen, B. Okazaki fragment maturation: Nucleases take centre stage. J. Mol. Cell Biol. 2011, 3, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, T.; Tomkinson, A.E. Eukaryotic DNA ligases: Structural and functional insights. Annu. Rev. Biochem. 2008, 77, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, P.V.; Pavlov, Y.I. 3′→5′ exonucleases of DNA polymerases ϵ and δ correct base analog induced DNA replication errors on opposite DNA strands in Saccharomyces cerevisiae. Genetics 1996, 142, 717–726. [Google Scholar] [PubMed]
- Jin, Y.H.; Obert, R.; Burgers, P.M.; Kunkel, T.A.; Resnick, M.A.; Gordenin, D.A. The 3′→5′ exonuclease of DNA polymerase δ can substitute for the 5′ flap endonuclease Rad27/Fen1 in processing Okazaki fragments and preventing genome instability. Proc. Natl. Acad. Sci. USA 2001, 98, 5122–5127. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Stith, C.M.; Sabouri, N.; Johansson, E.; Burgers, P.M. Idling by DNA polymerase delta maintains a ligatable nick during lagging-strand DNA replication. Genes Dev. 2004, 18, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, R.E.; Langston, L.; Yao, N.Y.; Yurieva, O.; Zhang, D.; Finkelstein, J.; Agarwal, T.; O’Donnell, M.E. Mechanism of asymmetric polymerase assembly at the eukaryotic replication fork. Nat. Struct. Mol. Biol. 2014, 21, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Nick McElhinny, S.A.; Gordenin, D.A.; Stith, C.M.; Burgers, P.M.; Kunkel, T.A. Division of labor at the eukaryotic replication fork. Mol. Cell 2008, 30, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Pursell, Z.F.; Isoz, I.; Lundstrom, E.B.; Johansson, E.; Kunkel, T.A. Yeast DNA polymerase e participates in leading-strand DNA replication. Science 2007, 317, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Lujan, S.A.; Clausen, A.R.; Clark, A.B.; MacAlpine, H.K.; MacAlpine, D.M.; Malc, E.P.; Mieczkowski, P.A.; Burkholder, A.B.; Fargo, D.C.; Gordenin, D.A.; et al. Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition. Genome Res. 2014, 24, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, Y.I.; Shcherbakova, P.V. DNA polymerases at the eukaryotic fork-20 years later. Mutat. Res. 2010, 685, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Reijns, M.A.; Kemp, H.; Ding, J.; de Proce, S.M.; Jackson, A.P.; Taylor, M.S. Lagging-strand replication shapes the mutational landscape of the genome. Nature 2015, 518, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Niimi, A.; Limsirichaikul, S.; Yoshida, S.; Iwai, S.; Masutani, C.; Hanaoka, F.; Kool, E.T.; Nishiyama, Y.; Suzuki, M. Palm mutants in DNA polymerases alpha and eta alter DNA replication fidelity and translesion activity. Mol. Cell. Biol. 2004, 24, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, Y.I.; Frahm, C.; Nick McElhinny, S.A.; Niimi, A.; Suzuki, M.; Kunkel, T.A. Evidence that errors made by DNA polymerase alpha are corrected by DNA polymerase delta. Curr. Biol. 2006, 16, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Shinbrot, E.; Henninger, E.E.; Weinhold, N.; Covington, K.R.; Goksenin, A.Y.; Schultz, N.; Chao, H.; Doddapaneni, H.; Muzny, D.M.; Gibbs, R.A.; et al. Exonuclease mutations in DNA polymerase epsilon reveal replication strand specific mutation patterns and human origins of replication. Genome Res. 2014, 24, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Johnson, R.E.; Prakash, L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu. Rev. Biochem. 2005, 74, 317–353. [Google Scholar] [CrossRef] [PubMed]
