Application of the Passive Sampler Developed for Atmospheric Mercury and Its Limitation
Abstract
:1. Introduction
2. Experiments
2.1. Theory
2.2. Sampling and Analysis
2.2.1. Design of Sampler
2.2.2. Sampling
2.2.3. Analysis
2.2.4. Cleaning and Others
2.2.5. Theoretical SR
3. Results and Discussion
3.1. Sampler Performance
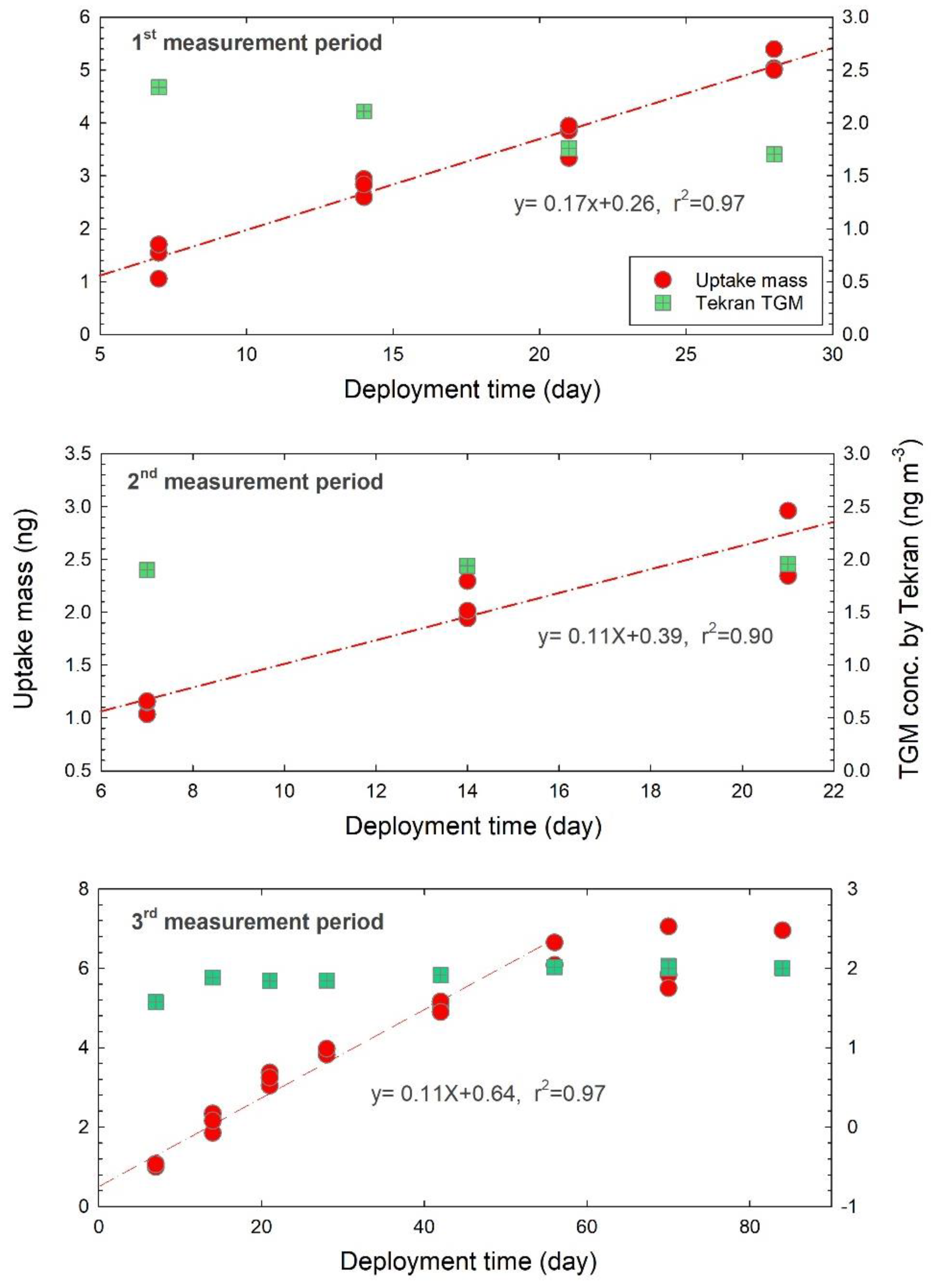
3.2. Sampling Rate
3.3. Effect of Meteorological Factors
3.4. Comparison to Active Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Pehkonen, S.O. The chemistry of atmospheric mercury: A riview. Atmos. Environ. 1999, 33, 2067–2079. [Google Scholar] [CrossRef]
- Si, L.; Ariya, P.A. Recent advances in atmospheric chemistry of mercury. Atmosphere 2018, 9, 76. [Google Scholar] [CrossRef]
- Horowitz, H.M.; Jacob, D.J.; Zhang, Y.; Dibble, T.S.; Slemr, F.; Amos, H.M.; Schmidt, J.A.; Corbitt, E.S.; Marais, E.A.; Sunderland, E.M. A new mechanism for atmospheric mercury redox chemistry: Implications for the global mercury budget. Atmos. Chem. Phys. 2017, 17, 6353–6371. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, J.E.; Kim, P.R.; Kim, W.J.; Yi, S.M.; Seo, Y.S.; Kim, S.H. General trend of atmospheric mercury concentrations in urban and rural areas in Korea and characteristics of high-concenration events. Atmos. Environ. 2014, 94, 754–764. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013; pp. 1–44. [Google Scholar]
- Huang, J.; Lyman, S.N.; Hartman, J.S.; Gustin, M.S. A review of passive sampling systems for ambient air mercury measurements. Environ. Sci. Processes Impact 2014, 16, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tong, Y.; Hu, D.; Ou, L.; Wang, X. Characterization of atmospheric mercury concentration along an urban-rural gradient using a newly developed passive sampler. Atmos. Environ. 2012, 47, 26–32. [Google Scholar] [CrossRef]
