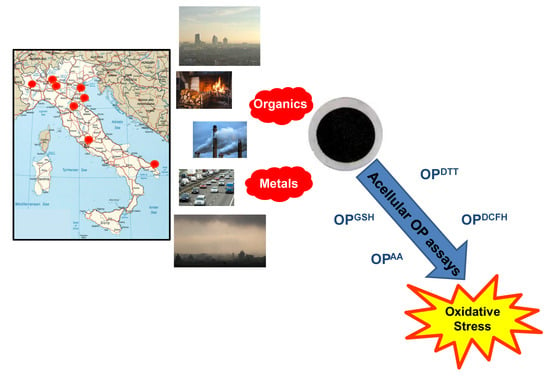
Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy
Abstract
:1. Introduction
2. Methodology
2.1. PM Sampling and Filter Extraction
2.2. Quantification of PM Oxidative Potential Using Acellular Assays
2.2.1. DTT Assay
2.2.2. AA and GSH Assays
2.2.3. DCFH Assay
2.3. Analytical Methodologies for Chemical Characterization
3. Results and Discussion
3.1. Study Overview
3.2. Association of Oxidative Potential with PM Chemical Composition
- -
- Metals. The major PM components, such as alkali (Na, K) and hearth metals (Ca, Mg, Ba, Al) can be originated from resuspension of road dust, road abrasion, and soil dust emissions. Trace elements are several transition metals (i.e., Fe Cu, Zn, Pb, Cr, Ni, Mn, Sn, Cd) associated with non-tail pipe traffic emissions, mainly related to brake and tire wear [11,25,39,41,42,43,44]. Finally, K and Rb can be considered as tracers of the biomass burning [23,26].
- -
- Carbonaceous components. They include elemental, EC, organic, OC, and water soluble organic carbon, WSOC. OC may be discriminated between primary (POC) and secondary organic species (SOC), based on the quantification of individual compounds, as tracers of specific sources, i.e., sugars, levoglucosan—widely used tracer of biomass burning [22,31,37,43]; n-alkane and PAHs—tracers of tail pipe traffic emissions [20]; carboxylic acids and quinones—markers of photochemical formation of SOA [45,46,47,48,49,50,51,52].
- -
3.2.1. Association with Metals
3.2.2. Association with Organic Species
3.2.3. Intercorrelation among Species
3.3. Comparison among Different Acellular Assays
3.3.1. Sensitivity of Different Acellular Assays
3.3.2. Correlation between OP Responses from Different Acellular Assays
3.3.3. PM Size Distribution of OP Responses
- ■
- Similar OPDTTV values were measured in Lecce for PM2.5 and PM10 fractions (close to 0.20 ± 0.04 nmol min−1 m−3), while the intrinsic OPDTTm value was larger for PM2.5 than for PM10 [24];
- ■
- An average ratio of 0.86 (±0.10 standard deviation) was found between OPDTTV of PM2.5 and PM10 particles in an independent study in Lecce, with the differences between the two fractions maximized for Saharan dust events and minimized for high carbon content samples [27];
- ■
- By investigating OP distribution of size-segregated PM samples collected in Rome and Ferrara, Simonetti found that OPDTTV, as well as OPDCFHV responses, shows a size distribution profile characterized by of a broad maximum in the 0.32–1.8 μm PM, that is similar to that of the markers of BB emissions [25];
- ■
- In a study concerning PM3, PM3–7, and PM>7 in 17 sites in Europe, including Milan, Shafer found that OPDTTV is dominated by the PM3 fraction, since it represents 76% of total OP activity, with the PM3-7 contributing on average 17% and PM>7 7%. Accordingly, PM3 fraction showed a higher intrinsic OPDTTm in comparison with the larger particles. No chemical components were found associated with DTT activity (Spearman r > 0.7) in the PM3 size cut, while tracers of biomass burning—K and Rb—and of non-tailpipe vehicle emission—Fe, Sn, Cu, Sb, and Ba—exhibited good correlations with OPDTTV in the PM3–7 size fraction [16].