- Northam, M.R.; Garg, P.; Baitin, D.M.; Burgers, P.M.; Shcherbakova, P.V. A novel function of DNA polymerase ζ regulated by PCNA. EMBO J. 2006, 25, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Northam, M.R.; Robinson, H.A.; Kochenova, O.V.; Shcherbakova, P.V. Participation of DNA polymerase zeta in replication of undamaged DNA in Saccharomyces cerevisiae. Genetics 2010, 184, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, M.S.; Rada, C. Somatic hypermutation: Activation-induced deaminase for C/G followed by polymerase eta for A/T. J. Exp. Med. 2007, 204, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Despras, E.; Sittewelle, M.; Pouvelle, C.; Delrieu, N.; Cordonnier, A.M.; Kannouche, P.L. Rad18-dependent sumoylation of human specialized DNA polymerase eta is required to prevent under-replicated DNA. Nat. Commun. 2016, 7, 13326. [Google Scholar] [CrossRef] [PubMed]
- Urban, V.; Dobrovolna, J.; Janscak, P. Distinct functions of human recq helicases during DNA replication. Biophys. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bansbach, C.E.; Betous, R.; Lovejoy, C.A.; Glick, G.G.; Cortez, D. The annealing helicase smarcal1 maintains genome integrity at stalled replication forks. Genes Dev. 2009, 23, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Betous, R.; Mason, A.C.; Rambo, R.P.; Bansbach, C.E.; Badu-Nkansah, A.; Sirbu, B.M.; Eichman, B.F.; Cortez, D. Smarcal1 catalyzes fork regression and holliday junction migration to maintain genome stability during DNA replication. Genes Dev. 2012, 26, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Blastyak, A.; Hajdu, I.; Unk, I.; Haracska, L. Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol. Cell. Biol. 2010, 30, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Burkovics, P.; Sebesta, M.; Balogh, D.; Haracska, L.; Krejci, L. Strand invasion by HLTF as a mechanism for template switch in fork rescue. Nuclic Acids Res. 2014, 42, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Nimonkar, A.V.; Hu, Y.; Hajdu, I.; Achar, Y.J.; Izhar, L.; Petit, S.A.; Adamson, B.; Yoon, J.C.; Kowalczykowski, S.C.; et al. Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress. Mol. Cell 2012, 47, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ghosal, G.; Chen, J. The HARP-like domain-containing protein AH2/ZRANB3 binds to PCNA and participates in cellular response to replication stress. Mol. Cell 2012, 47, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Yusufzai, T.; Kadonaga, J.T. Harp is an atp-driven annealing helicase. Science 2008, 322, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Rassool, F.V.; North, P.S.; Mufti, G.J.; Hickson, I.D. Constitutive DNA damage is linked to DNA replication abnormalities in bloom’s syndrome cells. Oncogene 2003, 22, 8749–8757. [Google Scholar] [CrossRef] [PubMed]
- Urban, V.; Dobrovolna, J.; Huhn, D.; Fryzelkova, J.; Bartek, J.; Janscak, P. Recq5 helicase promotes resolution of conflicts between replication and transcription in human cells. J. Cell Biol. 2016, 214, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, E.; Bauer, R.; Raub, U.; Schinzel, M.; Ruprecht, K.W.; Jonas, J.B. Spontaneous and induced chromosomal instability in werner syndrome. Hum. Genet. 1988, 80, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lauper, J.M.; Krause, A.; Vaughan, T.L.; Monnat, R.J., Jr. Spectrum and risk of neoplasia in werner syndrome: A systematic review. PLoS ONE 2013, 8, e59709. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Heravi, A.; Raams, A.; Lubieniecka, J.; Cho, K.S.; DeHaai, K.A.; Basiratnia, M.; Mari, P.O.; Xue, Y.; Rauth, M.; Olney, A.H.; et al. Smarcal1 deficiency predisposes to non-hodgkin lymphoma and hypersensitivity to genotoxic agents in vivo. Am. J. Med. Genet. A 2012, 158A, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Ellis, N.A.; Groden, J.; Ye, T.Z.; Straughen, J.; Lennon, D.J.; Ciocci, S.; Proytcheva, M.; German, J. The bloom’s syndrome gene product is homologous to recq helicases. Cell 1995, 83, 655–666. [Google Scholar] [CrossRef]
