Riding the Plumes: Characterizing Bubble Scavenging Conditions for the Enrichment of the Sea-Surface Microlayer by Transparent Exopolymer Particles
Abstract
:1. Introduction
2. Methods & Materials
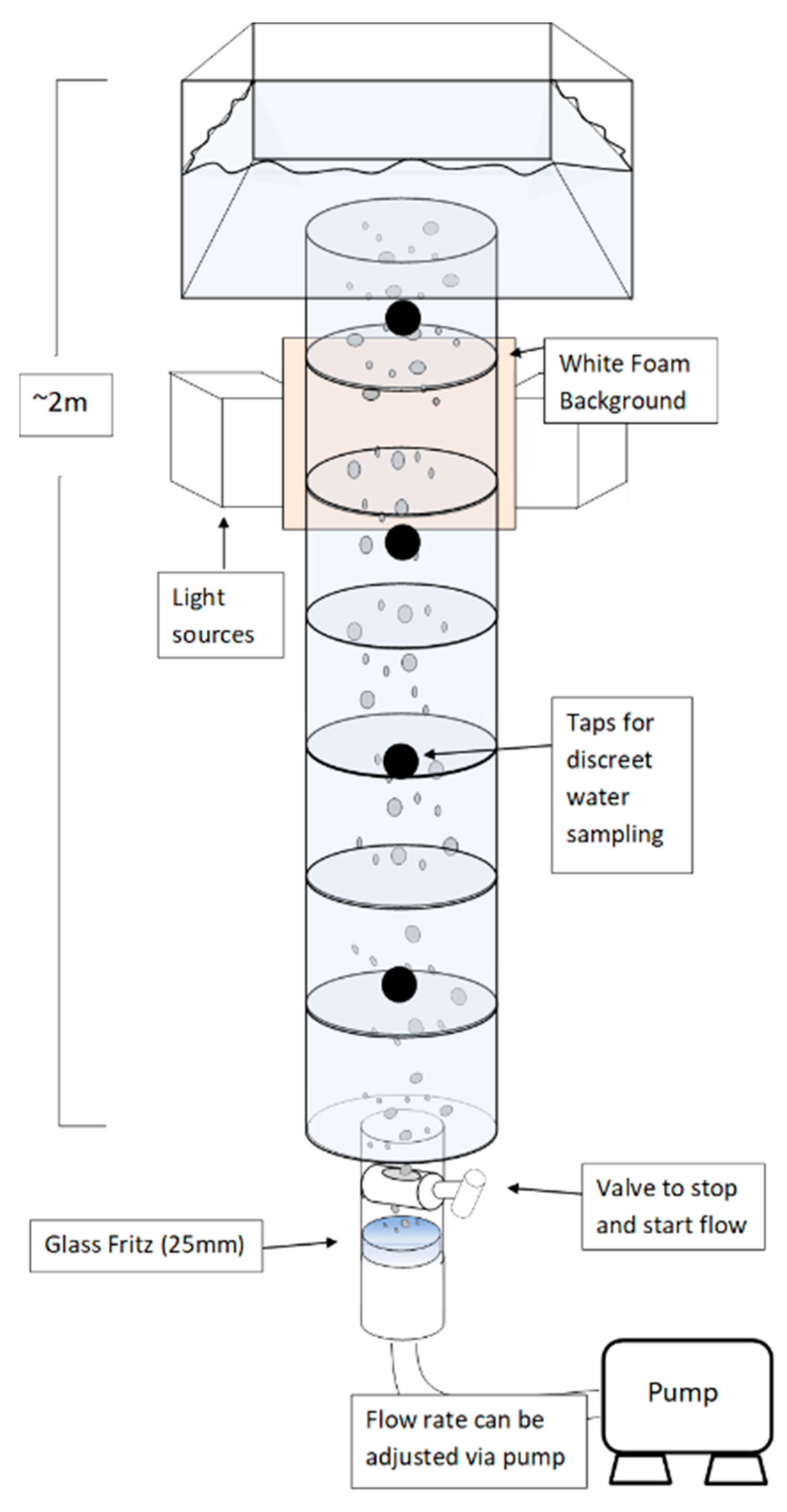
2.1. Tower Schematic
2.2. Experimental Setup
2.2.1. Overall Setup
2.2.2. Experiment 1—Bubbling Time and Production Rate