3.4. Spatial Variability of OP in Different Areas across Italy
3.4.1. OP Responses of PM10 Particles
3.4.2. OP Responses of PM2.5 Particles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrams, J.Y.; Weber, R.J.; Klein, M.; Samat, S.E.; Chang, H.H.; Strickland, M.J.; Verma, V.; Fang, T.; Bates, J.T.; Mulholland, J.A.; et al. Associations between Ambient Fine Particulate Oxidative Potential and Cardiorespiratory Emergency Department Visits. Environ. Health Perspect. 2017, 5, 10. [Google Scholar]
- Antinolo, M.; Willis, M.D.; Zhou, S.; Abbatt, J.P.D. Connecting the oxidation of soot to its redox cycling abilities. Nat. Commum. 2015, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 5–133. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 16–1167. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Global Perspective on the Oxidative Potential of Airborne Particulate Matter: A Synthesis of Research Findings. Environ. Sci. Technol. 2014, 48, 7576–7583. [Google Scholar] [CrossRef]
- Strak, M.; Janssen, N.A.H.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; Harrison, R.M.; Brunekreef, B.; et al. Respiratory Health Effects of Airborne Particulate Matter: The Role of Particle Size, Composition, and Oxidative Potential-The RAPTES Project. Environ. Health Perspect. 2012, 120, 1183–1189. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef]
- Sauvain, J.; Rossi, M.J.; Riediker, M. Comparison of three acellular tests for assessing the oxidation potential of nanomaterials. Aerosol Sci. Technol. 2013, 47, 218–227. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Ottria, G.; Perdelli, F.; Cristina, M.L. Chemical Characterization of the Coarse and Fine Particulate Matter in the Environment of an Underground Railway System: Cytotoxic Effects and Oxidative Stress—A Preliminary Study. Int. J. Environ. Res. Public Health 2015, 12, 4031–4046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ahmed, C.M.S.; Canchola, A.; Chen, J.Y.; Lin, Y.-H. Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols. Atmosphere 2019, 10, 571. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Hedayat, F.; Stevanovic, S.; Miljevic, B.; Bottle, S.; Ristovski, Z.D. Review—Evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Eng. Q. 2015, 21, 201–210. [Google Scholar] [CrossRef]
- Hellack, B.; Nickel, C.; Albrecht, C.; Kuhlbusch, T.A.J.; Boland, S.; Baeza-Squiban, A.; Wohlleben, W.; Schins, R.P.F. Analytical methods to assess the oxidative potential of nanoparticles: A review. Environ. Sci. Nano 2017, 4, 1920–1926. [Google Scholar] [CrossRef]
- Shafer, M.M.; Hemming, J.D.C.; Antkiewicz, D.S.; Schauer, J.J. Oxidative potential of size-fractionated atmospheric aerosol in urban and rural sites across Europe. Faraday Discuss. 2016, 189, 381–405. [Google Scholar] [CrossRef]
- Szigeti, T.; Dunster, C.; Cattaneo, A.; Cavallo, D.; Spinazzè, A.; Saraga, D.E.; Sakellaris, I.A.; de Kluizenaar, Y.; Cornelissen, E.J.M.; Hänninen, O.; et al. Oxidative potential and chemical composition of PM2.5 in office buildings across Europe—The OFFICAIR study. Environ. Int. 2016, 92–93, 324–333. [Google Scholar] [CrossRef]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef]
- Gulliver, J.; Morley, D.; Dunster, C.; McCrea, A.; van Nunen, E.; Tsai, M.-Y.; Probst-Hensch, N.; Eeftens, M.; Imboden, M.; Ducret-Stich, R.; et al. Land use regression models for the oxidative potential of fine particles (PM2.5) in five European areas. Environ. Res. 2018, 160, 247–255. [Google Scholar] [CrossRef]