- Cybulski, C.; Carrot-Zhang, J.; Kluzniak, W.; Rivera, B.; Kashyap, A.; Wokolorczyk, D.; Giroux, S.; Nadaf, J.; Hamel, N.; Zhang, S.; et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat. Genet. 2015, 47, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Futami, K.; Furuichi, Y. RECQL1 and WRN DNA repair helicases: Potential therapeutic targets and proliferative markers against cancers. Front. Genet. 2014, 5, 441. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Sommers, J.A.; Shoemaker, R.H.; Brosh, R.M., Jr. Inhibition of helicase activity by a small molecule impairs werner syndrome helicase (WRN) function in the cellular response to DNA damage or replication stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L.A.; Springgate, C.F.; Battula, N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974, 34, 2311–2321. [Google Scholar] [PubMed]
- Kunkel, T.A.; Erie, D.A. DNA mismatch repair. Annu. Rev. Biochem. 2005, 74, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Marsischky, G.T.; Filosi, N.; Kane, M.F.; Kolodner, R. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996, 10, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Harfe, B.D.; Jinks-Robertson, S. DNA mismatch repair and genetic instability. Annu. Rev. Genet. 2000, 34, 359–399. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Kucherlapati, R.; Edelmann, W. Mouse models for human DNA mismatch-repair gene defects. Trends Mol. Med. 2002, 8, 346–353. [Google Scholar] [CrossRef]
- Lynch, H.T.; de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [PubMed]
- Peltomäki, P.; Vasen, H. Mutations associated with HNPCC predisposition—Update of ICG-HNPCC/insight mutation database. Dis. Mark. 2004, 20, 269–276. [Google Scholar] [CrossRef]
- Imai, K.; Yamamoto, H. Carcinogenesis and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2008, 29, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Johnson, A.L.; Johnston, L.H.; Sugino, A. Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J. 1993, 12, 1467–1473. [Google Scholar] [PubMed]
- Williams, L.N.; Herr, A.J.; Preston, B.D. Emergence of DNA polymerase e antimutators that escape error-induced extinction in yeast. Genetics 2013, 193, 751–770. [Google Scholar] [CrossRef] [PubMed]
- Prindle, M.J.; Schmitt, M.W.; Parmeggiani, F.; Loeb, L.A. A substitution in the fingers domain of DNA polymerase d reduces fidelity by altering nucleotide discrimination in the catalytic site. J. Biol. Chem. 2013, 288, 5572–5580. [Google Scholar] [CrossRef] [PubMed]
- Palles, C.; Cazier, J.B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; Guarino, E.; Salguero, I.; et al. Germline mutations affecting the proofreading domains of pole and pold1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2013, 45, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, K.; Beilken, A.; Nustede, R.; Ripperger, T.; Lamottke, B.; Ure, B.; Steinmann, D.; Reineke-Plaass, T.; Lehmann, U.; Zschocke, J. A novel germline pole mutation causes an early onset cancer prone syndrome mimicking constitutional mismatch repair deficiency. Fam. Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Aoude, L.G.; Heitzer, E.; Johansson, P.; Gartside, M.; Wadt, K.; Pritchard, A.L.; Palmer, J.M.; Symmons, J.; Gerdes, A.M.; Montgomery, G.W.; et al. Pole mutations in families predisposed to cutaneous melanoma. Fam. Cancer 2015, 14, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bellido, F.; Pineda, M.; Aiza, G.; Valdes-Mas, R.; Navarro, M.; Puente, D.A.; Pons, T.; Gonzalez, S.; Iglesias, S.; Darder, E.; et al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: Review of reported cases and recommendations for genetic testing and surveillance. Genet. Med. 2016, 18, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Darmawan, H.; Schultz, A.; Fidalgo da Silva, E.; Reha-Krantz, L.J. A method to select for mutator DNA polymerase deltas in Saccharomyces cerevisiae. Genome 2006, 49, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Abdus Sattar, A.K.; Lin, T.C.; Jones, C.; Konigsberg, W.H. Functional consequences and exonuclease kinetic parameters of point mutations in bacteriophage T4 DNA polymerase. Biochemistry 1996, 35, 16621–16629. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.P.; Shcherbakova, P.V. A common cancer-associated DNA polymerase ε mutation causes an exceptionally strong mutator phenotype, indicating fidelity defects distinct from loss of proofreading. Cancer Res. 2014, 74, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Rohlin, A.; Zagoras, T.; Nilsson, S.; Lundstam, U.; Wahlstrom, J.; Hulten, L.; Martinsson, T.; Karlsson, G.B.; Nordling, M. A mutation in pole predisposing to a multi-tumour phenotype. Int. J. Oncol. 2014, 45, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Chubb, D.; Broderick, P.; Frampton, M.; Kinnersley, B.; Sherborne, A.; Penegar, S.; Lloyd, A.; Ma, Y.P.; Dobbins, S.E.; Houlston, R.S. Genetic diagnosis of high-penetrance susceptibility for colorectal cancer (CRC) is achievable for a high proportion of familial CRC by exome sequencing. J. Clin. Oncol. 2015, 33, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Spier, I.; Holzapfel, S.; Altmuller, J.; Zhao, B.; Horpaopan, S.; Vogt, S.; Chen, S.; Morak, M.; Raeder, S.; Kayser, K.; et al. Frequency and phenotypic spectrum of germline mutations in pole and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int. J. Cancer 2015, 137, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.F.; Johansen, J.; Bjornevoll, I.; Sylvander, A.E.; Steinsbekk, K.S.; Saetrom, P.; Sandvik, A.K.; Drablos, F.; Sjursen, W. A novel POLE mutation associated with cancers of colon, pancreas, ovaries and small intestine. Fam. Cancer 2015, 14, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; Hernández-Illán, E.; Bellido, F.; Aiza, G.; Castillejo, A.; Castillejo, M.-I.; Navarro, M.; Seguí, N.; Vargas, G.; Guarinos, C. New insights into pole and pold1 germline mutations in familial colorectal cancer and polyposis. Hum. Mol. Genet. 2014, 23, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Rayner, E.; van Gool, I.C.; Palles, C.; Kearsey, S.E.; Bosse, T.; Tomlinson, I.; Church, D.N. A panoply of errors: Polymerase proofreading domain mutations in cancer. Nat. Rev. Cancer 2016, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Church, D.N.; Briggs, S.E.; Palles, C.; Domingo, E.; Kearsey, S.J.; Grimes, J.M.; Gorman, M.; Martin, L.; Howarth, K.M.; Hodgson, S.V.; et al. DNA polymerase ɛ and δ exonuclease domain mutations in endometrial cancer. Hum. Mol. Genet. 2013, 22, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Seshagiri, S.; Stawiski, E.W.; Durinck, S.; Modrusan, Z.; Storm, E.E.; Conboy, C.B.; Chaudhuri, S.; Guan, Y.; Janakiraman, V.; Jaiswal, B.S.; et al. Recurrent R-spondin fusions in colon cancer. Nature 2012, 488, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Briggs, S.; Tomlinson, I. Germline and somatic polymerase ɛ and δ mutations define a new class of hypermutated colorectal and endometrial cancers. J. Pathol. 2013, 230, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Seshagiri, S. The burden of faulty proofreading in colon cancer. Nat. Genet. 2013, 45, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Daee, D.L.; Mertz, T.M.; Shcherbakova, P.V. A cancer-associated DNA polymerase d variant modeled in yeast causes a catastrophic increase in genomic instability. Proc. Natl. Acad. Sci. USA 2010, 107, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cbio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013. [Google Scholar] [CrossRef] [PubMed]
