Synthesis of Biomaterials Utilizing Microfluidic Technology
Abstract
:1. Introduction
2. Liposomes for Artificial Cell Systems and Organelles
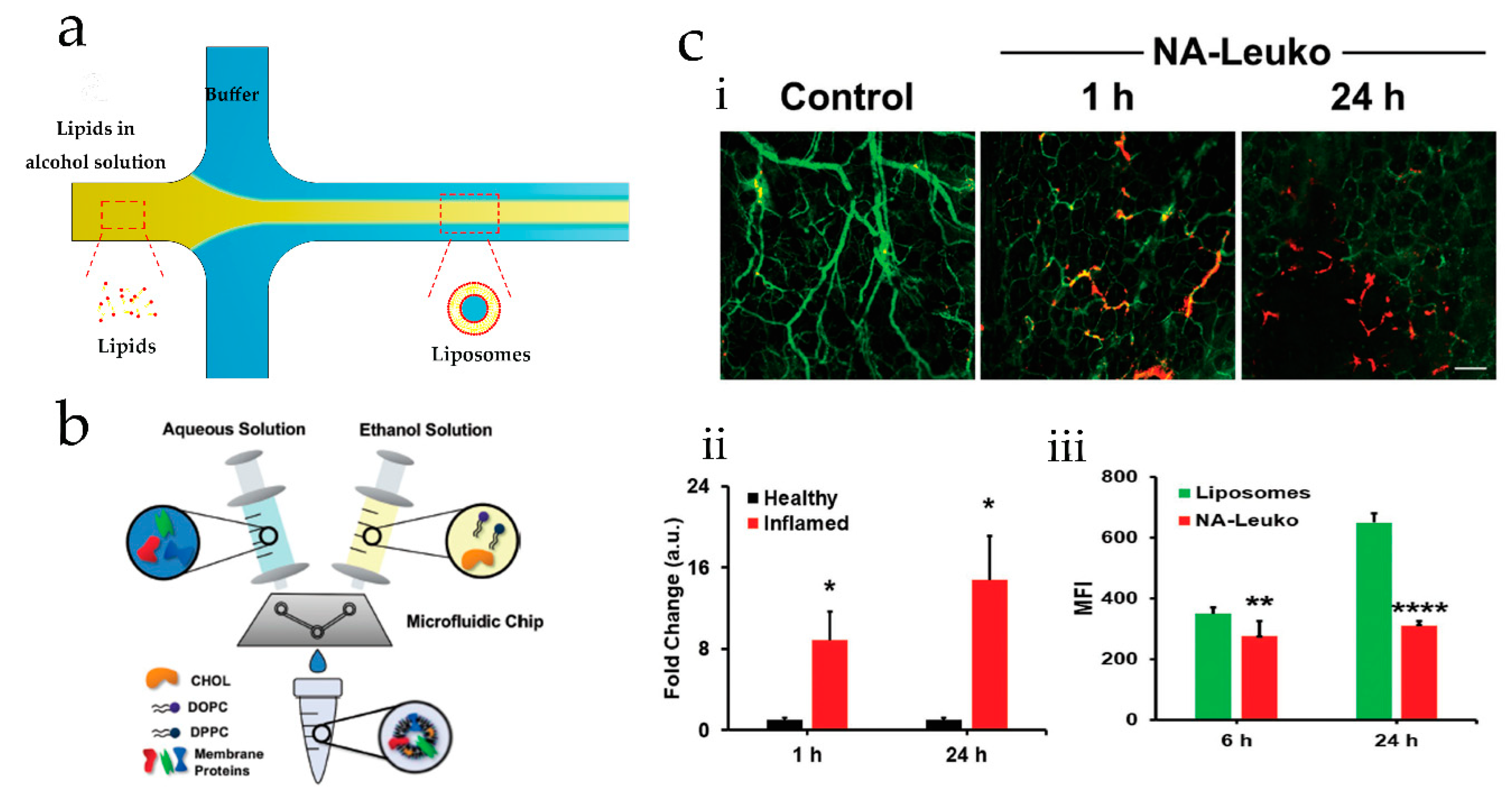
2.1. Microfluidic Hydrodynamic Focusing for Liposomes Production
2.2. Droplet Emulsion-Based Technologies for the Generation of Artificial Cells
2.3. Recent Efforts towards Cost-Effective Production of Liposomes in Microfluidic Systems
3. Microfluidic Spinning of Micro-/Nanofibers for Tissue Engineering
3.1. Photopolymerization
3.2. Diffusion-Controlled Ionic Cross-Linking
3.3. Solvent Extraction and Other Methods Based on Microfluidic Spinning
4. Microparticles/Nanomaterials for Drugs Delivery System
4.1. Nanoparticles for Drugs Delivery System
4.2. Microparticles for Drugs Delivery System
4.2.1. Microspheres
4.2.2. Non-Spherical Microparticles
5. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.-A.; Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- DeMello, A.J.; Wootton, R.C.R. Chemistry at the crossroads. Nat. Chem. 2009, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- DeMello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394. [Google Scholar] [CrossRef] [PubMed]
- Edel, J.B.; Fortt, R.; deMello, J.C.; deMello, A.J. Microfluidic routes to the controlled production of nanoparticles. Chem. Commun. 2002, 10, 1136–1137. [Google Scholar] [CrossRef]
- Onoe, H.; Takeuchi, S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today 2015, 20, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; deMello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; Solvas, X.C.I.; Wootton, R.C.R.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nam-Trung, N.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Choi, T.M.; Shim, T.S.; Frijns, R.A.M.; Kim, S.-H. Microfluidic production of multiple emulsions and functional microcapsules. Lab Chip 2016, 16, 3415–3440. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Seo, K.D.; Kim, D.W.; Kim, B.C.; Kim, D.S. Recent advances in engineering microparticles and their nascent utilization in biomedical delivery and diagnostic applications. Lab Chip 2017, 17, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cheng, Y.; Shang, L.; Wang, J.; Xie, Z.; Gu, Z. Microfluidic synthesis of barcode particles for multiplex assays. Small 2015, 11, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.-H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.-H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Carrara, P.; Kuruma, Y.; Pereira de Souza, T.; Luisi, P.L. Compartmentalized reactions as a case of soft-matter biotechnology: Synthesis of proteins and nucleic acids inside lipid vesicles. J. Mater. Chem. 2011, 21, 18887. [Google Scholar] [CrossRef]
- An, S.Y.; Bui, M.P.; Nam, Y.J.; Han, K.N.; Li, C.A.; Choo, J.; Lee, E.K.; Katoh, S.; Kumada, Y.; Seong, G.H. Preparation of monodisperse and size-controlled poly(ethylene glycol) hydrogel nanoparticles using liposome templates. J. Colloid Interface Sci. 2009, 331, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Van Swaay, D.; deMello, A. Microfluidic methods for forming liposomes. Lab Chip 2013, 13, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Carugo, D.; Mazzitelli, S.; Nastruzzi, C.; Zhang, X. Microfluidic and lab-on-a-chip preparation routes for organic nanoparticles and vesicular systems for nanomedicine applications. Adv. Drug Deliv. Rev. 2013, 65, 1496–1532. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S. Synthesizing artificial cells from giant unilamellar vesicles: State-of-the art in the development of microfluidic technology. Bioessays 2012, 34, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Andar, A.U.; Hood, R.R.; Vreeland, W.N.; DeVoe, D.L.; Swaan, P.W. Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms. Pharm. Res. 2014, 31, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, K.; Tresset, G.; Coquet, P.; Fujita, H.; Takeuchi, S. Electroformation of giant liposomes in microfluidic channels. Meas. Sci. Technol. 2006, 17, 3121–3126. [Google Scholar] [CrossRef]
