Comparative Analysis of Soybean Root Proteome Reveals Molecular Basis of Differential Carboxylate Efflux under Low Phosphorus Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Growth Traits and Tissue Phosphorus Status
2.3. Collection and Quantification of Root Carboxylate Efflux
2.4. Protein Isolation and Quantification
2.5. Two-Dimensional Gel Electrophoresis and Image Analysis
2.6. Trypsin Digestion of Proteins
2.7. Mass Spectrometry for Protein Identification
2.8. In-Silico Analysis for Protein Annotation
2.9. Validation of DAPs at Transcript Level by Reverse Transcription-qPCR
2.10. Experimental Design and Statistical Rationale
3. Results
3.1. Biomass Accumulation, Root System Traits and Tissue PosphorusStatus
3.2. Carboxylate Efflux in Response to Low Posphorus Stress
3.3. Comparative Analysis of Soybean Root Proteome
3.3.1. Differentially Abundant Proteins in Response to Low Posphorus Stress
3.3.2. Validation of Expression of Genes Encoding DAPs in Response to Low P Stress
4. Discussion
4.1. Soybean Genotypes Exhibit Improved Root System Traitsand Carboxylate Efflux under Low P Stress
4.2. Soybean Genotypes Exhibit Differential Molecular Regulation under Low Posphorus Stress
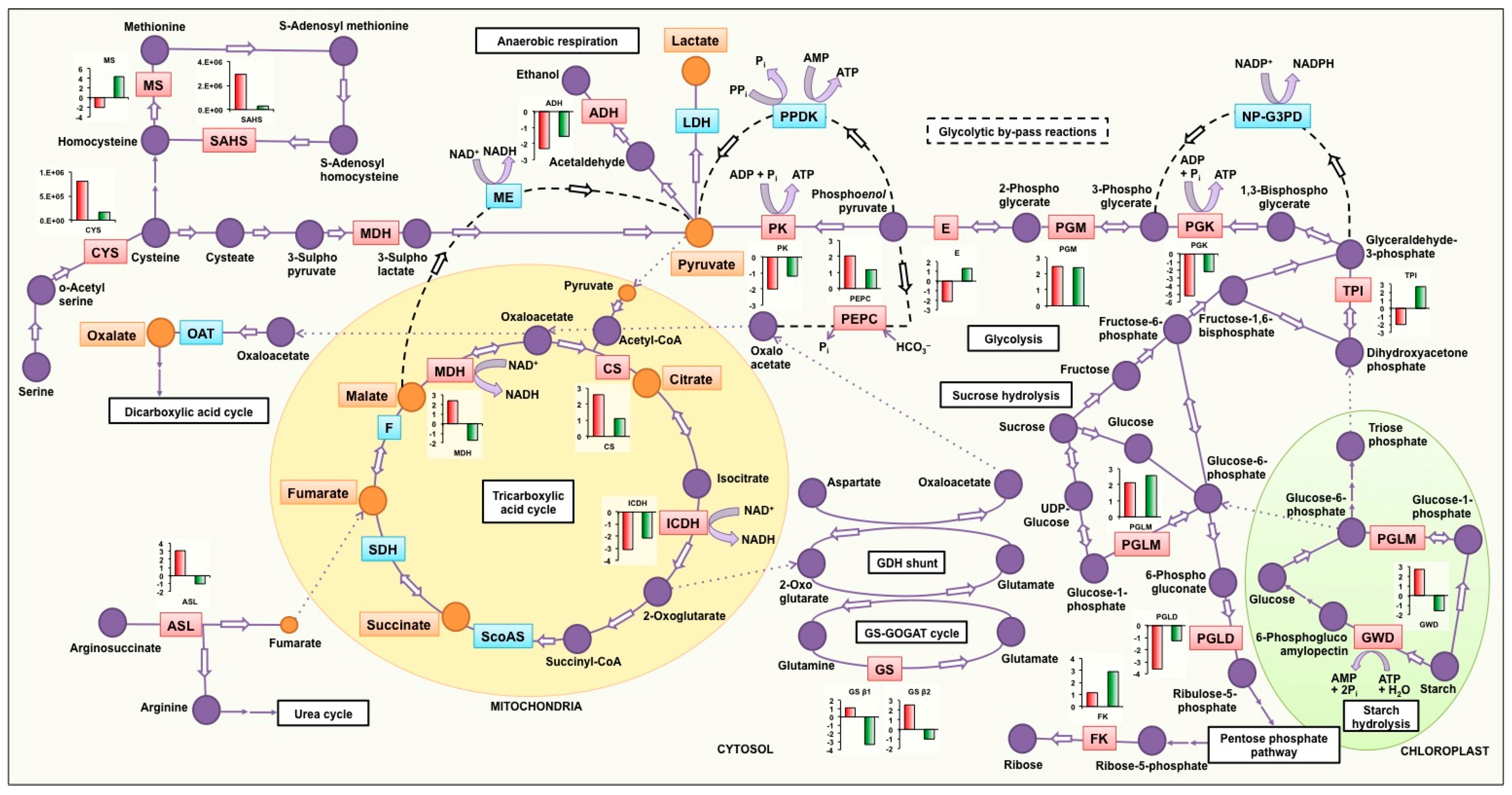
4.2.1. Tricarboxylic Acid Cycle and Glycolysis
4.2.2. Starch Hydrolysis
4.2.3. Anaerobic Respiration
4.2.4. Other Anaplerotic Reactions Replenishing the TCA Cycle Intermediates
4.2.5. Synthesis of Sulfur Containing Amino Acids
4.2.6. Other Pathways Affected by Low P Stress
4.3. Future Prospects
- Differentially abundant proteins with known physiological function may be tested rigorously for imparting P acquisition efficiency by creating overexpression/knockout mutant lines in soybean or other model systems.