- Shlien, A.; Campbell, B.B.; de Borja, R.; Alexandrov, L.B.; Merico, D.; Wedge, D.; van Loo, P.; Tarpey, P.S.; Coupland, P.; Behjati, S.; et al. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat. Genet. 2015, 47, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune activation and response to pembrolizumab in pole-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Refsland, E.W.; Harris, R.S. The APOBEC3 family of retroelement restriction factors. Curr. Top. Microbiol. Immunol. 2013, 371, 1–27. [Google Scholar] [PubMed]
- Bransteitter, R.; Pham, P.; Scharff, M.D.; Goodman, M.F. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl. Acad. Sci. USA 2003, 100, 4102–4107. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.K.; Market, E.; Besmer, E.; Papavasiliou, F.N. Aid mediates hypermutation by deaminating single stranded DNA. J. Exp. Med. 2003, 197, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Suspene, R.; Sommer, P.; Henry, M.; Ferris, S.; Guetard, D.; Pochet, S.; Chester, A.; Navaratnam, N.; Wain-Hobson, S.; Vartanian, J.P. APOBEC3G is a single-stranded DNA cytidine deaminase and functions independently of HIV reverse transcriptase. Nuclic Acids Res. 2004, 32, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Konig, R.; Pillai, S.; Chiles, K.; Kearney, M.; Palmer, S.; Richman, D.; Coffin, J.M.; Landau, N.R. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat. Struct. Mol. Biol. 2004, 11, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Resnick, M.A.; Gordenin, D.A. The choice of nucleotide inserted opposite abasic sites formed within chromosomal DNA reveals the polymerase activities participating in translesion DNA synthesis. DNA Repair 2013, 12, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Tian, M.; Khuong, C.; Chua, K.; Pinaud, E.; Alt, F.W. Transcription-targeted DNA deamination by the aid antibody diversification enzyme. Nature 2003, 422, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.S.; Petersen-Mahrt, S.K.; Neuberger, M.S. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol. Cell 2002, 10, 1247–1253. [Google Scholar] [CrossRef]
- Love, R.P.; Xu, H.; Chelico, L. Biochemical analysis of hypermutation by the deoxycytidine deaminase APOBEC3A. J. Biol. Chem. 2012, 287, 30812–30822. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, A.R.; Stavropoulos, P.; Jankovic, M.; Nussenzweig, M.C. Transcription enhances aid-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat. Immunol. 2003, 4, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.J.M.; Nik-Zainal, S.; Wu, Y.L.; Stebbings, L.A.; Raine, K.; Campbell, P.J.; Rada, C.; Stratton, M.R.; Neuberger, M.S. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. eLife 2013, 2, e00534. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, J.I.; Cortez, L.M.; Mertz, T.M.; Malc, E.P.; Mieczkowski, P.A.; Roberts, S.A. APOBEC3A and APOBEC3B preferentially deaminate the lagging strand template during DNA replication. Cell Rep. 2016, 14, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Landry, S.; Budagyan, K.; Avgousti, D.C.; Shalhout, S.; Bhagwat, A.S.; Weitzman, M.D. APOBEC3A damages the cellular genome during DNA replication. Cell Cycle 2016, 15, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Refsland, E.W.; Stenglein, M.D.; Shindo, K.; Albin, J.S.; Brown, W.L.; Harris, R.S. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: Implications for HIV-1 restriction. Nuclic Acids Res. 2010, 38, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Bogerd, H.P.; Wiegand, H.L.; Hulme, A.E.; Garcia-Perez, J.L.; O’Shea, K.S.; Moran, J.V.; Cullen, B.R. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. USA 2006, 103, 8780–8785. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.B.; Temiz, N.A.; Harris, R.S. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat. Genet. 