- Le Berre, M.; Yamada, A.; Reck, L.; Chen, Y.; Baigl, D. Electroformation of giant phospholipid vesicles on a silicon substrate: Advantages of controllable surface properties. Langmuir 2008, 24, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Pereno, V.; Carugo, D.; Bau, L.; Sezgin, E.; de la Serna, J.B.; Eggeling, C.; Stride, E. Electroformation of giant unilamellar vesicles on stainless steel electrodes. ACS Omega 2017, 2, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Ma, S.; Zhang, Y.; Han, X. Electroformation of giant unilamellar vesicles in saline solution. Colloids Surf. B-Biointerfaces 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Polenz, I.; Baret, J.-C.; Herminghaus, S.; Baeumchen, O. Vesicles-on-a-chip: A universal microfluidic platform for the assembly of liposomes and polymersomes. Eur. Phys. J. E 2016, 39, 59. [Google Scholar] [CrossRef] [PubMed]
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phapal, S.M.; Sunthar, P. Influence of micro-mixing on the size of liposomes self-assembled from miscible liquid phases. Chem. Phys. Lipids 2013, 172, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, R.R.; DeVoe, D.L.; Atencia, J.; Vreeland, W.N.; Omiatek, D.M. A facile route to the synthesis of monodisperse nanoscale liposomes using 3Dd microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip 2014, 14, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; DeVoe, D.L. High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, R.; Evangelopoulos, M.; Hoffman, J.R.; Corbo, C.; Taraballi, F.; Martinez, J.O.; Hartman, K.A.; Cosco, D.; Costa, G.; Romeo, I.; et al. Design and development of biomimetic nanovesicles using a microfluidic approach. Adv. Mater. 2018, 30, e1702749. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.G.S.; Briuglia, M.L.; Niosi, F.; Lamprou, D.A. Microfluidic manufacturing of phospholipid nanoparticles: Stability, encapsulation efficacy, and drug release. Int. J. Pharm. 2017, 516, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, R.R.; Shao, C.; Omiatek, D.M.; Vreeland, W.N.; DeVoe, D.L. Microfluidic synthesis of peg- and folate-conjugated liposomes for one-step formation of targeted stealth nanocarriers. Pharm. Res. 2013, 30, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Middelberg, A.P.J.; Zhao, C.-X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B-Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.C.; Hettiarachchi, K.; Siu, M.; Pan, Y.P. Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles. J. Am. Chem. Soc. 2006, 128, 5656–5658. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Paegel, B.M. Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line. J. Am. Chem. Soc. 2011, 133, 2798–2800. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Caspi, Y.; Meijering, A.E.C.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 10447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, N.-N.; Yelleswarapu, M.; Huck, W.T.S. Monodisperse uni- and multicompartment liposomes. J. Am. Chem. Soc. 2016, 138, 7584–7591. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-N.; Yelleswarapu, M.; Zheng, L.; Huck, W.T.S. Microfluidic assembly of monodisperse vesosomes as artificial cell models. J. Am. Chem. Soc. 2017, 139, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-N.; Huck, W.T.S. Microfluidic formation of monodisperse coacervate organelles in liposomes. Angew. Chem.-Int. Ed. 2017, 56, 9736–9740. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Frohnmayer, J.P.; Benk, L.T.; Haller, B.; Janiesch, J.