Variations in Greenhouse Gas Fluxes in Response to Short-Term Changes in Weather Variables at Three Elevation Ranges, Wakiso District, Uganda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Layout
2.3. Measurement of Precipitation and Soil Temperature
2.4. Soil Sampling and Analysis
2.5. Greenhouse Gas Fluxes: Sampling and Analysis
2.6. Statistical Analysis
3. Results
3.1. Precipitation and Soil Temperatures in the Study Sites
3.2. Physical–Chemical Characterization of the Soil Sampled at the Studied Sites
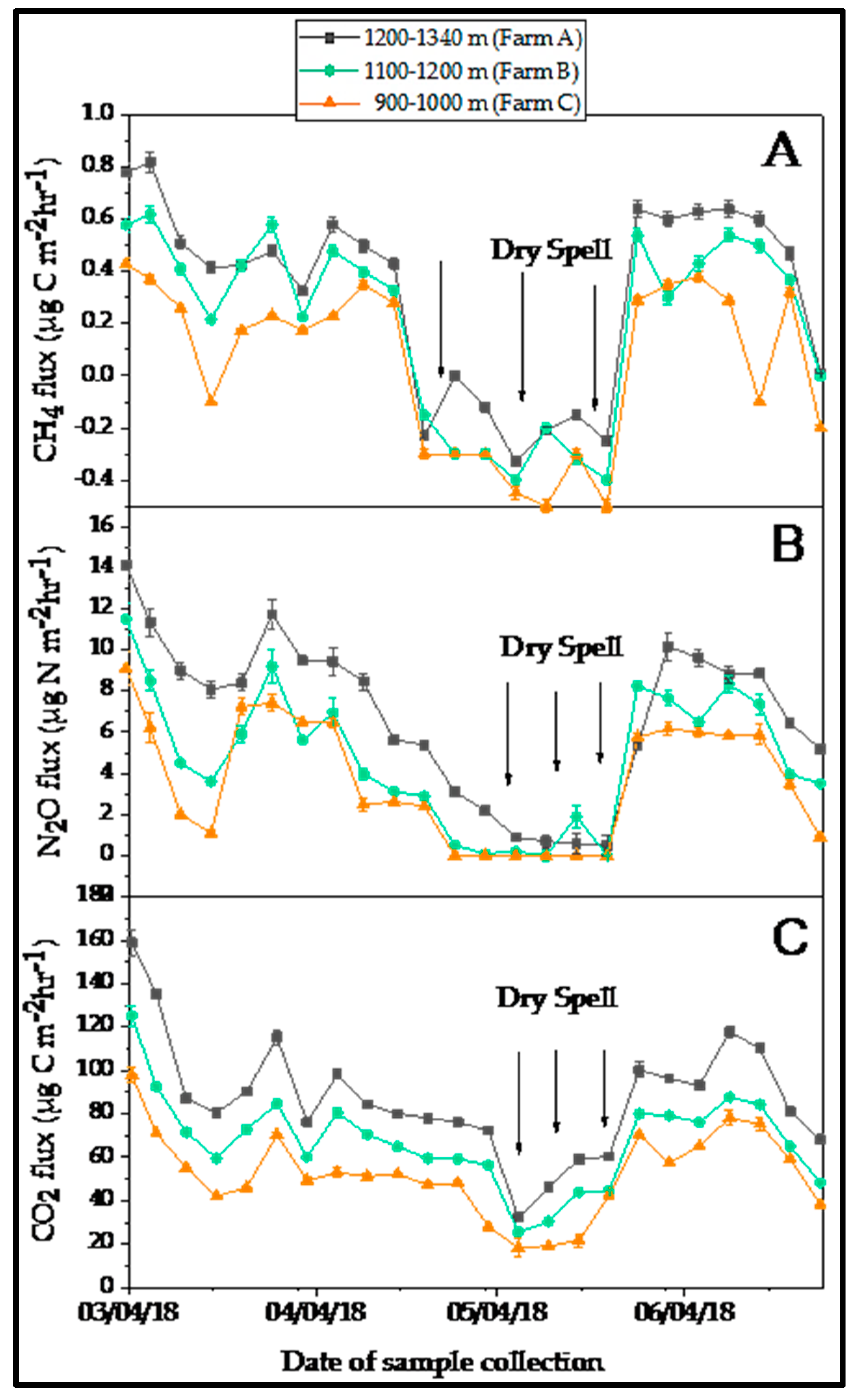
3.3. Spatial and Temporal Variation in the Soil Greenhouse-Gas Fluxes
3.4. Effect of the Daily Precipitation and Soil Temperatures on the Soil Greenhouse-Gas Fluxes
3.5. Relationship between the Daily Precipitation, Soil Temperatures, and Soil Greenhouse-Gas Fluxes
4. Discussion
4.1. Spatial and Temporal Variation in the Soil Greenhouse Gas Fluxes
4.2. The Effect of Daily Precipitation on the Soil Greenhouse-Gas Fluxes
4.3. The Effect of the Soil Temperatures on the Soil Greenhouse-Gas Fluxes
4.4. Influence of the Combined Effect of Daily Precipitation and Soil Temperatures on the Soil Greenhouse-Gas Fluxes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Hansen, J.; Sato, M.; Kharecha, P.; Russell, G.; Lea, D.W.; Siddall, M. Climate change and trace gases. Philos. Trans. R. Soc. A 2007, 365, 1925–1954. [Google Scholar] [CrossRef] [PubMed]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A. Chapter 11—Agriculture, forestry and other land use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. IPCC Working Group III Contribution to AR5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- IPCC. Summary for Policymakers. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Donat, M.G.; Lowry, A.L.; Alexander, L.V.; O’Gorman, P.A.; Maher, N. More extreme precipitation in the world’s dry and wet regions. Nat. Clim. Chang. 2016, 6, 508–513. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Prior, S.A.; Runion, G.B.; Torbert, H.A.; Tian, H.; Lu, C.; Venterea, R.T. Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: Evidence from field experiments. Front. Ecol. Environ. 2012, 10, 520–527. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Bloor, J.M.G.; Bardgett, R.D. Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: Interactions with plant species diversity and soil nitrogen availability. Perspect. Plant. Ecol. Evol. Syst. 2012, 14, 193–204. [Google Scholar] [CrossRef]
- Birch, H.F. Mineralisation of plant nitrogen following alternate wet and dry conditions. Plant. Soil 1964, 20, 43–49. [Google Scholar] [CrossRef]
- Fan, Z.; Neff, J.C.; Hanan, N.P. Modeling pulsed soil respiration in an African savanna ecosystem. Agric. For. Meteorol. 2015, 200, 282–292. [Google Scholar] [CrossRef]
- López-Ballesteros, A.; Serrano-Ortiz, P.; Sánchez-Cañete, E.P.; Oyonarte, C.; Kowalski, A.S.; Pérez-Priego, Ó.; Domingo, F. Enhancement of the net CO2 release of a semiarid grassland in SE Spain by rain pulses. J. Geophys. Res. 2016, 121, 52–66. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, X.J.; Li, Y. The response of CO2 flux to rain pulses at a saline desert. Hydrol. Process. 2012, 26, 4029–4037. [Google Scholar] [CrossRef]