- McLagan, D.S.; Mazur, M.E.E.; Mitchell, C.P.J.; Wania, F. Passive air sampling of gaseous elemental mercury: A critical review. Atmos. Chem. Phys. 2016, 16, 3061–3076. [Google Scholar] [CrossRef]
- McLagan, D.S.; Mitchell, C.P.J.; Huang, H.; Lei, Y.D.; Cole, A.S.; Steffen, A.; Hung, H.; Wania, F. A high-precision passive air sampler for gasoues mercury. Environ. Sci. Technol. 2016, 3, 24–29. [Google Scholar] [CrossRef]
- McLagan, D.S.; Mitchell, C.P.J.; Steffen, A.; Hung, H.; Shin, C.; Stupple, G.W.; Olson, M.L.; Luke, W.T.; Kelley, P.; Howard, D.; et al. Global evaluation and calibration of a passive air sampler for gaseous mercury. Atmos. Chem. Phys. 2018, 18, 5905–5919. [Google Scholar] [CrossRef] [Green Version]
- McLagan, D.S.; Monaci, M.; Huang, H.; Lei, Y.D.; Mitchell, C.P.J.; Wania, F. Characterization and quantification of atmospheric mercury sources using passive air samplers. J. Geophys. Res. Atmos. 2019, 124, 2351–2362. [Google Scholar] [CrossRef]
- Skov, H.; Sorensen, B.T.; Landis, M.S.; Johnson, M.S.; Sacco, P.; Goodsite, M.E.; Lohse, C.; Christiansen, K.S. Performance of a new diffusive sampler for Hg0 determination in the troposphere. Environ. Chem. 2007, 4, 75–80. [Google Scholar] [CrossRef]
- Lyman, S.N.; Gustin, M.S.; Prestbo, E.M. A passive sampler for ambient gaseous oxidized mercury concentrations. Atmos. Environ. 2010, 44, 246–252. [Google Scholar] [CrossRef]
- May, A.A.; Ashman, P.; Huang, J.; Dhaniyala, S.; Holson, T.M. Evaluation of the polyurethane foam (PUF) disk passive air sampler: Computational modeling and experimental measurements. Atmos. Environ. 2011, 45, 4354–4359. [Google Scholar] [CrossRef]
- Scott, J.E.; Ottaway, J.M. Determination of mercury vapour in air using a passive gold wire sampler. Analyst 1981, 106, 1076–1081. [Google Scholar] [CrossRef]