-W.; Heitkamp, T.; Borsch, M.; Lira, R.B.; Dimova, R.; Lipowsky, R.; et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mat. 2018, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, D.A.; Yanar, F.; Mosayyebi, A.; García-Manrique, P.; Stulz, E.; Carugo, D.; Zhang, X. Easy-to-perform and cost-effective fabrication of continuous-flow reactors and their application for nanomaterials synthesis. New Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electro spinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Kim, J.; Kim, S.; Lee, S.; Mensing, G.; Beebe, D.J. Hydrodynamic microfabrication via “on the fly” photopolymerization of microscale fibers and tubes. Lab Chip 2004, 4, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Choi, C.-H.; Chung, S.; Chung, Y.-M.; Lee, C.-S. Microfluidic synthesis of a cell adhesive janus polyurethane microfiber. Lab Chip 2009, 9, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ostrovidov, S.; Zhao, Y.; Liang, X.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Wu, H.; Khademhosseini, A. Microfluidic spinning of cell-responsive grooved microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, K.H.; Kang, E.; Kim, D.-S.; Lee, S.-H. Microfluidic wet spinning of chitosan-alginate microfibers and encapsulation of HepG2 cells in fibers. Biomicrofluidics 2011, 5, 22208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelozzi, M.; Miotto, M.; Penolazzi, L.; Mazzitelli, S.; Keane, T.; Badylak, S.F.; Piva, R.; Nastruzzi, C. Composite ECM-alginate microfibers produced by microfluidics as scaffolds with biomineralization potential. Mater. Sci. Eng. C 2015, 56, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yu, Y.; Fu, F.; Wang, J.; Shang, L.; Gu, Z.; Zhao, Y. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 2016, 8, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Naganuma, Y.; Yajima, Y.; Yamada, M.; Seki, M. Patterned hydrogel microfibers prepared using multilayered microfluidic devices for guiding network formation of neural cells. Biofabrication 2014, 6, 035011. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.-Y.; Lee, J.-Y.; Park, H.; Park, Y.-D.; Lee, K.-B.; Whang, C.-M.; Lee, S.-H. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.-H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Choi, Y.Y.; Chae, S.-K.; Moon, J.-H.; Chang, J.-Y.; Lee, S.-H. Microfluidic spinning of flat alginate fibers with grooves for cell-aligning scaffolds. Adv. Mater. 2012, 24, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; He, X.; Yang, Y.; Wei, D.; Sun, J.; Zhong, M.; Xie, R.; Fan, H.; Zhang, X. Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction. Acta Biomater. 2016, 38, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, W.; Wang, Y.; Xu, C.; Guo, Y.; Qin, J. Simple spinning of heterogeneous hollow microfibers on chip. Adv. Mater. 2016, 28, 6649–6655. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic generation of hollow Ca-alginate microfibers. Lab Chip 2016, 16, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Tottori, S.; Takeuchi, S. Formation of liquid rope coils in a coaxial microfluidic device. RSC Adv. 2015, 5, 33691–33695. [Google Scholar] [CrossRef]
- Nie, M.; Takeuchi, S. Microfluidics based synthesis of coiled hydrogel microfibers with flexible shape and dimension control. Sens. Actuators B-Chem. 2017, 246, 358–362. [Google Scholar] [CrossRef]
- He, X.-H.; Wang, W.; Deng, K.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic fabrication of chitosan microfibers with controllable internals from tubular to peapod-like structures. RSC Adv. 2015, 5, 928–936. [Google Scholar] [CrossRef]