- Unger, S.; Máguas, C.; Pereira, J.S.; David, T.S.; Werner, C. The influence of precipitation pulses on soil respiration - Assessing the Birch effect by stable carbon isotopes. Soil Biol. Biochem. 2010, 42, 1800–1810. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Spatiotemporal measurement and modeling of stand-level boreal forest soil temperatures. Agric. For. Meteorol. 2005, 131, 27–40. [Google Scholar] [CrossRef]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L.; Schwinning, S. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Miao, R.; Liu, Y.; Wang, D.; Zhao, C. Seasonal variations in the response of soil CO2 efflux to precipitation pulse under mild drought in a temperate oak (Quercus variabilis) forest. Agric. For. Meteorol. 2019, 271, 240–250. [Google Scholar] [CrossRef]
- Ortiz-Gonzalo, D.; de Neergaard, A.; Vaast, P.; Suárez-Villanueva, V.; Oelofse, M.; Rosenstock, T.S. Multi-scale measurements show limited soil greenhouse gas emissions in Kenyan smallholder coffee-dairy systems. Sci. Total Environ. 2018, 626, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, X.; Peng, C.; Mu, Y.; Ouyang, Z.; Lu, F.; Zhang, H.; Zhang, S.; Liu, W. Nitrous oxide emissions from soils under traditional cropland and apple orchard in the semi-arid Loess Plateau of China. Agric. Ecosyst. Environ. 2019, 269, 116–124. [Google Scholar] [CrossRef]
- Kim, D.G.; Thomas, A.D.; Pelster, D.; Rosenstock, T.S.; Sanz-Cobena, A. Greenhouse gas emissions from natural ecosystems and agricultural lands in sub-Saharan Africa: Synthesis of available data and suggestions for further research. Biogeosciences 2016, 13, 4789–4809. [Google Scholar] [CrossRef]
- Pfahl, S.; O’Gorman, P.A.; Fischer, E.M. Understanding the regional pattern of projected future changes in extreme precipitation. Nat. Clim. Chang. 2017, 7, 423–427. [Google Scholar] [CrossRef]
- Adhikari, U.; Nejadhashemi, A.P.; Woznicki, S.A. Climate change and eastern Africa: A review of the impact on major crops. Food Energy Secur. 2015, 4, 110–132. [Google Scholar] [CrossRef]
- Sasson, A. Food security for Africa: An urgent global challenge. Agric. Food Secur. 2012, 1, 1–16. [Google Scholar] [CrossRef]
- Rosenstock, T.S.; Rufino, M.C.; Butterbach-Bahl, K.; Wollenberg, E. Toward a protocol for quantifying the greenhouse gas balance and identifying mitigation options in smallholder farming systems. Environ. Res. Lett. 2013, 8, 021003. [Google Scholar] [CrossRef]
- WDLG. Higher Local Government Statistical Abstract: Wakiso District Local Government (WDLG); WDLG: Wakiso, Uganda, 2009; pp. 2–3. [Google Scholar]
- UBOS. Uganda Bureau of Statistics 2018 Statistical Abstract. Kampala. Available online: https://www.ubos.org/wp-content/.../05_2019STATISTICAL_ABSTRACT_2018.pdf (accessed on 5 April 2019).