- Jedynska, A.; Hoek, G.; Wang, M.; Yang, A.; Eeftens, M.; Cyrys, J.; Keuken, M.; Ampe, C.; Beelen, R.; Cesaroni, G.; et al. Spatial variations and development of land use regression models of oxidative potential in ten European study areas. Atmos. Environ. 2017, 150, 24–32. [Google Scholar] [CrossRef]
- Costabile, F.; Alas, H.; Aufderheide, M.; Avino, P.; Amato, F.; Argentini, S.; Barnaba, F.; Berico, M.; Bernardoni, V.; Biondi, R.; et al. First Results of the “Carbonaceous Aerosol in Rome and Environs (CARE)” Experiment: Beyond Current Standards for PM10. Atmosphere 2017, 8, 249. [Google Scholar] [CrossRef]
- Sandrini, S.; Fuzzi, S.; Piazzalunga, A.; Prati, P.; Bonasoni, P.; Cavalli, F.; Bove, M.C.; Calvello, M.; Cappelletti, D.; Colombi, C.; et al. Spatial and seasonal variability of carbonaceous aerosol across Italy. Atmos. Environ. 2014, 99, 587–598. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Dalpiaz, C.; Dell’Anna, R.; Lazzeri, P.; Manarini, F.; Visentin, M.; Toninandel, G. Chemical composition and oxidative potential of atmospheric coarse particles at an industrial and urban background site in the alpine region of northern Italy. Atmos. Environ. 2018, 191, 340–350. [Google Scholar] [CrossRef]
- Perrone, M.R.; Bertoli, I.; Romano, S.; Russo, M.; Rispoli, G.; Pietrogrande, M.C. PM2.5 and PM10 oxidative potential at a Central Mediterranean Site: Contrasts between dithiothreitol—And ascorbic acid—Measured values in relation with particle size and chemical composition. Atmos. Environ. 2019, 210, 143–155. [Google Scholar] [CrossRef]
- Simonetti, G.; Conte, E.; Perrino, C.; Canepari, S. Oxidative potential of size-segregated PM in an urban and an industrial area of Italy. Atmos. Environ. 2018, 187, 292–300. [Google Scholar] [CrossRef]
- Simonetti, G.; Conte, E.; Massimi, L.; Frasca, D.; Perrino, C.; Canepari, S. Oxidative potential of particulate matter components generated by specific emission sources. Atmos. Environ. 2018, 6, 99–109. [Google Scholar] [CrossRef]
- Chirizzi, D.; Cesari, D.; Guascito, M.R.; Dinoi, A.; Giotta, L.; Donateo, A.; Contini, D. Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2.5 and PM10. Atmos. Environ. 2017, 163, 1–8. [Google Scholar] [CrossRef]
- Perrone, M.G.; Zhou, J.; Malandrino, M.; Sangiorgi, G.; Rizzi, C.; Ferrero, L.; Dommen, J.; Bolzacchini, E. PM chemical composition and oxidative potential of the soluble fraction of particles at two sites in the urban area of Milan, Northern Italy. Atmos. Environ. 2016, 8, 104–113. [Google Scholar] [CrossRef]
- Ciriello, F.; Gualtieri, M.; Longhin, E.; Ruffo, R.; Camatini, M.; Parenti, P. A new method and tool for detection and quantification of PM oxidative potential. Environ. Sci. Pollut. Res. 2015, 22, 469–478. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bertoli, I.; Manarini, F.; Russo, M. Ascorbate assay as a measure of oxidative potential for ambient particles: Evidence for the importance of cell-free surrogate lung fluid composition. Atmos. Environ. 2019, 211, 103–112. [Google Scholar] [CrossRef]