- Gustin, M.S.; Lyman, S.N.; Kilner, P.; Prestbo, E. Development of passive sampler for gaseous mercury. Atmos. Environ. 2011, 45, 5805–5812. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A General 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Oudar, J.; Wise, H. Deactivation of Catalysis; Academic Press: London, UK, 1984; Chapter 8. [Google Scholar]
- McLagan, D.S.; Huang, H.; Ying, D.; Wania, F.; Michell, C.P.J. Application of sodium carbonate prevents sulphur poisoning of catalysts in automated total mercury analysis. Spectrochim. Acta Part B 2017, 133, 60–62. [Google Scholar] [CrossRef]
- McLagan, D.S.; Mitchell, C.P.J.; Huang, H.; Hussain, B.A.; Lei, Y.D.; Wania, F. The effects of meteorological parameters and diffusive barrier reuse on the sampling rate of a passive air sampler for gaseous mercury. Atmos. Meas. Tech. 2017, 10, 3651–3660. [Google Scholar] [CrossRef] [Green Version]
- Bartkow, M.E.; Booij, K.; Kennedy, K.E.; Muller, J.F.; Hawker, D.W. Passive air sampling theory for semivolatile organic compounds. Chemosphere 2005, 60, 170–176. [Google Scholar] [CrossRef]
- Naval Facilities Engineering Command. Passive sampling for vapor intrusion assessment. In Technical Memorandum; TM-NAVFAC EXWC-EV-1503; Naval Facilities Engineering Command: Port Hueneme, CA, USA, 2015; pp. 1–14. [Google Scholar]
- Wania, F.; Shen, L.; Lei, Y.D.; Teixeira, C.; Muir, D.C.G. Development and calibration of a Resin-based passive sampling system for monitoring persistent organic pollutants in the atmosphere. Environ. Sci. Technol. 2003, 37, 1352–1359. [Google Scholar] [CrossRef]
- Gan, S.Y.; Yi, S.Y.; Han, Y.J. Characteristics of atmospheric speciated gaseous mercury in Chuncheon, Korea. J. Korean Soc. Environ. Eng. 2009, 31, 382–391. [Google Scholar]
- Stupple, G.W.; McLagan, D.S.; Steffen, A. In situ reactive gaseous mercury uptake on radiello diffusive barrier, cation exchange membrane and Teflon filter membrane during atmospheric mercury depletion events. In Proceedings of the 14th International Conference on Mercury as a Global Pollutant, Krakow, Poland, 8–13 September 2019. [Google Scholar]