- Hypothetical proteins with putative or unknown function identified in the proteomic analysis may be functionally characterized to ascertain their role(s) under low P stress.
- The identified genotypes may be potential donors in crop improvement programs to develop high-yielding P-efficient cultivars, an asset to low-input sustainable agriculture.
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandey, R.; Zinta, G.; AbdElgawad, H.; Ahmad, A.; Jain, V.; Janssens, I.A. Physiological and molecular alterations in plants exposed to high [CO2] under phosphorus stress. Biotech. Adv. 2015, 33, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behavior in soils—Misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Neumann, G.; Romheld, V. The release of root exudates as affected by the plant physiological status. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 23–71. [Google Scholar]
- Lambers, H.; Martinoia, E.; Renton, M. Plant adaptations to severely phosphorus-impoverished soils. Curr. Opin. Plant Biol. 2015, 25, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.R.; Delhaize, E.; Jones, D.L. Function and mechanism of organic acid exudation from plant roots. Ann. Rev. Plant Physiol. Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Lipton, D.S.; Blanchar, R.W.; Blevins, D.G. Citrate, malate and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiol. 1987, 85, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Ohwaki, Y.; Hirata, H. Differences in carboxylic acid exudation among P-starved leguminous crops in relation to carboxylic acid contents in plant tissues and phospholipid level in roots. Soil Sci. Plant Nutr. 1992, 38, 235–243. [Google Scholar] [CrossRef]
- Dong, D.; Peng, X.; Yan, X. Organic acid exudation induced by phosphorus deficiency and/or aluminum toxicity in two contrasting soybean genotypes. Physiol. Plant. 2004, 122, 190–199. [Google Scholar] [CrossRef]
- Hoffland, E.; Wei, C.Z.; Wissuwa, M. Organic anion exudation by lowland rice (Oryzasativa L.) at zinc and phosphorus deficiency. Plant Soil 2006, 283, 155–162. [Google Scholar] [CrossRef]
- Pandey, R.; Meena, S.K.; Krishnapriya, V.; Ahmad, A.; Kishora, N. Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep. 2014, 33, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Marschner, P. Nutrient availability and management in the rhizosphere: Exploiting genotypic differences. New Phytol. 2005, 168, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K. Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol. 2006, 169, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Tyerman, S.D.; Sasaki, T.; Furuichi, T.; Yamamoto, Y.; Zhang, W.H.; Delhaize, E. The identification of aluminum-resistance genes provides opportunities for enhancing crop production on acid soils. J. Exp. Bot. 2011, 62, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Raman, H.; Gupta, S.; Horst, W.J.; Delhaize, E. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol. 2009, 149, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Taylor, P.; Hocking, P.J.; Simpson, R.J.; Ryan, P.R.; Richardson, A.E. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminum resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotech. J. 2009, 7, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Pineros, M.A.