- FAO. World Reference Base for Soil Resources; FAO: Rome, Italy, 1998. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3—Chemical Methods; Madison: SSSA Book Ser. 5; Sparks, D.L., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Rayment, G.E.; Higginson, F.R. Soil survey standard test method for electrical conductivity. In Australian Laboratory Handbook of Soil and Water Chemical Methods; Australian Soil L. Surv. Handbooks; Inkata Press: Melbourne, Australian, 1992. [Google Scholar]
- Blake, G. Bulk density. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 1, p. 770. [Google Scholar]
- Smemo, K.A.; Ostrom, N.E.; Opdyke, M.R.; Ostrom, P.H.; Sven, B.; Robertson, G.P. Improving process-based estimates of N2O emissions from soil using temporally extensive chamber techniques and stable isotopes. Nutr. Cycl. Agroecosyst. 2011, 91, 91–145. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Xie, B.; Liu, C.; Mei, B.; Dong, H.; Butterbach-Bahl, K.; Zhu, J. Comparison of manual and automated chambers for field measurements of N2O, CH4, CO2 fluxes from cultivated land. Atmos. Environ. 2009, 43, 1888–1896. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Livingston, G.P. Vents and seals in non-steady state chambers used for measuring gas exchange between the soil and the atmosphere. Eur. J. Soil Sci. 2001, 52, 675–682. [Google Scholar] [CrossRef]
- Xu, L.; Furtaw, M.D.; Madsen, R.A.; Garcia, R.L.; Anderson, D.J.; McDermitt, D.K. On maintaining pressure equilibrium between a soil CO2 flux chamber and the ambient air. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolisable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Rochette, P. Towards a standard non-steady-state chamber methodology for measuring soil N2O emissions. Anim. Feed Sci. Technol. 2011, 166, 141–146. [Google Scholar] [CrossRef]
- Dong, Y.S.; Zhang, S.; Qi, Y.C.; Chen, Z.Z.; Geng, Y.B. Fluxes of CO2, N2O, and CH4 from a typical temperate grassland in Inner Mongolia and its daily variation. Chin. Sci. Bull. 2000, 45, 1590–1594. [Google Scholar] [CrossRef]
- Cosentino, V.R.N.; Fernandez, P.L.; Aureggi, S.A.F.; Taboada, M.A. NO2 emission from a cultivated mollisol: Optimal time of day for sampling and the role of soil temperature. Rev. Bras. Cienc. Solo 2012, 36, 1814–1819. [Google Scholar] [CrossRef]
- Arnold, S.; Parkin, T.B.; Doran, J.W.; Mosier, A.R. Automated gas sampling system for laboratory analysis of CH4 and N2O. Commun. Soil Sci. Plant. Anal. 2001, 32, 2795–2807. [Google Scholar] [CrossRef] [Green Version]
- Giovannelli, D.; Black, B.A.; Cox, A.D.; Sheik, C.S. Deep Carbon in Earth: Early Career Scientist Contributions to the Deep Carbon Observatory; Frontiers Media SA: Lausanne, Switzerland, 2017. [Google Scholar]
- Liu, J.; Shen, J.; Li, Y.; Su, Y.; Ge, T.; Jones, D.L.; Wu, J. Effects of biochar amendment on the net greenhouse gas emission and greenhouse gas intensity in a Chinese double rice cropping system. Eur. J. Soil Biol. 2014, 65, 30–39. [Google Scholar] [CrossRef]
- Schwarz, G.E. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Novara, A.; La Mantia, T.; Barbera, V.; Gristina, L. Paired-site approach for studying soil organic carbon dynamics in a Mediterranean semiarid environment. Catena 2012, 89, 1–7. [Google Scholar] [CrossRef]