2013, 45, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Lawrence, M.S.; Klimczak, L.J.; Grimm, S.A.; Fargo, D.; Stojanov, P.; Kiezun, A.; Kryukov, G.V.; Carter, S.L.; Saksena, G.; et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 2013, 45, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.; Hart, S.N.; Burns, M.B.; Carpenter, M.A.; Temiz, N.A.; Rathore, A.; Vogel, R.I.; Nikas, J.B.; Law, E.K.; Brown, W.L.; et al. APOBEC3B upregulation and genomic mutation patterns in serous ovarian carcinoma. Cancer Res. 2013, 73, 7222–7231. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Gordenin, D.A. Clustered and genome-wide transient mutagenesis in human cancers: Hypermutation without permanent mutators or loss of fitness. BioEssays 2014, 36, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.; Chakravarthy, A.; Su, X.P.; Boshoff, C.; Fenton, T.R. Apobec-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014, 7, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Marusawa, H.; Matsumoto, T.; Ueda, Y.; Matsumoto, Y.; Endo, Y.; Takai, A.; Chiba, T. Excessive activity of apolipoprotein B mRNA editing enzyme catalytic polypeptide 2 (APOBEC2) contributes to liver and lung tumorigenesis. Int. J. Cancer 2012, 130, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Balestra, M.E.; Ferrell, L.D.; Fan, J.; Arnold, K.S.; Taylor, S.; Taylor, J.M.; Innerarity, T.L. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc. Natl. Acad. Sci. USA 1995, 92, 8483–8487. [Google Scholar] [CrossRef] [PubMed]
- Lackey, L.; Law, E.K.; Brown, W.L.; Harris, R.S. Subcellular localization of the APOBEC3 proteins during mitosis and implications for genomic DNA deamination. Cell Cycle 2013, 12, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.B.; Lackey, L.; Carpenter, M.A.; Rathore, A.; Land, A.M.; Leonard, B.; Refsland, E.W.; Kotandeniya, D.; Tretyakova, N.; Nikas, J.B.; et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature 2013, 494, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Middlebrooks, C.D.; Banday, A.R.; Matsuda, K.; Udquim, K.I.; Onabajo, O.O.; Paquin, A.; Figueroa, J.D.; Zhu, B.; Koutros, S.; Kubo, M.; et al. Association of germline variants in the APOBEC3 region with cancer risk and enrichment with APOBEC-signature mutations in tumors. Nat. Genet. 2016, 48, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Caval, V.; Suspene, R.; Shapira, M.; Vartanian, J.P.; Wain-Hobson, S. A prevalent cancer susceptibility APOBEC3A hybrid allele bearing APOBEC3B 3′ UTR enhances chromosomal DNA damage. Nat. Commun. 2014, 5, 5129. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Wedge, D.C.; Alexandrov, L.B.; Petljak, M.; Butler, A.P.; Bolli, N.; Davies, H.R.; Knappskog, S.; Martin, S.; Papaemmanuil, E.; et al. Association of a germline copy number polymorphism of APOBEC3A and APOBEC3B with burden of putative APOBEC-dependent mutations in breast cancer. Nat. Genet. 2014, 46, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Cescon, D.W.; Haibe-Kains, B.; Mak, T.W. Apobec3B expression in breast cancer reflects cellular proliferation, while a deletion polymorphism is associated with immune activation. Proc. Natl. Acad. Sci. USA 2015, 112, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.X.; Soo, J.S.-S.; Kwan, P.Y.; Hong, E.; Khang, T.F.; Mariapun, S.; Lee, C.S.-M.; Hasan, S.N.; Rajadurai, P.; Yip, C.H. Germline APOBEC3B deletion is associated with breast cancer risk in an Asian multi-ethnic cohort and with immune cell presentation. Breast Cancer Res. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cai, J.; Chang, J.; Yu, D.; Wu, C.; Yan, T.; Zhai, K.; Bi, X.; Zhao, H.; Xu, J.; et al. Evidence of associations of APOBEC3B gene deletion with susceptibility to persistent HBV infection and hepatocellular carcinoma. Hum. Mol. Genet. 