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, L.V.; Liao, H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, C.; Li, Z.; Zhang, K.; Yang, A.; Zhang, J. Comparative proteome analysis of phosphorus responses in maize (Zea mays L.) roots of wild-type and a low-P-tolerant mutant reveal root characteristics associated with phosphorus efficiency. Plant J. 2008, 55, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, H.; Xu, F.; Zhang, X.; Liu, S. Comparative proteome analysis of metabolic changes by low phosphorus stress in two Brassica napus genotypes. Planta 2011, 233, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Li, W.; Schmidt, W. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol. Cell. Proteom. 2012, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Brechenmacher, L.; Lee, J.; Sachdev, S.; Song, Z.; Nguyen, T.H.N.; Joshi, T.; Oehrle, N.; Libault, M.; Mooney, B.; Xu, D.; et al. Establishment of a protein reference map for soybean root hair cells. Plant Physiol. 2009, 149, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Ohyanagi, H.; Nobori, H.; Nakamura, T.; Hashiguchi, A.; Nanjo, Y.; Mikami, Y.; Yunokawa, H.; Komatsu, S. Soybean Proteome Database: A data resource for plant differential omics. J. Proteome Res. 2009, 8, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Ohyanagi, H.; Sakata, K.; Komatsu, S. Soybean Proteome Database 2012: Update on the comprehensive data repository for soybean proteomics. Front. Plant Sci. 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Khatoon, A.; Komatsu, S. Soybean proteomics for unravelling abiotic stress response mechanism. J. Proteome Res. 2013, 12, 4670–4684. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Salekdeh, G.H.; Yang, P.; Woo, S.H.; Chin, C.F.; Gehring, C.; Haynes, P.A.; Mirzaei, M.; Komatsu, S. Proteomics of important food crops in the Asia Oceania region: Current status and future perspectives. J. Proteome Res. 2015, 14, 2723–2744. [Google Scholar] [CrossRef] [PubMed]
- Vengavasi, K.; Pandey, R.; Kumar, A. Transcript abundance, enzyme activity and metabolite concentration governing differential carboxylate efflux in soybean under low phosphorus stress. Indian J. Plant Physiol. 2016, 21, 179–188. [Google Scholar] [CrossRef]
- Vengavasi, K.; Pandey, R. Root exudation index as a physiological marker for efficient phosphorus acquisition in soybean: An effective tool for plant breeding. Crop Pasture Sci. 2016, 67, 1096–1109. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S. Blast2GO: A comprehensive suite for functional analysis in Plant Genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef] [PubMed]
- Myhre, S.; Tveit, H.; Mollestad, T.; Laegreid, A. Additional gene ontology structure for improved biological reasoning. Bioinformatics 2006, 22, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Integrated DNA Technologies. Available online: http://eu.idtdna.com/scitools/Applications/RealTimePCR. (accessed on 23 November 2015).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gahoonia, T.S.; Ali, O.; Sarker, A.; Nielsen, N.E.; Rahman, M.M. Genetic variation in root traits and nutrient acquisition of lentil genotypes. J. Plant Nutr. 2006, 29, 643–655. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, Z.; Shi, L.; Duan, H.; Xu, F. Genotypic differences in root morphology and phosphorus uptake kinetics in Brassica napus under low phosphorus supply. J. Plant Nutr. 2010, 33, 889–901. [Google Scholar] [CrossRef]
- de Sousa, S.M.; Clark, R.T.; Mendes, F.F.; de Oliveira, A.C.; de Vasconcelos, M.J.V.; Parentoni, S.N.; Kochian, L.V.; Guimaraes, C.T.; Magalhaes, J.V. A role for root morphology and related candidate genes in P acquisition efficiency in maize. Func. Plant Biol. 2012, 39, 925–935. [Google Scholar] [CrossRef]