- McClain, M.E.; Boyer, E.W.; Dent, C.L.; Gergel, S.E.; Grimm, N.B.; Groffman, P.M.; Hart, S.C.; Harvey, J.W.; Johnston, C.A.; Mayorga, E.; et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 2003, 6, 301–312. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- Liu, L.; Hu, C.; Yang, P.; Ju, Z.; Olesen, J.E.; Tang, J. Effects of experimental warming and nitrogen addition on soil respiration and CH4 fluxes from crop rotations of winter wheat–soybean/fallow. Agric. For. Meteorol. 2015, 207, 38–47. [Google Scholar] [CrossRef]
- Savage, K.; Phillips, R.; Davidson, E. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences 2014, 11, 2709–2720. [Google Scholar] [CrossRef] [Green Version]
- Scott-Denton, L.E.; Rosenstiel, T.N.; Monson, R.K. Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob. Chang. Biol. 2006, 12, 205–216. [Google Scholar] [CrossRef]
- Wan, S.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.A.; Zechmeister-Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Yang, H.; Zhang, P.; Gao, Y. Soil respiration of biologically-crusted soils in response to simulated precipitation pulses in the Tengger Desert, Northern China. Pedosphere 2018, 28, 103–113. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Hereş, A.M.; Ojeda, G.; Paz, A.; Pizano, C.; García-Angulo, D.; Lasso, E. Soil heterotrophic CO2 emissions from tropical high-elevation ecosystems (Páramos) and their sensitivity to temperature and moisture fluctuations. Soil Biol. Biochem. 2017, 110, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Li, Y.; Su, Y.G. Effects of increasing precipitation on soil microbial community composition and soil respiration in a temperate desert, Northwestern China. Soil Biol. Biochem. 2015, 83, 52–56. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Bond-Lamberty, B.; Benscoter, B.W.; Tfaily, M.M.; Hinkle, C.R.; Liu, C.; Bailey, V.L. Shifts in pore connectivity from precipitation versus groundwater rewetting increases soil carbon loss after drought. Nat. Commun. 2017, 8, 1335. [Google Scholar] [CrossRef] [PubMed]
- McCulley, R.L.; Boutton, T.W.; Archer, S.R. Soil respiration in a subtropical savanna parkland: Response to water additions. Soil Sci. Soc. Am. J. 2007, 71, 820–828. [Google Scholar] [CrossRef]
- Linfeng, L.; Fan, W.; Kang, X.; Wang, Y.; Cui, X.; Xu, C.; Griffin, K.L.; Hao, Y. Responses of greenhouse gas fluxes to climate extremes in a semiarid grassland. Atmos. Environ. 2016, 142, 32–42. [Google Scholar] [CrossRef]
- Morse, J.L.; Bernhardt, E.S. Using 15N tracers to estimate N2O and N2 emissions from nitrification and denitrification in coastal plain wetlands under contrasting land-uses. Soil Biol. Biochem. 2013, 57, 635–643. [Google Scholar] [CrossRef]
- Liu, B.; Frostegård, Å.; Bakken, L.R. Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. mBio 2014, 5, e01383-14. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zheng, X.; Chen, Q.; Wolf, B.; Butterbach-Bahl, K.; Brüggemann, N.; Lin, S. Effects of increasing precipitation and nitrogen deposition on CH4 and N2O fluxes and ecosystem respiration in a degraded steppe in Inner Mongolia, China. Geoderma 2013, 192, 335–340. [Google Scholar] [CrossRef]