- Hwang, C.M.; Khademhosseini, A.; Park, Y.; Sun, K.; Lee, S.-H. Microfluidic chip-based fabrication of PLGA microfiber scaffolds for tissue engineering. Langmuir 2008, 24, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Thangawng, A.L.; Howell, P.B., Jr.; Richards, J.J.; Erickson, J.S.; Ligler, F.S. A simple sheath-flow microfluidic device for micro/nanomanufacturing: Fabrication of hydrodynamically shaped polymer fibers. Lab Chip 2009, 9, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; VanDersarl, J.J.; Dashtimoghadam, E.; Bahlakeh, G.; Majedi, F.S.; Mokarram, N.; Bertsch, A.; Jacob, K.I.; Renaud, P. A microfluidic approach to synthesizing high-performance microfibers with tunable anhydrous proton conductivity. Lab Chip 2013, 13, 4549–4553. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.-K.; Kang, E.; Khademhosseini, A.; Lee, S.-H. Micro/nanometer-scale fiber with highly ordered structures by mimicking the spinning process of silkworm. Adv. Mater. 2013, 25, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Guo, M. Microfluidic controlled mass-transfer and buckling for easy fabrication of polymeric helical fibers. Macromol. Rapid Commun. 2016, 37, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-Y.; Ju, X.-J.; Deng, K.; Fan, X.-X.; He, X.-H.; Wu, F.; He, F.; Liu, Z.; Wang, W.; Xie, R.; et al. The microfluidic synthesis of composite hollow microfibers for k+-responsive controlled release based on a host–guest system. J. Mater. Chem. B 2016, 4, 3925–3935. [Google Scholar] [CrossRef]
- Daniele, M.A.; North, S.H.; Naciri, J.; Howell, P.B.; Foulger, S.H.; Ligler, F.S.; Adams, A.A. Rapid and continuous hydrodynamically controlled fabrication of biohybrid microfibers. Adv. Funct. Mater. 2013, 23, 698–704. [Google Scholar] [CrossRef]
- Lee, K.H.; Shin, S.J.; Kim, C.-B.; Kim, J.K.; Cho, Y.W.; Chung, B.G.; Lee, S.-H. Microfluidic synthesis of pure chitosan microfibers for bio-artificial liver chip. Lab Chip 2010, 10, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, Z.; Ma, J.; Shi, Y.; Xu, H.; Lykkemark, S.; Qin, J. Flexible fabrication of shape-controlled collagen building blocks for self-assembly of 3d microtissues. Small 2015, 11, 3666–3675. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wen, H.; Ma, J.; Lykkemark, S.; Xu, H.; Qin, J. Flexible fabrication of biomimetic bamboo- like hybrid microfibers. Adv. Mater. 2014, 26, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Gong, X. Pdms Based Microfluidic Chips and Their Application in Material Synthesis; Hong Kong University of Science and Technology: Hong Kong, China, 2009. [Google Scholar]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hormes, J.; Kumar, C.S. Microfluidic synthesis of nanomaterials. Small 2008, 4, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cito, S.; Zhang, Y.; Wang, C.; Sikanen, T.M.; Santos, H.A. A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties. Adv. Mater. 2015, 27, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.D.; Liu, D.; Lin, W. Are high drug loading nanoparticles the next step forward for chemotherapy? Nanomedicine 2012, 7, 303–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.C.W. Nanomedicine 2.0. Acc. Chem. Res. 2017, 50, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Liu, D.; Bernuz, C.R.; Fan, J.; Li, W.; Correia, A.; Hirvonen, J.; Santos, H.A. A nano-in-nano vector: Merging the best of polymeric nanoparticles and drug nanocrystals. Adv. Funct. Mater. 2017, 27, 13. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Cito, S.; Fan, J.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; Sikanen, T.M.; Weitz, D.A.; Santos, H.A. Core/shell nanocomposites produced by superfast sequential microfluidic nanoprecipitation. Nano Lett. 2017, 17, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Herranz-Blanco, B.; Mäkilä, E.; Lehto, V.-P.; Salonen, J.; Hirvonen, J.; Santos, H.A. Microfluidic assembly of monodisperse multistage ph-responsive polymer/porous silicon composites for precisely controlled multi-drug delivery. Small 2014, 10, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Mäkilä, E.; Fan, J.; Herranz-Blanco, B.; Wang, C.-F.; Rosa, R.; Ribeiro, A.J.; Salonen, J.; Hirvonen, J.; et al. Microfluidic assisted one-step fabrication of porous silicon@acetalated dextran nanocomposites for precisely controlled combination chemotherapy. Biomaterials 2015, 39, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kodzius, R.; Xiao, K.; Wu, J.; Yi, X.; Gong, X.; Foulds, I.G.; Wen, W. Inhibitory effect of common microfluidic materials on pcr outcome. Sens. Actuators B-Chem. 2012, 161, 349–358. [Google Scholar] [CrossRef]