- Lal, M.K. Effect of High [CO2] on Phosphorus Efficiency in Wheat Grown under Phosphorus Stress with Different Sulphur Levels. Master’s Thesis, ICAR-Indian Agricultural Research Institute, New Delhi, India, 2015. [Google Scholar]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Asmar, F.; Giese, H.; Gissel-Nielsen, G.; Nielsen, N.E. Root-released organic acids and phosphorus uptake of two barley cultivars in laboratory and field experiments. Eur. J. Agron. 2000, 12, 281–289. [Google Scholar] [CrossRef]
- Gaume, A.; Machler, F.; Leon, D.; Narro, L.; Frossard, E. Low-P tolerance by maize (Zea mays) genotypes: Significance of root growth, and organic acids and acid phosphatase root exudation. Plant Soil 2001, 228, 253–264. [Google Scholar] [CrossRef]
- Liao, H.; Wan, H.; Shaff, J.; Wang, X.; Yan, X.; Kochian, L.V. Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance, Exudation of specific organic acids from different regions of the intact root system. Plant Physiol. 2006, 141, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Ramirez, M.; Valdes-Lopez, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; Wu, H.C.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Merewitz, E.B.; Gianfagnam, T.; Huang, B. Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J. Exp. Bot. 2011, 62, 5311–5333. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yamaji, N.; Che, J.; Shen, R.F.; Ma, J.F. Normal root elongation requires arginine produced by argininosuccinate lyase in rice. Plant J. 2014, 78, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Odanaka, S.; Bennett, A.B.; Kanayama, Y. Distinct physiological roles of fructokinase isozymes revealed by gene-specific suppression of Frk1 and Frk2 expression in tomato. Plant Physiol. 2002, 129, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Essigmann, B.; Guler, S.; Narang, R.A.; Linke, D.; Benning, C. Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipids biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1998, 95, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Tjellström, H.; Andersson, M.X.; Larsson, K.E.; Sandelius, A.S. Membrane phospholipids as a phosphate reserve: The dynamic nature of phospholipid-to-digalactosyldiacylglycerol exchange in higher plants. Plant Cell Environ. 2008, 31, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, S.T.; Wang, Y.; Kim, S.K.; Lee, C.H.; Kim, K.K.; Kim, J.K.; Lee, S.Y.; Kang, K.Y. Overexpression of rice isoflavone reductase-like gene (OsIRL) confers tolerance to reactive oxygen species. Physiol. Plant. 2010, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]









| Spot ID | Accession Number | Description | Taxonomic Database | E | Mr (kDa) | pI | Mascot Score | #P | C (%) |
|---|---|---|---|---|---|---|---|---|---|
| Carbohydrate metabolic process | |||||||||
| 1a | XP_003534616.1 | Predicted: 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase-like | Glycine max | 0.00013 | 61.1 | 5.51 | 106 | 10/10 | 31 |
| 2a | NP_001243291.1 | Malate dehydrogenase, cytoplasmic-like | Glycine max | 0.00017 | 35.5 | 5.91 | 105 | 8/12 | 38 |
| 3a | XP_018674024.1 | Predicted: α-glucan water dikinase 2 isoform X2 | Musa acuminata subsp. malaccensis | 0.44 | 144.4 | 8.55 | 71 | 10/10 | 8 |
| 4b | XP_003537935.1 | Predicted: Fructokinase-2-like | Glycine max | 5.3 × 10−12 | 35.6 | 4.96 | 180 | 12/8 | 47 |
| 5b | XP_003552336.1 | Predicted: 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | Glycine max | 2.6 × 10−5 | 61.1 | 5.58 | 113 | 10/10 | 25 |
| 6b | BAS92721.1 | Predicted protein | Oryza sativa Japonica | 0.17 | 14.1 | 10.27 | 75 | 5/15 | 34 |