- Homyak, P.M.; Allison, S.D.; Huxman, T.E.; Goulden, M.L.; Treseder, K.K. Effects of drought manipulation on soil nitrogen cycling: A meta-analysis. J. Geophys. Res. 2017, 122, 3260–3272. [Google Scholar] [CrossRef]
- Wu, D.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Vieira, F.C.B.; Zanatta, J.A.; Piccolo, M.C.; Dieckow, J. Methane emission from soil under long-term no-till cropping systems. Soil Tillage Res. 2012, 124, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.K.; Parkin, T.B. Effect of land use on methane flux from soil. J. Environ. Qual. 2001, 30, 786–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggs, E.M.; Chebii, J.; Ndufa, J.K. A short-term investigation of trace gas emissions following tillage and no-tillage of agroforestry residues in western Kenya. Soil Tillage Res. 2006, 90, 69–76. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Levy-Booth, D.; Prescott, C.E.; Grayston, S.J. Different soil moisture control of net methane oxidation and production in organic upland and wet forest soils of the pacific coastal rainforest in Canada. Can. J. For. Res. 2017, 47, 628–635. [Google Scholar] [CrossRef]
- Teh, Y.A.; Silver, W.L.; Conrad, M.E. Oxygen effects on methane production and oxidation in humid tropical forest soils. Glob. Chang. Biol. 2005, 11, 1283–1297. [Google Scholar] [CrossRef]
- Hall, S.J.; McDowell, W.H.; Silver, W.L. When wet gets wetter: Decoupling of moisture, redox biogeochemistry, and greenhouse gas fluxes in a humid tropical forest soil. Ecosystems 2013, 16, 576–589. [Google Scholar] [CrossRef] [Green Version]
- Topp, E.; Pattey, E. Soils as sources and sinks for atmospheric methane. Can. J. Soil Sci. 1997, 77, 167–178. [Google Scholar] [CrossRef]
- Levy, P.E.; Burden, A.; Cooper, M.D.A.; Dinsmore, K.J.; Drewer, J.; Evans, C.; Fowler, D.; Gaiawyn, J.; Gray, A.; Jones, S.K.; et al. Methane emissions from soils: Synthesis and analysis of a large UK data set. Glob. Chang. Biol. 2012, 18, 1657–1669. [Google Scholar] [CrossRef] [Green Version]
- Sgouridis, F.; Ullah, S. Soil Greenhouse Gas Fluxes, Environmental Controls, and the Partitioning of N2O Sources in UK Natural and Seminatural Land Use Types. J. Geophys. Res. 2017, 122, 2617–2633. [Google Scholar] [CrossRef]
- Chamberlain, S.D.; Gomez-Casanovas, N.; Walter, M.T.; Boughton, E.H.; Bernacchi, C.J.; DeLucia, E.H.; Groffman, P.M.; Keel, E.W.; Sparks, J.P. Influence of transient flooding on methane fluxes from subtropical pastures. J. Geophys. Res. Biogeosci. 2016, 121, 965–977. [Google Scholar] [CrossRef] [Green Version]
- Conrad, M.E.; Templeton, A.S.; Daley, P.F.; Alvarez-Cohen, L. Seasonally-induced fluctuations in microbial production and consumption of methane during bioremediation of aged subsurface refinery contamination. Environ. Sci. Technol. 1999, 33, 4061–4068. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Treat, C.C.; Waldrop, M.P.; Waddington, J.M.; Harden, J.W.; McGuire, A.D. Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. J. Geophys. Res.-Biogeosci. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.B.; Egerer, M.H.; Liere, H.; Jha, S.; Philpott, S.M. Soil management is key to maintaining soil moisture in urban gardens facing changing climatic conditions. Sci. Rep. 2018, 8, 17565. [Google Scholar] [CrossRef]