- Massman, W.J. Molecular diffusivities of Hg vapor in air, O2 and N2 near STP and the kinematic viscosity and thermal diffusivity of air near STP. Atmos. Environ. 1999, 33, 453–457. [Google Scholar] [CrossRef]
- Fuller, E.N.; Schettler, P.D.; Giddings, J.C. New method for prediction of binary gas-phase diffusion coefficients. Ind. Eng. Chem. 1966, 58, 18–27. [Google Scholar] [CrossRef]
- Chapman, S.; Cowling, T.G. The Mathematical Theory of Non-Uniform Gases, 3rd ed.; Cambridge University Press: Cambridge, UK, 1970. [Google Scholar]
- Guo, H.; Lin, H.; Zhang, W.; Deng, C.; Wang, H.; Zhang, Q.; Shen, Y.; Wang, X. Influence of meteolorogical factors on the atmospheric mercury measurement by a novel passive sampler. Atmos. Environ. 2014, 97, 310–315. [Google Scholar] [CrossRef]
- Huang, C.; Tong, L.; Dai, X.; Xiao, H. Evaluation and application of a passive air sampler for atmospheric volatile organic compounds. Aerosol Air Qual. Res. 2018, 18, 3047–3055. [Google Scholar] [CrossRef]
- Armitage, J.M.; Hayward, S.J.; Wania, F. Modeling the uptake of neutral organic chemicals on XAD passive air samplers under variable temperatures, external wind speeds and ambient air concentrations (PAS-SIM). Environ. Sci. Technol. 2013, 47, 13546–13554. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Burdon, M.K.; Brown, A.S.; Kim, K.H. Assessment of pumped mercury vapour adsorption tubes as passive samplers using a micro-exposure chamber. J. Environ. Monit. 2012, 14, 2456–2463. [Google Scholar] [CrossRef]
- Huang, J.; Choi, H.D.; Landis, M.S.; Holsen, T.M. An application of passive samplers to understand atmospheric mercury concentration and dry deposition spatial distributions. J. Environ. Monit. 2012, 14, 2976–2982. [Google Scholar] [CrossRef]