| 7b | XP_001758882.1 | Predicted: β-galactosyltransferase 7-like | Physcomitrella patens | 0.44 | 45.1 | 8.25 | 71 | 6/9 | 16 |
| 8b | XP_003531483.1 | Phosphoglycerate kinase isomerase, cytosolic | Glycine max | 6.6 | 42.4 | 6.28 | 59 | 5/15 | 18 |
| 9b | XP_021745158.1 | SW15-dependent HO expression protein 3-like | Chenopodium quinoa | 3.0 | 21.2 | 10.00 | 62 | 6/14 | 28 |
| 10b | XP_019436544.1 | Predicted: Fructokinase-2-like | Lupinus angustifolius | 5.4 | 35.8 | 5.28 | 60 | 6/14 | 25 |
| 11a,b | XP_006580435.1 | Predicted: Phosphoglucomutase, cytoplasmic-like | Glycine max | 2.1 × 10−5 | 63.7 | 5.33 | 114 | 11/9 | 20 |
| Protein metabolic process | |||||||||
| 12a | XP_003525105.1 | Predicted: Actin-7-like | Glycine max | 5.3 × 10−6 | 41.9 | 5.37 | 120 | 10/10 | 38 |
| 13a | KHN39675.1 | Stromal 70 kDa heat shock-related protein, chloroplastic | Glycine soja | 0.0021 | 65.5 | 4.84 | 94 | 10/10 | 21 |
| 14a | XP_010063241.1 | Predicted: Heat shock cognate protein 80-like | Eucalyptus grandis | 0.016 | 80.8 | 4.94 | 85 | 9/11 | 11 |
| 15a | XP_006422243.1 | Hypothetical: Proteasome subunit α type-2-a | Citrus clementia | 0.12 | 21.6 | 7.88 | 76 | 6/14 | 31 |
| 16a | CDY43475.1 | Predicted protein | Brassica napus | 21.0 | 6.2 | 4.78 | 54 | 4/16 | 38 |
| 17b | XP_015954976.1 | Proteasome subunit β type-2-b | Arachis duranensis | 1.7 × 10−5 | 22.7 | 6.30 | 115 | 7/13 | 37 |
| Biosynthetic process | |||||||||
| 18a | NP_001242332.2 | Glutamine synthetase β2, cytosolic | Glycine max | 6.6 × 10−6 | 39.3 | 5.48 | 119 | 9/11 | 40 |
| 19a | XP_003555932.1 | Predicted: ATP synthase subunit β, mitochondrial-like | Glycine max | 0.00011 | 59.9 | 5.80 | 107 | 10/10 | 26 |
| 20a | XP_014514203.1 | ATP synthase subunit, mitochondrial | Vigna radiata var. radiata | 0.12 | 59.8 | 5.90 | 76 | 8/12 | 19 |
| 21a | XP_009122013.1 | Argininosuccinate lyase | Brassica rapa | 0.17 | 58.3 | 5.41 | 75 | 8/12 | 18 |
| 22a | AQK43314.1 | Hypothetical: Nucleoside diphosphate kinase 1 | Glycine max | 0.11 | 44.0 | 9.56 | 77 | 8/12 | 20 |
| 23a | KHN48251.1 | Adenosine kinase 2 | Glycine soja | 0.33 | 38.1 | 5.29 | 72 | 6/14 | 29 |
| 24a | XP_009394624.1 | Uncharacterized protein | Musa acuminata subsp. malaccensis | 1.1 | 64.9 | 9.87 | 67 | 8/12 | 16 |
| 25b | XP_003536650.1 | Predicted: ATP synthase subunit β, mitochondrial | Glycine max | 5.3 × 10−11 | 59.9 | 5.80 | 170 | 14/6 | 38 |
| 26b | KZM83344.1 | Hypothetical protein | Daucus carota subsp. sativus | 0.33 | 14.2 | 6.41 | 72 | 5/15 | 44 |
| 27a,b | KHN32353.1 | Cysteine synthase, chloroplastic/chromoplastic | Glycine soja | 0.014 | 37.3 | 5.67 | 86 | 7/13 | 27 |
| 28a,b | XP_020889182.1 | Glutamine synthetase cytosolic isozyme 1-3-like | Arabidopsis lyrata subsp. lyrata | 0.16 | 38.7 | 5.72 | 75 | 7/13 | 18 |
| Lipid metabolic process | |||||||||
| 29a,b | CDY19393.1 | Hypothetical: Oxysterol-binding protein 1d | Brassica napus | 0.34 | 88.7 | 6.07 | 72 | 9/11 | 11 |
| 30a,b | KXG23463.1 | Hypothetical: 3-hydroxy-3-methylglutaryl-coenzyme a reductase 1-like | Sorghum bicolor | 2e+2 | 9.8 | 5.88 | 44 | 3/9 | 24 |
| Cellular homeostasis | |||||||||
| 31a | NP_001236037.2 | Isoflavone reductase | Glycine max | 1.7 × 10−11 | 35.7 | 5.30 | 175 | 11/9 | 47 |
| 32a | XP_003557022.1 | Predicted: Monodehydroascorbate reductase | Glycine max | 0.057 | 47.1 | 5.49 | 80 | 7/13 | 21 |
| Nucleobase-containing compound metabolic process | |||||||||
| 33a | XP_010932834.1 | Predicted: ATP sulfurylase 1, chloroplastic-like | Elaeis guineensis | 23.0 | 53.8 | 9.36 | 54 | 6/9 | 8 |
| Cellular process | |||||||||