- Zhou, X.; Sherry, R.A.; An, Y.; Wallace, L.L.; Luo, Y. Main and interactive effects of warming, clipping, and doubled precipitation on soil CO2 efflux in a grassland ecosystem. Glob. Biogeochem. Cycles 2006, 20, 1–12. [Google Scholar] [CrossRef]
- Shi, F.; Chen, H.; Chen, H.; Wu, Y.; Wu, N. The combined effects of warming and drying suppress CO2 and N2O emission rates in an alpine meadow of the eastern Tibetan Plateau. Ecol. Res. 2012, 27, 725–733. [Google Scholar] [CrossRef]
- Ahmad, W.; Singh, B.; Dijkstra, F.A.; Dalal, R.C.; Geelan-Small, P. Temperature sensitivity and carbon release in an acidic soil amended with lime and mulch. Geoderma 2014, 214, 168–176. [Google Scholar] [CrossRef]
- Conen, F.; Leifeld, J.; Seth, B.; Alewell, C. Warming mineralises young and old soil carbon equally. Biogeosciences 2006, 3, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Butterbach-Bahl, K.; Stange, F.; Papen, H.; Grell, G.; Li, C. Impact of changes in temperature and precipitation on N2O and NO emissions from forest soils. In Non-CO2 Greenhouse Gases: Scientific Understanding, Control, and Implementation; van Ham, J., Baede, A.P.M., Meyer, L.A., Ybema, R., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 165–171. [Google Scholar]
- Hu, Z.; Cui, H.; Chen, S.; Shen, S.; Li, H.; Yang, Y.; Li, C. Soil respiration and N2O flux response to UV-B radiation and straw incorporation in a soybean-winter wheat rotation system. Water Air Soil Pollut. 2013, 224, 1394. [Google Scholar] [CrossRef]
- Hu, Y.; Chang, X.; Lin, X.; Wang, Y.; Wang, S.; Duan, J.; Zhang, Z.; Yang, X.; Luo, C.; Xu, G.; et al. Effects of warming and grazing on N2O fluxes in an alpine meadow ecosystem on the Tibetan plateau. Soil Biol. Biochem. 2010, 42, 944–952. [Google Scholar] [CrossRef]
- Hartmann, A.A.; Niklaus, P.A. Effects of simulated drought and nitrogen fertilizer on plant productivity and nitrous oxide (N2O) emissions of two pastures. Plant. Soil 2012, 361, 411–426. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H. Four years continuous record of CH4-exchange between the atmosphere and untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany. Plant. Soil 2002, 240, 77–90. [Google Scholar] [CrossRef]
- Oelbermann, M.; Schiff, S.L. Inundating contrasting boreal forest soils: CO2 and CH4 production rates. Ecoscience 2010, 17, 216–224. [Google Scholar] [CrossRef]