- Künzli, N.; Mudway, I.S.; Götschi, T.; Shi, T.; Kelly, F.J.; Cook, S.; Burney, P.; Forsberg, B.; Gauderman, J.W.; Hazenkamp, M.E.; et al. Comparison of oxidative Properties, Light Absorbance, and Total and Elemental Mass Concentration of Ambient PM2.5 Collected at 20 European Sites. Environ. Health Perspect. 2006, 114, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Mihucz, V.G.; Szigeti, T.; Dunster, C.; Giannoni, M.; de Kluizenaar, Y.; Cattaneo, A.; Mandin, C.; Bartzis, J.G.; Lucarelli, F.; Kelly, F.J.; et al. An integrated approach for the chemical characterization and oxidative potential assessment of indoor PM2.5. Microchem. J. 2015, 119, 22–29. [Google Scholar] [CrossRef]
- Conte, E.; Canepari, S.; Frasca, D.; Simonetti, G. Oxidative Potential of Selected PM Components. In Proceedings of the 2nd International Electronic Conference on Atmospheric Sciences, Basel, Switzerland, 16–31 July 2017; Volume 2. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Perrone, M.R.; Manarini, F.; Romano, S.; Udisti, R.; Becagli, S. PM10 oxidative potential at a Central Mediterranean Site: Association with chemical composition and meteorological parameters. Atmos. Environ. 2018, 188, 97–111. [Google Scholar] [CrossRef]
- Daher, N.; Ruprecht, A.; Invernizzi, G.; De Marco, C.; Miller-Schulze, J.; Heo, J.B.; Shafer, M.M.; Shelton, B.R.; Schauer, J.J.; Sioutas, C. Characterization, sources and redox activity of fine and coarse particulate matter in Milan, Italy. Atmos. Environ. 2012, 49, 130–141. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Martins, J.M.F.; Voisin, D.; Spadini, L.; Lacroix, T.; Jaffrezo, J.L. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci. Rep. 2017, 7, 2045–2322. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Guo, H.; King, L.E.; Edgerton, E.S.; Weber, R.J. A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: Results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE). Atmos. Meas. Tech. 2015, 8, 471–482. [Google Scholar] [CrossRef]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.-M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef]
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 2491–2504. [Google Scholar] [CrossRef]
- Lyu, Y.; Guo, H.; Cheng, T.; Li, X. Particle size distributions of oxidative potential of lung-deposited particles: Assessing contributions from quinones and water-soluble metals. Environ. Sci. Technol. 2018, 52, 6592–6600. [Google Scholar] [CrossRef]
- Shuster-Meiseles, T.; Shafer, M.M.; Heo, J.; Pardo, M.; Antkiewicz, D.S.; Schauer, J.J.; Rudich, A.; Rudich, Y. ROS-generating/ARE-activating capacity of metals in roadway particulate matter deposited in urban environment. Environ. Res. 2016, 146, 252–262. [Google Scholar] [CrossRef]
- Diapouli, E.; Manousakas, M.I.; Vratolis, S.; Vasilatou, V.; Pateraki, S.; Bairachtari, K.A.; Querol, X.; Amato, F.; Alastuey, A.; Karanasiou, A.A.; et al. AIRUSE-LIFE +: Estimation of natural source contributions to urban ambient air PM10 and PM2. 5 concentrations in southern Europe—Implications to compliance with limit values. Atmos. Chem. Phys. 2017, 17, 3673–3685. [Google Scholar] [CrossRef]
- Taghvae, S.; Sowlat, M.H.; Diapouli, E.; Manousakas, M.I.; Vasilatou, V.; Eleftheriadis, K.; Sioutas, C. Source apportionment of the oxidative potential of fine ambient particulate matter (PM2.5) in Athens, Greece. Sci. Total Environ. 2019, 653, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.C.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 20, 9321–9333. [Google Scholar] [CrossRef]