| 34a | KRH58847.1 | Predicted: S-adenosyl-homocysteinase hydrolase | Glycine max | 0.13 | 55.7 | 5.79 | 76 | 8/12 | 21 |
| 35a | KOM56630.1 | Hypothetical: Mediator of RNA polymerase II transcription subunit 32 | Vigna angularis | 1.1 | 61.9 | 11.06 | 67 | 8/12 | 16 |
| 36a,b | XP_011039269.1 | Predicted: Tubulin beta chain-like | Populus euphratica | 0.0019 | 50.7 | 4.76 | 94 | 8/12 | 18 |
| 37a,b | NP_001237543.1 | Trypsin inhibitor | Glycine max | 0.0047 | 18.3 | 6.12 | 90 | 6/14 | 53 |
| 38a,b | OMO89133.1 | Shoot gravitropism protein | Corchorus capsularis | 0.34 | 105.8 | 5.32 | 72 | 10/10 | 9 |
| Generation of precursor metabolites and energy | |||||||||
| 39a,b | AGV54452.1 | NADPH-specific isocitrate dehydrogenase | Phaseolus vulgaris | 1.3 | 46.4 | 6.00 | 66 | 8/12 | 17 |
| Signal transduction | |||||||||
| 40a | OTG28653.1 | Hypothetical protein | Helianthus annuus | 0.028 | 91.3 | 5.20 | 83 | 9/11 | 12 |
| 41a,b | XP_002959041.1 | Small ARF-related GTPase | Volvox carteri f. nagariensis | 1.6e+2 | 20.1 | 6.74 | 45 | 4/16 | 30 |
| Nucleic acid/protein binding | |||||||||
| 42b | OWM88181.1 | Hypothetical protein | Punica granatum | 3.3 | 57.5 | 9.61 | 62 | 7/13 | 17 |
| 43a,b | GAU40109.1 | Hypothetical protein | Trifolium subterraneum | 0.46 | 53.1 | 6.19 | 71 | 7/13 | 14 |
| 44a,b | XP_019085342.1 | Uncharacterized protein | Camelina sativa | 0.48 | 101.2 | 9.42 | 70 | 7/13 | 7 |
| Translation | |||||||||
| 45a,b | AAY62839.1 | Small ribosomal protein subunit 4, partial | Haplohymenium triste | 0.092 | 22.3 | 10.17 | 78 | 6/14 | 33 |
| Transport | |||||||||
| 46a,b | KHN32468.1 | Patellin-5 | Glycine soja | 0.024 | 35.2 | 8.73 | 84 | 7/13 | 18 |
| Transferase activity | |||||||||
| 47b | OIW11558.1 | Hypothetical: Methyltransferase | Lupinus angustifolius | 5.9 | 76.6 | 4.93 | 60 | 8/12 | 11 |
| Unknown | |||||||||
| 48a | XP_003064461.1 | Predicted protein | Micromonas pusilla | 0.11 | 46.7 | 9.84 | 77 | 8/12 | 19 |
| 49a | KDP45225.1 | Hypothetical protein | Jatropha curcas | 0.32 | 27.0 | 5.33 | 72 | 6/14 | 27 |
| 50a | XP_002953155.1 | Hypothetical protein | Volvox carteri f. nagariensis | 0.28 | 44.4 | 6.96 | 71 | 7/13 | 14 |
| 51a | OWM69200.1 | Hypothetical protein | Punica granatum | 3.1 | 76.6 | 7.72 | 62 | 6/11 | 8 |
| 52a | BAD15857.1 | Hypothetical protein | Oryza sativa Japonica | 5.2e+2 | 14.7 | 12.21 | 40 | 4/16 | 31 |
| 53a | XP_020159678.1 | Predicted: Vacuolar-sorting receptor 1-like isoform X3 | Aegilopos tauschii subsp. Tauschii | 1.7e+3 | 25.5 | 5.57 | 35 | 3/14 | 8 |
| 54b | OAE31941.1 | Hypothetical protein | Marchantia polymorpha subsp. ruderalis | 0.34 | 18.0 | 7.90 | 72 | 6/14 | 25 |
| 55b | XP_017621778.1 | Uncharacterized protein | Gossypium arboreum | 0.31 | 18.1 | 11.28 | 72 | 6/14 | 39 |
| 56b | XP_018726619.1 | Predicted: Disease resistance protein | Eucalyptus grandis | 6.2 | 33.1 | 7.01 | 59 | 6/14 | 17 |
| 57b | XP_020253187.1 | R3H and coiled-coil domain-containing protein | Asparagus officinalis | 6.3 | 39.1 | 4.78 | 59 | 6/14 | 18 |
| 58a,b | XP_018450448.1 | Lysine-specific demethylase | Raphanus sativus | 7.4 | 99.2 | 6.12 | 58 | 6/14 | 7 |
| 59a,b | EPS63431.1 | Hypothetical: Probable membrane-associated kinase regulator 1 | Genlisea aurea | 25.0 | 23.9 | 10.24 | 53 | 5/15 | 26 |
| 60a,b | KHG04359.1 | Hypothetical protein | Gossypium arboreum | 3.6 | 3.4 | 3.87 | 62 | 3/17 | 90 |
| 61a,b | XP_011087585.1 | Uncharacterized protein | Sesamum indicum | 2.1e+2 | 43.8 | 4.77 | 44 | 5/15 | 8 |