2013, 22, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Roberts, S.A.; Klimczak, L.J.; Sterling, J.F.; Saini, N.; Malc, E.P.; Kim, J.; Kwiatkowski, D.J.; Fargo, D.C.; Mieczkowski, P.A.; et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat. Genet. 2015, 47, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R. Activation-induced cytidine deaminase in B cell immunity and cancers. Immune Netw. 2012, 12, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Saraconi, G.; Severi, F.; Sala, C.; Mattiuz, G.; Conticello, S.G. The RNA editing enzyme APOBEC1 induces somatic mutations and a compatible mutational signature is present in esophageal adenocarcinomas. Genome Biol. 2014, 5, 417. [Google Scholar] [CrossRef]
- Starrett, G.J.; Luengas, E.M.; McCann, J.L.; Ebrahimi, D.; Temiz, N.A.; Love, R.P.; Feng, Y.Q.; Adolph, M.B.; Chelico, L.; Law, E.K.; et al. The DNA cytosine deaminase APOBEC3H haplotype I likely contributes to breast and lung cancer mutagenesis. Nat. Commun. 2016, 7, 12918. [Google Scholar] [CrossRef] [PubMed]
- Lada, A.G.; Dhar, A.; Boissy, R.J.; Hirano, M.; Rubel, A.A.; Rogozin, I.B.; Pavlov, Y.I. Aid/APOBEC cytosine deaminase induces genome-wide kataegis. Biol. Direct 2012, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.J.; Wu, Y.L.; Rada, C. Active RNAP pre-initiation sites are highly mutated by cytidine deaminases in yeast, with aid targeting small RNA genes. eLife 2014, 3, e03553. [Google Scholar] [CrossRef] [PubMed]
- Kotsantis, P.; Silva, L.M.; Irmscher, S.; Jones, R.M.; Folkes, L.; Gromak, N.; Petermann, E. Increased global transcription activity as a mechanism of replication stress in cancer. Nat. Commun. 2016, 7, 13087. [Google Scholar] [CrossRef] [PubMed]
- Mimitou, E.P.; Symington, L.S. DNA end resection—Unraveling the tail. DNA Repair 2011, 10, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Caron, P.; Legube, G.; Paull, T.T. Quantitation of DNA double-strand break resection intermediates in human cells. Nuclic Acids Res. 2014, 42, e19. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Ramakrishnan, S.; Elango, R.; Ayyar, S.; Zhang, Y.; Deem, A.; Ira, G.; Haber, J.E.; Lobachev, K.S.; Malkova, A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 2013, 502, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Sakofsky, C.J.; Roberts, S.A.; Malc, E.; Mieczkowski, P.A.; Resnick, M.A.; Gordenin, D.A.; Malkova, A. Break-induced replication is a source of mutation clusters underlying kataegis. Cell Rep. 2014, 7, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.S.; Hao, W.L.; Townes, J.P.; Lee, H.; Tang, H.X.; Foster, P.L. Strand-biased cytosine deamination at the replication fork causes cytosine to thymine mutations in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Maizels, N. Cell cycle regulates nuclear stability of aid and determines the cellular response to aid. PLoS Genet. 2015, 11, e1005411. [Google Scholar] [CrossRef] [PubMed]
- Haradhvala, N.J.; Polak, P.; Stojanov, P.; Covington, K.R.; Shinbrot, E.; Hess, J.M.; Rheinbay, E.; Kim, J.; Maruvka, Y.E.; Braunstein, L.Z.; et al. Mutational strand asymmetries in cancer genomes reveal mechanisms of DNA damage and repair. Cell 2016, 164, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Morganella, S.; Alexandrov, L.B.; Glodzik, D.; Zou, X.; Davies, H.; Staaf, J.; Sieuwerts, A.M.; Brinkman, A.B.; Martin, S.; Ramakrishna, M.; et al. The topography of mutational processes in breast cancer genomes. Nat. Commun. 2016, 7, 11383. [Google Scholar] [CrossRef] [PubMed]