- Samara, C. On the redox activity of urban aerosol particles: Implications for size distribution and relationships with organic aerosol components. Atmosphere 2017, 8, 205. [Google Scholar] [CrossRef]
- Bae, M.; Schauer, J.J.; Lee, T.; Jeong, J.; Kim, Y.; Ro, C.; Song, S.; Shon, Z. Relationship between reactive oxygen species and water-soluble organic compounds: Time-resolved benzene carboxylic acids measurement in the coastal area during the KORUS-AQ campaign. Environ. Pollut. 2017, 231, 1–12. [Google Scholar] [CrossRef]
- Decesari, S.; Sowlat, M.H.; Hasheminassab, S.; Sandrini, S.; Gilardoni, S.; Facchini, M.C.; Fuzzi, S.; Sioutas, C. Enhanced toxicity of aerosol in fog conditions in the Po Valley, Italy. Atmos. Chem. Phys. Discuss. 2017, 17, 7721–7731. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.H.; Jang, M.; Sabo-Attwood, T.; Robinson, S.E. Oxidative potential of secondary organic aerosols produced from photooxidation of different hydrocarbons using outdoor chamber under ambient sunlight. Atmos. Environ. 2016, 131, 382–389. [Google Scholar] [CrossRef]
- Tuet, W.Y.; Chen, Y.; Xu, L.; Fok, S.; Gao, D.; Weber, R.J.; Ng, N.L. Chemical oxidative potential of secondary organic aerosol (SOA) generated from the photooxidation of biogenic and anthropogenic volatile organic compounds. Atmos. Chem. Phys. 2017, 17, 839–853. [Google Scholar] [CrossRef] [Green Version]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox activity and in vitro bioactivity of the water-soluble fraction of urban particulate matter in relation to particle size and chemical composition. Environ. Pollut. 2016, 208, 774–786. [Google Scholar] [CrossRef]
- Perrone, M.R.; Becagli, S.; Gracia Orza, J.A.; Vecchi, R.; Dinoi, A.; Udisti, R.; Cabello, M. The impact of long-range-transport on PM1 and PM2.5 at a Central Mediterranean site. Atmos. Environ. 2013, 71, 176–186. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bacco, D.; Ferrari, S.; Ricciardelli, I.; Scotto, F.; Trentini, A.; Visentin, M. Characteristics and major sources of carbonaceous aerosols in PM2.5 in Emilia Romagna Region (Northern Italy) from four-year observations. Sci. Total Environ. 2016, 553, 172–183. [Google Scholar] [CrossRef] [PubMed]




| PM Fraction | Ref. | Location | Sampling Period | Sampling Duration | OP Assay | Chemical Characterization |
|---|---|---|---|---|---|---|
| TSP | [28] | Milan | January, June, October 2013 | 24 h | DTT, DCFH | EC, OC, inorganic ions, metals, trace organic compounds |
| PM10 | [23] | Trento | April–May 2016 | 24 h | DTT, AA | WSTC, ions, metals, sugars |
| [34] | Lecce | December 2014–October 2015 | 24 h | DTT, AA | metals, ions, EC, OC, POC, LW carboxylic acids | |
| [29] | Milan | Winter 2009 | 24 h | DTT (cit-c) | OC, metals, quinones | |
| PM10 and PM2.5 | [24] | Lecce | December 2014–October 2015 | 24 h | DTT, AA | metals, ions, EC, OC, POC, LW carboxylic acids |
| [27] | Lecce | Fall/Winter 2013–2016 | 24 h | DTT | OC, EC, POC, SOC, TC | |
| [35] | Milan | December 2009–November 2010 | 24 h | DCFH | OC, EC, WSOC, ions, metals, levoglucosan, PAHs, hopanes, alkanes | |
| PM2.5 | [18] | Bologna | February–July 2013 | 24 h | DTT, AA | metals, EC, OC |
| [30] | Bologna | mar 2018 | 24 h | AA | metals, EC, OC | |
| [20] | Rome | January 2010–January 2011 | 24h | DTT | OC, EC, PAH, hopanes | |