- Tu, C.; Li, F. Responses of greenhouse gas fluxes to experimental warming in the wheat season under conventional tillage and no-tillage fields. J. Environ. Sci.-China 2017, 54, 314–327. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Romero, A.J.B.; Jørgensen, N.O.G.; Glaring, M.A.; Jørgensen, C.J.; Berg, L.K.; Elberling, B. Methane fluxes and the functional groups of methanotrophs and methanogens in a young Arctic landscape on Disko Island, West Greenland. Biogeochemistry 2015, 122, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Sjögersten, S.; Aplin, P.; Gauci, V.; Peacock, M.; Siegenthaler, A.; Turner, B. Temperature response of ex-situ greenhouse gas emissions from tropical peatlands: Interactions between forest type and peat moisture conditions. Geoderma 2018, 324, 47–55. [Google Scholar] [CrossRef]
- Harper, C.W.; Blair, J.M.; Fay, P.A.; Knapp, A.K.; Carlisle, J.D. Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Glob. Chang. Biol. 2005, 11, 322–334. [Google Scholar] [CrossRef]
- Yiqi, L.; Zhou, X. Soil Respiration and the Environment; Elsevier Academic Press: London, UK, 2006. [Google Scholar] [CrossRef]




| Elevation Range (m) | N | C | Sand | Clay | Bulk Density | pH |
|---|---|---|---|---|---|---|
| g kg−1 | % | g cm−3 | - | |||
| 1200–1340 (Farm A) | 4.8 ± 2.1 | 17.8 ± 5.8 | 52.5 ± 7.2 | 35.3 ± 8.1 | 1.5 ± 0.2 | 5.9 |
| 1100–1200 (Farm B) | 3.6 ± 1.1 | 15.9 ± 5.5 | 52.0 ± 6.5 | 34.9 ± 0.2 | 1.3 ± 0.1 | 5.4 |
| 900–1000 (Farm C) | 2.4 ± 1.2 | 12.9 ± 3.4 | 52.1 ± 6.3 | 35.0 ± 7.6 | 1.0 ± 0.1 | 5.0 |
| Elevation Range (m) | P (mm/day) | T (°C) | CO2 (µg C m−2 h−1) | N2O (µg N m−2 h−1) | CH4 (µg C m−2 h−1) | |
|---|---|---|---|---|---|---|
| 1200–1340 (Farm A) | 3.5 ± 0.15 a | 20.5 ± 0.5 a | 87.42 ± 5.6 a | 6.82 ± 0.8 a | 0.31 ± 0.01 a | |
| 1100–1200 (Farm B) | 3.0 ± 0.15 b | 21.1 ± 0.5 a | 67.62 ± 4.4 b | 4.75 ± 0.7 b | 0.20 ± 0.01 b | |
| 900–1000 (Farm C) | 2.6 ± 0.10 c | 23.2 ± 0.7 b | 52.41 ± 3.9 c | 3.65 ± 0.6 c | 0.04 ± 0.00 c | |
| ANOVA | ||||||
| Factor | CO2 (µg C m−2 h−1) | N2O (µg N m−2 h−1) | CH4 (µg C m−2 h−1) | |||
| F-value | p-value | F-value | p-value | F-value | p-value | |
| T | 142.263 | 0.000 | 56.781 | 0.010 | 53.011 | 0.010 |
| P | 235.521 | 0.000 | 201.026 | 0.000 | 115.078 | 0.000 |
| T × P | 4.521 | 0.001 | 5.610 | 0.014 | 18.023 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatumah, N.; Munishi, L.K.; Ndakidemi, P.A. Variations in Greenhouse Gas Fluxes in Response to Short-Term Changes in Weather Variables at Three Elevation Ranges, Wakiso District, Uganda. Atmosphere 2019, 10, 708. https://doi.org/10.3390/atmos10110708
Fatumah N, Munishi LK, Ndakidemi PA. Variations in Greenhouse Gas Fluxes in Response to Short-Term Changes in Weather Variables at Three Elevation Ranges, Wakiso District, Uganda. Atmosphere. 2019; 10(11):708. https://doi.org/10.3390/atmos10110708
Chicago/Turabian StyleFatumah, Nakiguli, Linus K. Munishi, and Patrick A. Ndakidemi. 2019. "Variations in Greenhouse Gas Fluxes in Response to Short-Term Changes in Weather Variables at Three Elevation Ranges, Wakiso District, Uganda" Atmosphere 10, no. 11: 708. https://doi.org/10.3390/atmos10110708
APA StyleFatumah, N., Munishi, L. K., & Ndakidemi, P. A. (2019). Variations in Greenhouse Gas Fluxes in Response to Short-Term Changes in Weather Variables at Three Elevation Ranges, Wakiso District, Uganda. Atmosphere, 10(11), 708. https://doi.org/10.3390/atmos10110708