| Spot ID | Accession Number | Description | Taxonomic Database | E | Mr (kDa) | pI | Mascot Score | #P | C(%) |
|---|---|---|---|---|---|---|---|---|---|
| Carbohydrate metabolic process | |||||||||
| 1a | XP_003547334.1 | Predicted: Triosephosphate isomerase isoform X1 | Glycine max | 5.3 × 10−6 | 27.4 | 5.87 | 120 | 9/11 | 36 |
| 2a | XP_003531895.1 | Predicted: 6-Phosphogluconate dehydrogenase, decarboxylating 3-like isoform 1 | Glycine max | 5.3 × 10−6 | 53.8 | 6.11 | 120 | 11/9 | 31 |
| 3a | AAS18240.1 | Enolase | Glycine max | 0.017 | 48.0 | 5.31 | 85 | 9/11 | 23 |
| 4a | OQU84669.1 | Hypothetical protein | Sorghum bicolor | 0.028 | 4.0 | 10.04 | 83 | 4/16 | 94 |
| Protein metabolic process | |||||||||
| 5a | XP_003552094.1 | Predicted: Probable mitochondrial-processing peptidase subunit β-like | Glycine max | 1.1 × 10−7 | 58.8 | 6.49 | 137 | 12/8 | 27 |
| 6a | OMO96428.1 | Hypothetical protein | Glycine max | 0.24 | 85.7 | 5.22 | 73 | 9/11 | 11 |
| Biosynthetic process | |||||||||
| 7a | NP_001235794.1 | Methionine synthase | Glycine max | 3.3 × 10−11 | 84.4 | 5.93 | 172 | 15/5 | 25 |
| 8a | NP_001235219.1 | Chalcone isomerase A | Glycine max | 0.0046 | 23.3 | 6.23 | 91 | 7/13 | 41 |
| 9b | NP_001238531.2 | Cytosolic glutamine synthetase | Glycine max | 4.2 × 10−5 | 39.1 | 5.48 | 111 | 9/11 | 31 |
| 10b | XP_003546821.1 | Predicted: Phosphoglycerate kinase, cytosolic-like | Glycine max | 0.00074 | 42.4 | 5.48 | 98 | 9/11 | 28 |
| 11b | XP_016685735.1 | L10-interacting MYB domain-containing protein-like, isoform X1 | Gossypium hirsutum | 0.14 | 36.0 | 5.96 | 76 | 7/13 | 20 |
| 12a,b | XP_003554033.1 | Predicted: 5-Methytetrahydropteroyltriglutamate-homocysteine methyltransferase 1 isoform 1 | Glycine max | 4.2 × 10−6 | 84.4 | 8.73 | 121 | 12/8 | 23 |
| 13a,b | XP_003529397.1 | Predicted: Ferredoxin-nitrite reductase, chloroplastic-like | Glycine max | 0.036 | 66.5 | 5.97 | 82 | 9/11 | 14 |
| 14a,b | XP_016752752.1 | Uncharacterized: ATP synthase CF0 subunit I | Gossypium hirsutum | 0.29 | 26.4 | 6.31 | 73 | 7/13 | 21 |
| Nucleobase-containing compound metabolic process | |||||||||
| 15a | Gm_SSP8106a | Predicted protein | Ostreococcus lucimarinus | 0.28 | 46.3 | 6.30 | 73 | 6/9 | 19 |
| 16a,b | XP_021735866.1 | Uncharacterized: ATP sulfurylase 2 | Chenopodium quinoa | 2.6 | 92.0 | 5.49 | 63 | 7/13 | 8 |
| Cellular process | |||||||||
| 17b | XP_009141007.1 | Predicted: Tubulin β-7 chain-like | Brassica napus | 0.00017 | 51.2 | 4.73 | 105 | 9/11 | 22 |
| 18b | XP_016580376.1 | Sulfite oxidase | Capsicum annuum | 3.0 | 69.1 | 9.45 | 62 | 6/14 | 11 |
| 19b | OQU86022.1 | Hypothetical: Kinesin heavy chain | Sorghum bicolor | 3.5 | 14.6 | 9.35 | 62 | 5/15 | 48 |
| Generation of precursor metabolites and energy | |||||||||
| 20a | AAA33978.1 | Isocitrate dehydrogenase | Glycine max | 5.3 × 10−9 | 49.5 | 6.13 | 150 | 13/7 | 32 |
| 21a,b | NP_001241237.1 | Uncharacterized protein | Glycine max | 6.6 × 10−9 | 46.4 | 5.87 | 149 | 13/7 | 32 |
| Metabolic process | |||||||||
| 22a | XP_013467839.1 | Pyridoxal-5′-phosphate-dependent enzyme family protein | Medicago truncatula | 0.21 | 22.2 | 7.66 | 74 | 6/14 | 30 |
| Signal transduction | |||||||||
| 23a | XP_006584765.1 | Predicted: Calcineurin B-like protein 2-like | Glycine max | 4.0 | 23.9 | 4.84 | 61 | 5/15 | 22 |
| Nucleotide/Nucleic acid binding | |||||||||
| 24a | XP_009797404.1 | Predicted: Glycine-rich RNA-binding protein 4 | Nicotiana sylvestris | 1.3 | 18.4 | 9.32 | 66 | 5/15 | 31 |
| 25a | NP_001276267.2 | Predicted: Elongation factor Tu, mitochondrial-like | Glycine max | 0.05 | 49.3 | 6.40 | 80 | 8/12 | 23 |