- Lone, S.; Cheong, I.W. Fabrication of polymeric janus particles by droplet microfluidics. RSC Adv. 2014, 4, 13322–13333. [Google Scholar] [CrossRef]
- Luo, G.; Du, L.; Wang, Y.; Lu, Y.; Xu, J. Controllable preparation of particles with microfluidics. Particuology 2011, 9, 545–558. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.-J.; Chu, L.-Y. Functional polymeric microparticles engineered from controllable microfluidic emulsions. Acc. Chem. Res. 2014, 47, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic devices for drug delivery systems and drug screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Xie, J.; Hou, Z.; Shezad, K.; Xu, J.; Wang, K.; Gao, Y.; Shen, L.; Zhu, J. Regulation of drug release by tuning surface textures of biodegradable polymer microparticles. ACS Appl. Mater. Interfaces 2017, 9, 14391–14400. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Peng, S.; Wen, W.; Sheng, P.; Li, W. Design and fabrication of magnetically functionalized core/shell microspheres for smart drug delivery. Adv. Funct. Mater. 2009, 19, 292–297. [Google Scholar] [CrossRef]
- Kim, D.H.; Choy, T.; Huang, S.; Green, R.M.; Omary, R.A.; Larson, A.C. Microfluidic fabrication of 6-methoxyethylamino numonafide-eluting magnetic microspheres. Acta Biomater. 2014, 10, 742–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J. Microfluidic-based synthesis of hydrogel particles for cell microencapsulation and cell-based drug delivery. Polymers 2012, 4, 1084–1108. [Google Scholar] [CrossRef]
- Choi, C.-H.; Wang, H.; Lee, H.; Kim, J.H.; Zhang, L.; Mao, A.; Mooney, D.J.; Weitz, D.A. One-step generation of cell-laden microgels using double emulsion drops with a sacrificial ultra-thin oil shell. Lab Chip 2016, 16, 1549–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.Y.; Praveenkumar, R.; Oh, Y.-K.; Lee, K.; Kim, S.-H. Alginate microgels created by selective coalescence between core drops paired with an ultrathin shell. J. Mater. Chem. B 2016, 4, 3232–3238. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Chen, C.; Zheng, Z.; Chang, H. Monodisperse water-in-oil-in-water emulsions generation for synthesising alginate hydrogel microspheres via locally hydrophobic modification to pmma microchannels. Sens. Actuators B Chem. 2018, 255, 1048–1056. [Google Scholar] [CrossRef]
- Marquis, M.; Alix, V.; Capron, I.; Cuenot, S.; Zykwinska, A. Microfluidic encapsulation of pickering oil microdroplets into alginate microgels for lipophilic compound delivery. ACS Biomater. Sci. Eng. 2016, 2, 535–543. [Google Scholar] [CrossRef]
- Foster, G.A.; Headen, D.M.; Gonzalez-Garcia, C.; Salmeron-Sanchez, M.; Shirwan, H.; Garcia, A.J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 2017, 113, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossow, T.; Heyman, J.A.; Ehrlicher, A.J.; Langhoff, A.; Weitz, D.A.; Haag, R.; Seiffert, S. Controlled synthesis of cell-laden microgels by radical-free gelation in droplet microfluidics. J. Am. Chem. Soc. 2012, 134, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.N.; Luo, R.; Kwek, K.Z.; Por, Y.C.; Zhang, Y.; Chen, C.-H. Sustained release of hydrophobic drugs by the microfluidic assembly of multistage microgel/poly (lactic-co-glycolic acid) nanoparticle composites. Biomicrofluidics 2015, 9, 052601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Yoshida, M.; Yanagi, K.; Hatate, Y.; Shiomori, K.; Kiyoyama, S. Preparation of acetamiprid-loaded polymeric microcapsules: Influence of preparation parameter in emulsion system on microcapsule characteristics. Polym. Bull. 2008, 61, 119–127. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Sakdapipanich, J.T. Development of a controlled release neem capsule with a sodium alginate matrix, crosslinked by glutaraldehyde and coated with natural rubber. Polym. Bull. 2009, 63, 609–622. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, T.Y.; Lee, S.S. Osmocapsules for direct measurement of osmotic strength. Small 2014, 10, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Byun, A.; Shim, J.; Han, S.W.; Kim, B.; Chae, P.S.; Shin, H.S.; Kim, J.W. One-pot microfluidic fabrication of graphene oxide-patched hollow hydrogel microcapsules with remarkable shell impermeability. Chem. Commun. 2015, 51, 12756–12759. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Chung, S.; Kim, Y.E.; Lee, K.S.; Lee, S.H.; Oh, K.W.; Kang, J.Y. Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid. Lab Chip 2011, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T.; et al. Microfluidic fabrication of colloidal nanomaterials-encapsulated microcapsules for biomolecular sensing. Nano Lett. 2017, 17, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, W.J.; Lin, T.; Abate, A.R.; Seiffert, S.; Shah, R.K.; Weitz, D.A. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip 2012, 12, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.S.; Abbaspourrad, A.; Amstad, E.; Fan, J.; Kim, S.-H.; Romanowsky, M.; Shum, H.C.; Sun, B.; Utada, A.S.; Windbergs, M.; et al. 25th anniversary article: Double emulsion templated solid microcapsules: Mechanics and controlled release. Adv. Mater. 2014, 26, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhao, G.; Panhwar, F.; Akbar, M.F.; Shu, Z. Well-designed microcapsules fabricated using droplet-based microfluidic technique for controlled drug release. J. Drug Deliv. Sci. Technol. 2017, 39, 379–384. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Ganan-Calvo, A.M. Micro/nano encapsutation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef]
- Lone, S.; Ghodake, G.S.; Lee, D.S.; Cheong, I.W. Facile preparation of highly monodisperse poly(NIPAAm)-AuNP composite hollow microcapsules by simple tubular microfluidics. New J. Chem. 2013, 37, 877–881. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Deng, R.; Xu, W.; Liu, S.; Liang, R.; Nie, Z.; Zhu, J. Construction of multifunctional photonic crystal microcapsules with tunable shell structures by combining microfluidic and controlled photopolymerization. Lab Chip 2012, 12, 2795–2798. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Boltyanskiy, R.; Nejati, S.; Thiam, A.R.; Loewenberg, M.; Dufresne, E.R.; Osuji, C.O. Single-step microfluidic fabrication of soft monodisperse polyelectrolyte microcapsules by interfacial complexation. Lab Chip 2014, 14, 3494–3497. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kimura, Y.; Ono, T. Monodisperse polylactide microcapsules with a single aqueous core prepared via spontaneous emulsification and solvent diffusion. Rsc Adv. 2014, 4, 4872–4877. [Google Scholar] [CrossRef]
- Choi, C.-H.; Jung, J.-H.; Kim, D.-W.; Chung, Y.-M.; Lee, C.-S. Novel one-pot route to monodisperse thermosensitive hollow microcapsules in a microfluidic system. Lab Chip 2008, 8, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, L.; Gu, Y.-Y.; Chu, L.-Y. Microfluidic fabrication of monodisperse microcapsules for glucose-response at physiological temperature. Soft Matter 2013, 9, 4150–4159. [Google Scholar] [CrossRef]