| [21] | Rome | January–February 2017 | 24 h | DCFH | OC, EC, BC, WSOC, water soluble BrC, metals, levoglucosan, PAH | |
| [19] | Turin | February 2010–January 2011 | 24 h | AA, GSH | metals, NO2 | |
| [17,32] | Milan, Florence | Summer/Winter 2012–2013 | 24 h | AA, GSH | OC, EC, ions, metals | |
| [31] | Turin, Pavia | June–December 2000 | 24 h | AA, GSH | metals | |
| PM3–PM3–7–PM7 | [16] | Milan | April–July 20 | 3–4 days | DTT, DCFH | metals, ions |
| 9 PM fractions 0.18–18 μm | [25] | Ferrara, Rome | February–March 2017 | 24 h | DTT, AA, DCFH | ions, metals |
| 50 µm dust | [26,33] | specific sources | - | - | DTT, AA, DCFH | OC, EC, WSOC, ions, metals |
| PM Fraction | Ref. | Correlation | Assay | Chemical Species | |
|---|---|---|---|---|---|
| TSP | [28] | Milan | Spearman p < 0.01 | DTT | Solar Radiation |
| DCFH | TSP mass, OC, TC, SO42−, NO3−, NH4+, Ca, Mn, Co, Zn, As | ||||
| PM10 | [23] | Trento | Pearson p < 0.01 | DTT | SO42−, NH4+, NO3−, Cl−, Ca, Mg, K, Mn, Cu, Rb, and Zn, Fe, Ni, Pb, Sr, V, WSOC, sugars, levoglucosan |
| AA | SO42−, NH4+, NO3−, Cl−, Ca, Mg, K, Mn, Cu, Rb and Zn, Fe, Ni, Pb, Sr, V, WSOC, sugars | ||||
| [34] | Lecce | Pearson p < 0.01 | DTT | AW: K+, Ca2+, Ba, Cd, Ce, Cr, Cu, Fe, Mn, OC, EC, POC | |
| SS: NO3−, NH4+, Cu, OC, EC, POC, LW carboxylic acids | |||||
| AA | AW: K+, Ca2+, Ba, Ce, Cr, Cu, Fe, Mn, OC, EC, POC | ||||
| SS: NH4+, nss-K+, nss-Mg2+, nss-Ca2+, nss-SO42−, Cu, Mn, P, Pb, LW carboxylic acids | |||||
| PM10 and PM2.5 | [24] | Lecce | Pearson p < 0.01 | DTT | AW: K+, NO3−, Ba, Cd, Cu, Fe, Mn, P, V, OC, EC, carboxylic acids |
| SS: NO3−, SO42−, OC, EC, TC, POC | |||||
| AA | AW: NO3−, Ba, Cd, Cu, Fe, Mn, P, V, OC, EC | ||||
| SS: NO3−, SO42−, OC, EC, TC, POC | |||||
| PM2.5 | [27] | Lecce | Pearson p < 0.05 | DTT | OC, EC |
| [35] | Milan | Spearman p < 0.05 | DCFH | Ni, Cr, Cu, OC | |
| [18] | Bologna | Pearson p < 0.01 | DTT | Mn, Fe, Cu, Cr, Zn, OC, EC | |
| AA | Mn, Cu, OC, EC | ||||
| [30] | Bologna | Pearson p < 0.01 | AA | Mn, Fe, Cu, Cr, Zn, OC, EC | |
| [20] | Rome | linear regression | DTT | OC, EC Levoglucosan, ∑PAHs | |
| [21] | Rome | linear regression | DCFH | equivalent Black Carbon, ∑PAHs | |
| [19] | Turin | Pearson p < 0.05 | AA | PM2.5 mass, NO2, Cu, Fe | |
| GSH | NO2, Cu, Fe | ||||
| [17] | Milan, Florence | Spearman p < 0.01 | AA | Indoor: Cu, Mo, OC | |
| Outdoor: Fe, Cu, Cr, Ni, Cd, Sn, Sb, K+ | |||||
| GSH | Indoor: Cu, Mo, OC | ||||
| Outdoor: Cu, Sn, OC | |||||
| [31] | Turin, Pavia | Pearson | AA | Fe, Cu, Zn | |
| GSH | Cu, Al | ||||
| PM3, PM3–7, PM7 | [16] | Milan | Spearman r > 0.70 | DTT | <3 µm: - |
| 3–7 µm: Fe, Sn, Cu, Sb, Ba | |||||
| >7 µm: As, Al, Ti, Sr, Li |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrogrande, M.C.; Russo, M.; Zagatti, E. Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere 2019, 10, 626. https://doi.org/10.3390/atmos10100626
Pietrogrande MC, Russo M, Zagatti E. Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere. 2019; 10(10):626. https://doi.org/10.3390/atmos10100626
Chicago/Turabian StylePietrogrande, Maria Chiara, Mara Russo, and Elisa Zagatti. 2019. "Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy" Atmosphere 10, no. 10: 626. https://doi.org/10.3390/atmos10100626
APA StylePietrogrande, M. C., Russo, M., & Zagatti, E. (2019). Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere, 10(10), 626. https://doi.org/10.3390/atmos10100626