- Nishikawa, M.; Shiraishi, H.; Yanase, R.; Tanida, K. Examination of an improved passive sampler for gaseous mercury on the landfill site. J. Environ. Chem. 1999, 9, 681–684. [Google Scholar] [CrossRef]
- Won, J.H.; Lee, T.G. Estimeation of total annual mercury emission from cement manufacturing facilities in Korea. Atmos. Environ. 2012, 62, 265–271. [Google Scholar] [CrossRef]

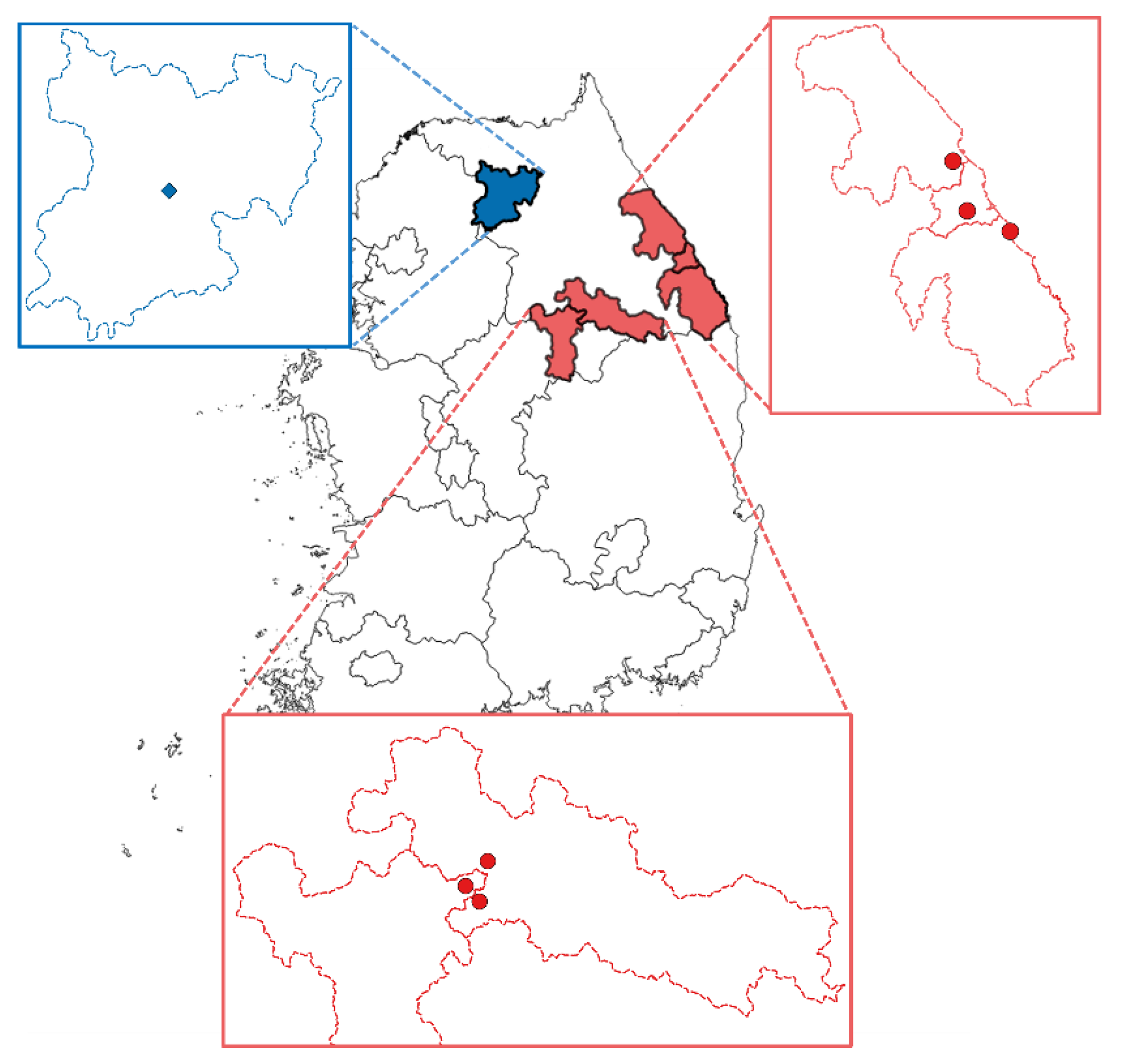







| Purpose | Location | Period | Deployment Time | No. of Samples |
|---|---|---|---|---|
| Intensive measurements | 1st period at KNU | 11 July–8 August 2017 | 1–4 weeks | 4 sets |
| 2nd period at KNU | 9 August–30 2017 | 1–3 weeks | 3 sets | |
| 3rd period at KNU | 12 September–5 December 2017 | 1–4, 6, 8, 10, 12 weeks | 8 sets | |
| Field application | Near cement plants | 23 March–18 April 2018 | 26 days | 6 sets |
| Chuncheon | 23 March–18 April 2018 | 26 days | 10 sets |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, J.-W.; Han, Y.-J.; Cha, S.-H.; Kim, P.-R.; Kim, Y.-H.; Kim, H.; Seok, G.-S.; Noh, S. Application of the Passive Sampler Developed for Atmospheric Mercury and Its Limitation. Atmosphere 2019, 10, 678. https://doi.org/10.3390/atmos10110678
Jeon J-W, Han Y-J, Cha S-H, Kim P-R, Kim Y-H, Kim H, Seok G-S, Noh S. Application of the Passive Sampler Developed for Atmospheric Mercury and Its Limitation. Atmosphere. 2019; 10(11):678. https://doi.org/10.3390/atmos10110678
Chicago/Turabian StyleJeon, Ji-Won, Young-Ji Han, Seung-Hwan Cha, Pyung-Rae Kim, Young-Hee Kim, Hyuk Kim, Gwang-Seol Seok, and Seam Noh. 2019. "Application of the Passive Sampler Developed for Atmospheric Mercury and Its Limitation" Atmosphere 10, no. 11: 678. https://doi.org/10.3390/atmos10110678
APA StyleJeon, J.-W., Han, Y.-J., Cha, S.-H., Kim, P.-R., Kim, Y.-H., Kim, H., Seok, G.-S., & Noh, S. (2019). Application of the Passive Sampler Developed for Atmospheric Mercury and Its Limitation. Atmosphere, 10(11), 678. https://doi.org/10.3390/atmos10110678