- Kim, M.; Yeo, S.J.; Highley, C.B.; Burdick, J.A.; Yoo, P.J.; Doh, J.; Lee, D. One-step generation of multifunctional polyelectrolyte microcapsules via nanoscale interfacial complexation in emulsion (NICE). ACS Nano. 2015, 9, 8269–8278. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, W.; Li, Y.; Luo, C.; Zeng, Y.; Xu, Y.; Zhou, J. Generation of uniform polymer eccentric and core-centered hollow microcapsules for ultrasound-regulated drug release. J. Mater. Chem. B 2014, 2, 6848–6854. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Reis, R.L.; Azevedo, H.S. Peptide-based microcapsules obtained by self-assembly and microfluidics as controlled environments for cell culture. Soft Matter 2013, 9, 9237–9248. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Zhao, S.; Bielecki, P.; Rao, W.; Choi, J.K.; Zhao, Y.; Yu, J.; Zhang, W.; He, X. One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip 2013, 13, 4525–4533. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jeong, Y.; Kwon, T.; Lee, D.; Oh, D.Y.; Park, T.-J.; Kim, J.; Kim, J.; Kwon, S. Liquid-capped encoded microcapsules for multiplex assays. Lab Chip 2017, 17, 738. [Google Scholar] [CrossRef] [PubMed]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Non-spherical micro- and nanoparticles: Fabrication, characterization and drug delivery applications. Expert Opin. Drug Deliv. 2015, 12, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Jeyhani, M.; Mak, S.Y.; Sammut, S.; Shum, H.C.; Hwang, D.K.; Tsai, S.S.H. Controlled electrospray generation of nonspherical alginate microparticles. Chemphyschem 2017, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alteriis, R.D.; Vecchione, R.; Attanasio, C.; Gregorio, M.D.; Porzio, M.; Battista, E.; Netti, P.A. A method to tune the shape of protein-encapsulated polymeric microspheres. Sci. Rep. 2015, 5, 12634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, W.G.; Pishko, M. Photoreaction injection molding of biomaterial microstructures. Langmuir 2003, 19, 10310–10316. [Google Scholar] [CrossRef]
- Burdick, J.A.; Khademhosseini, A.; Langer, R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 2004, 20, 5153–5156. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, D.C.; Chapin, S.C.; Srinivas, R.L.; Doyle, P.S. Bar-coded hydrogel microparticles for protein detection: Synthesis, assay and scanning. Nat. Protoc. 2011, 6, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Tsai, S.S.H.; Cheng, C.-H.; Hwang, D.K. One-step two-dimensional microfluidics-based synthesis of three-dimensional particles. Adv. Mater. 2014, 26, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Dendukuri, D.; Gu, S.S.; Pregibon, D.C.; Hatton, T.A.; Doyle, P.S. Stop-flow lithography in a microfluidic device. Lab Chip 2007, 7, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Bong, K.W.; Chapin, S.C.; Pregibon, D.C.; Baah, D.; Floyd-Smith, T.M.; Doyle, P.S. Compressed-air flow control system. Lab Chip 2011, 11, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of monodisperse particles by using microfluidics: Control over size, shape, and composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, K.W.; Zhang, H.Y.; Pang, B.; Choi, C.H.; Mao, A.S.; Liao, H.B.; Utech, S.; Mooney, D.J.; Wang, H.N.; et al. Microfluidic templated multicompartment microgels for 3D encapsulation and pairing of single cells. Small 2018, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, K.; Tresset, G.; Coquet, P.; Fujita, H.; Takeuchi, S. Electroformation of giant liposomes in microfluidic channels. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Seoul, Korea, 5–9 June 2005; pp. 1159–1162. [Google Scholar]
- Dittrich, P.S.; Heule, M.; Renaud, P.; Manz, A. On-chip extrusion of lipid vesicles and tubes through microsized apertures. Lab Chip 2006, 6, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, E.; Nastruzzi, C. “Off-the-shelf” microfluidic devices for the production of liposomes for drug delivery. Mater. Sci. Eng. C 2016, 64, 29–33. [Google Scholar] [CrossRef] [PubMed]