- Seplyarskiy, V.B.; Soldatov, R.A.; Popadin, K.Y.; Antonarakis, S.E.; Bazykin, G.A.; Nikolaev, S.I. Apobec-induced mutations in human cancers are strongly enriched on the lagging DNA strand during replication. Genome Res. 2016, 26, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Munger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.J.; Nguyen, M.; Liem, A.; Lee, D.; Montagna, C.; Lambert, P.F.; Ried, T.; Difilippantonio, M.J. E6 and E7 oncoproteins induce distinct patterns of chromosomal aneuploidy in skin tumors from transgenic mice. Cancer Res. 2004, 64, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Law, E.K.; Sieuwerts, A.M.; LaPara, K.; Leonard, B.; Starrett, G.J.; Molan, A.M.; Temiz, N.A.; Vogel, R.I.; Meijer-van Gelder, M.E.; Sweep, F.C.; et al. The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer. Sci. Adv. 2016, 2, e1601737. [Google Scholar] [CrossRef] [PubMed]
- Aird, K.M.; Zhang, G.; Li, H.; Tu, Z.; Bitler, B.G.; Garipov, A.; Wu, H.; Wei, Z.; Wagner, S.N.; Herlyn, M.; et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013, 3, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Angus, S.P.; Wheeler, L.J.; Ranmal, S.A.; Zhang, X.; Markey, M.P.; Mathews, C.K.; Knudsen, E.S. Retinoblastoma tumor suppressor targets dNTP metabolism to regulate DNA replication. J. Biol. Chem. 2002, 277, 44376–44384. [Google Scholar] [CrossRef] [PubMed]
- Onyenwoke, R.U.; Forsberg, L.J.; Liu, L.; Williams, T.; Alzate, O.; Brenman, J.E. Ampk directly inhibits NDPK through a phosphoserine switch to maintain cellular homeostasis. Mol. Biol. Cell 2012, 23, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Mannava, S.; Grachtchouk, V.; Wheeler, L.J.; Im, M.; Zhuang, D.; Slavina, E.G.; Mathews, C.K.; Shewach, D.S.; Nikiforov, M.A. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle 2008, 7, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhou, B.; Liu, X.; Qiu, W.; Jin, Z.; Yen, Y. Wild-type p53 regulates human ribonucleotide reductase by protein-protein interaction with p53R2 as well as hRRM2 subunits. Cancer Res. 2003, 63, 980–986. [Google Scholar] [PubMed]
- Eckert, K.A.; Kunkel, T.A. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Creighton, S.; Goodman, M.F. Gel kinetic analysis of DNA polymerase fidelity in the presence of proofreading using bacteriophage T4 DNA polymerase. J. Biol. Chem. 1995, 270, 4759–4774. [Google Scholar] [CrossRef] [PubMed]
- Mendelman, L.V.; Petruska, J.; Goodman, M.F. Base mispair extension kinetics. Comparison of DNA polymerase a and reverse transcriptase. J. Biol. Chem. 1990, 265, 2338–2346. [Google Scholar] [PubMed]
- Mertz, T.M.; Sharma, S.; Chabes, A.; Shcherbakova, P.V. Colon cancer-associated mutator DNA polymerase delta variant causes expansion of dNTP pools increasing its own infidelity. Proc. Natl. Acad. Sci. USA 2015, 112, E2467–E2476. [Google Scholar] [CrossRef] [PubMed]
- Gon, S.; Napolitano, R.; Rocha, W.; Coulon, S.; Fuchs, R.P. Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 19311–19316. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.J.; Rajagopal, I.; Mathews, C.K. Stimulation of mutagenesis by proportional deoxyribonucleoside triphosphate accumulation in Escherichia coli. DNA Repair 2005, 4, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Schmeits, J.L.; Amin, N.S.; Lau, P.J.; Myung, K.; Kolodner, R.D. Checkpoint-dependent activation of mutagenic repair in Saccharomyces cerevisiae pol3-01 mutants. Mol. Cell 2000, 6, 593–603. [Google Scholar] [CrossRef]
- Hills, S.A.; Diffley, J.F. DNA replication and oncogene-induced replicative stress. Curr. Biol. 2014, 24, R435–R444. [Google Scholar] [CrossRef] [PubMed]
- Kanu, N.; Cerone, M.A.; Goh, G.; Zalmas, L.P.; Bartkova, J.; Dietzen, M.; McGranahan, N.; Rogers, R.; Law, E.K.; Gromova, I.; et al. DNA replication stress mediates APOBEC3 family mutagenesis in breast cancer. Genome Biol. 2016, 17, 185. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Piovesan, A.; Facchin, F.; Beraudi, A.; Casadei, R.; Frabetti, F.; Vitale, L.; Pelleri, M.C.; Tassani, S.; Piva, F.; et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).