| 26b | XP_016674018.1 | Predicted: Coiled-coil domain-containing protein 22 homolog isoform X2 | Gossypium hirsutum | 2e+2 | 56.1 | 4.93 | 44 | 5/8 | 8 |
| Transport | |||||||||
| 27a | XP_007509415.1 | Unknown protein | Bathycoccus prasinos | 0.86 | 84.2 | 5.04 | 68 | 7/13 | 10 |
| 28a | XP_021753870.1 | Uncharacterized: ATP synthase CF1 α | Chenopodium quinoa | 0.18 | 20.2 | 8.91 | 75 | 5/7 | 19 |
| Protein binding | |||||||||
| 29a | NP_001239642.1 | Glutathione S-transferase | Glycine max | 3.3 × 10−5 | 24.9 | 5.73 | 112 | 7/13 | 36 |
| 30a | XP_021316845.1 | Acyl-binding domain-containing protein 5-like | Sorghum bicolor | 0.19 | 77.1 | 8.85 | 74 | 9/11 | 19 |
| 31a,b | XP_003531506.1 | Predicted: Nitrile-specifier protein 5-like | Glycine max | 3.3 × 10−15 | 36.0 | 5.59 | 212 | 14/6 | 55 |
| Catalytic activity | |||||||||
| 32a | KHN43834.1 | Aldo-keto reductase family 4 member C9-like | Glycine max | 1.3 × 10−9 | 35.0 | 6.40 | 156 | 12/8 | 47 |
| 33a | NP_001340170.1 | Predicted: Alcohol dehydrogenase 1 | Glycine max | 2.6 × 10−5 | 41.6 | 5.97 | 113 | 9/11 | 32 |
| 34a | KHN30856.1 | NADP-dependent alkenal double bond reductase | Glycine max | 0.036 | 38.0 | 5.94 | 82 | 7/13 | 25 |
| 35a | XP_003549946.1 | Predicted: Expansin-like B1-like | Glycine max | 0.00021 | 28.2 | 6.30 | 104 | 7/13 | 43 |
| 36b | KHN16790.1 | Monodehydroascorbate chloroplastic | Glycine max | 2.1 × 10−5 | 52.4 | 8.36 | 144 | 12/8 | 33 |
| 37b | XP_003521330.1 | Heat shock protein 70 | Glycine max | 6.6 × 10−7 | 71.8 | 5.05 | 129 | 12/8 | 26 |
| 38b | NP_001234990.1 | Peroxisomal betaine-aldehyde dehydrogenase | Glycine max | 0.00021 | 55.4 | 5.23 | 104 | 10/10 | 17 |
| 39b | KCW74152.1 | Hypothetical protein | Eucalyptus grandis | 0.046 | 52.8 | 9.73 | 81 | 10/10 | 18 |
| Kinase activity | |||||||||
| 40b | ONH93257.1 | Hypothetical: Serine threonine-protein kinase 19 | Prunus persica | 0.84 | 25.3 | 9.11 | 68 | 7/13 | 25 |
| Transferase activity | |||||||||
| 41b | XP_004968119.1 | Predicted: o-linked n-acetylglucosamine transferase-like | Setaria italica | 0.11 | 50.3 | 10.45 | 77 | 8/12 | 16 |
| Unknown | |||||||||
| 42a | XP_018476189.1 | Predicted: RRP12-like protein | Raphanus sativus | 0.44 | 141.5 | 8.88 | 71 | 9/11 | 11 |
| 43a | XP_016506991.1 | Uncharacterized protein | Nicotiana tabacum | 0.45 | 17.4 | 8.95 | 71 | 6/14 | 25 |
| 44b | XP_011040448.1 | Uncharacterized protein | Populus euphratica | 2.1 | 83.8 | 6.22 | 64 | 7/7 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vengavasi, K.; Pandey, R.; Abraham, G.; Yadav, R.K. Comparative Analysis of Soybean Root Proteome Reveals Molecular Basis of Differential Carboxylate Efflux under Low Phosphorus Stress. Genes 2017, 8, 341. https://doi.org/10.3390/genes8120341
Vengavasi K, Pandey R, Abraham G, Yadav RK. Comparative Analysis of Soybean Root Proteome Reveals Molecular Basis of Differential Carboxylate Efflux under Low Phosphorus Stress. Genes. 2017; 8(12):341. https://doi.org/10.3390/genes8120341
Chicago/Turabian StyleVengavasi, Krishnapriya, Renu Pandey, Gerard Abraham, and Ravindra Kumar Yadav. 2017. "Comparative Analysis of Soybean Root Proteome Reveals Molecular Basis of Differential Carboxylate Efflux under Low Phosphorus Stress" Genes 8, no. 12: 341. https://doi.org/10.3390/genes8120341
APA StyleVengavasi, K., Pandey, R., Abraham, G., & Yadav, R. K. (2017). Comparative Analysis of Soybean Root Proteome Reveals Molecular Basis of Differential Carboxylate Efflux under Low Phosphorus Stress. Genes, 8(12), 341. https://doi.org/10.3390/genes8120341