- TruongVo, T.N.; Kennedy, R.M.; Chen, H.; Chen, A.; Berndt, A.; Agarwal, M.; Zhu, L.; Nakshatri, H.; Wallace, J.; Na, S.; et al. Microfluidic channel for characterizing normal and breast cancer cells. J. Micromech. Microeng. 2017, 27, 035017. [Google Scholar] [CrossRef]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Formation of giant lipid vesiclelike compartments from a planar lipid membrane by a pulsed jet flow. J. Am. Chem. Soc. 2007, 129, 12608–12609. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibres. Angew. Chem.-Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Oakey, J.; Toner, M.; Arthur, J.A.; Anseth, K.S.; Lee, S.; Zeiger, A.; Van Vliet, K.J.; Doyle, P.S. Stop-flow lithography for the production of shape-evolving degradable microgel particles. J. Am. Chem. Soc. 2009, 131, 4499–4504. [Google Scholar] [CrossRef] [PubMed]




| Method | Advantages | Disadvantages | Liposomes Diameter | Reference |
|---|---|---|---|---|
| Electroformation | Simple and rapid | Large size polydisperisity, inapplicable to ionic solutions, low encapsulation efficiency | 5–150 μm | [24,25] |
| Hydration | Electric fields are unnecessary | Large size polydisperisity, products are multilamellar; sensitive to phospholipid type and physical conditions, low encapsulation efficiency | 1–10 μm | [24] |
| Extrusion | Reduced size polydispersity | Relatively complex in operation | 130–370 nm | [28] |
| Microfluidic jetting | Products are unilamellar and of controlled size, encapsulation efficiencies are high | Specialized equipment needed, sensitivity to operational parameters and types of materials used | Above 100 μm | [29] |
| Microfluidic hydrodynamic-focusing | Products are monodisperse, the size and lamellarity of liposomes are easily controlled, high-throughput production | Low liposome concentration in the end-product | 50–300 nm | [30] |
| Droplet emulsion templates | Polymerosomes can be generated, the size and structure of products can be controlled, high encapsulation efficiencies | Solvent may reside between monolayers | 20–200 μm | [31] |
| Method | Advantage | Disadvantage | Morphologies | Reference |
|---|---|---|---|---|
| Photopolymerization | Simple and fast | The biomaterials loaded on the fiber are limited because of the ultraviolet (UV) radiation | Solid cylinder | [53] |
| Microtube | [53] | |||
| Janus | [54] | |||
| Solid cylinder with grooved structures | [55] | |||
| Diffusion-Controlled Ionic Cross-linking | Reaction processing under mild conditions, more flexible control of structure of fibers, a wider range of biomaterials could be encapsulated loaded or within fibers | The reaction is affected by diffusion rate of ionic cross-linking agent | Solid cylinder | [56,57,58,59] |
| Spiral curls | [60] | |||
| Solid fiber with spindle-knots | [61] | |||
| Tubuliform fibers with nanogrooves | [61] | |||
| Flat microfibers | [62] | |||
| Hollow fibers | [58,63,64,65] | |||
| Core–shell microfibers | [66] [67,68] | |||
| Straight, folded, and coiled structure | ||||
| Cylinder with peapod-like internals | [69] | |||
| Solvent extraction | Reaction processing under mild conditions | Limit type of materials used to fabricate fibers because of special requirement of solvent | Solid cylinder | [70,71,72] |
| Ribbon-shaped fiber | [71] | |||
| Silk structures | [73] | |||
| Helical microfibers | [74] | |||
| Janus hollow microfiber | [11] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, J.; Wang, P.; DeMello, A.; Feng, L.; Zhu, X.; Wen, W.; Kodzius, R.; Gong, X. Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes 2018, 9, 283. https://doi.org/10.3390/genes9060283
Wang X, Liu J, Wang P, DeMello A, Feng L, Zhu X, Wen W, Kodzius R, Gong X. Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes. 2018; 9(6):283. https://doi.org/10.3390/genes9060283
Chicago/Turabian StyleWang, Xiaohong, Jinfeng Liu, Peizhou Wang, Andrew DeMello, Lingyan Feng, Xiaoli Zhu, Weijia Wen, Rimantas Kodzius, and Xiuqing Gong. 2018. "Synthesis of Biomaterials Utilizing Microfluidic Technology" Genes 9, no. 6: 283. https://doi.org/10.3390/genes9060283
APA StyleWang, X., Liu, J., Wang, P., DeMello, A., Feng, L., Zhu, X., Wen, W., Kodzius, R., & Gong, X. (2018). Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes, 9(6), 283. https://doi.org/10.3390/genes9060283