2.2.3. Experiment 2—Bubble Size
2.2.4. Experiment 3—Depth Profiles of TEP
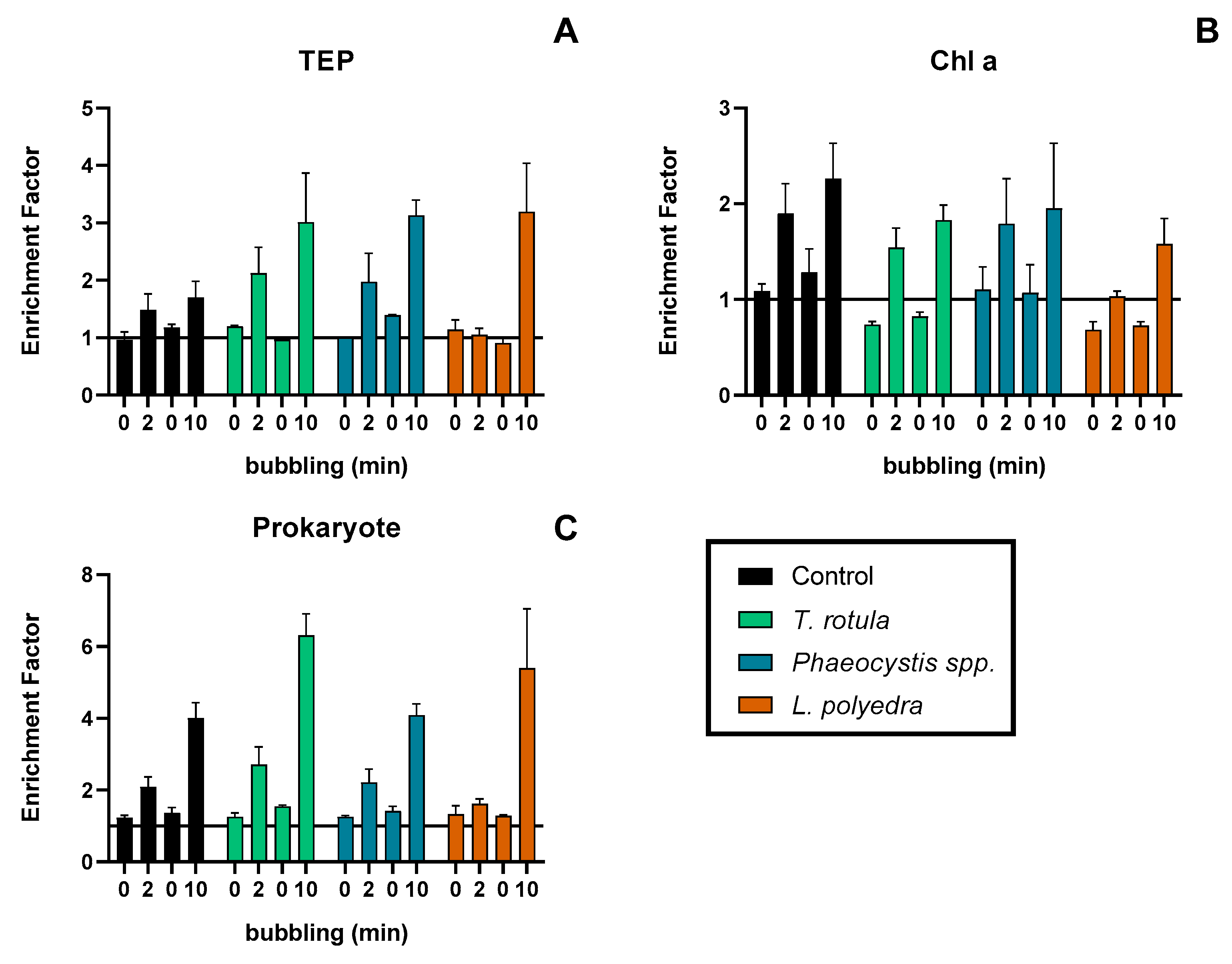
2.2.5. Experiment 4—Effect of Phytoplankton Species on TEP Enrichment from Bubble Scavenging
2.3. Measurements
2.3.1. Transparent Exopolymer Particles (TEP)
2.3.2. Chlorophyll a (Chl a)
2.3.3. Microbial Counts
2.3.4. Salinity/Temperature
2.4. Statistical Analyses
3. Results
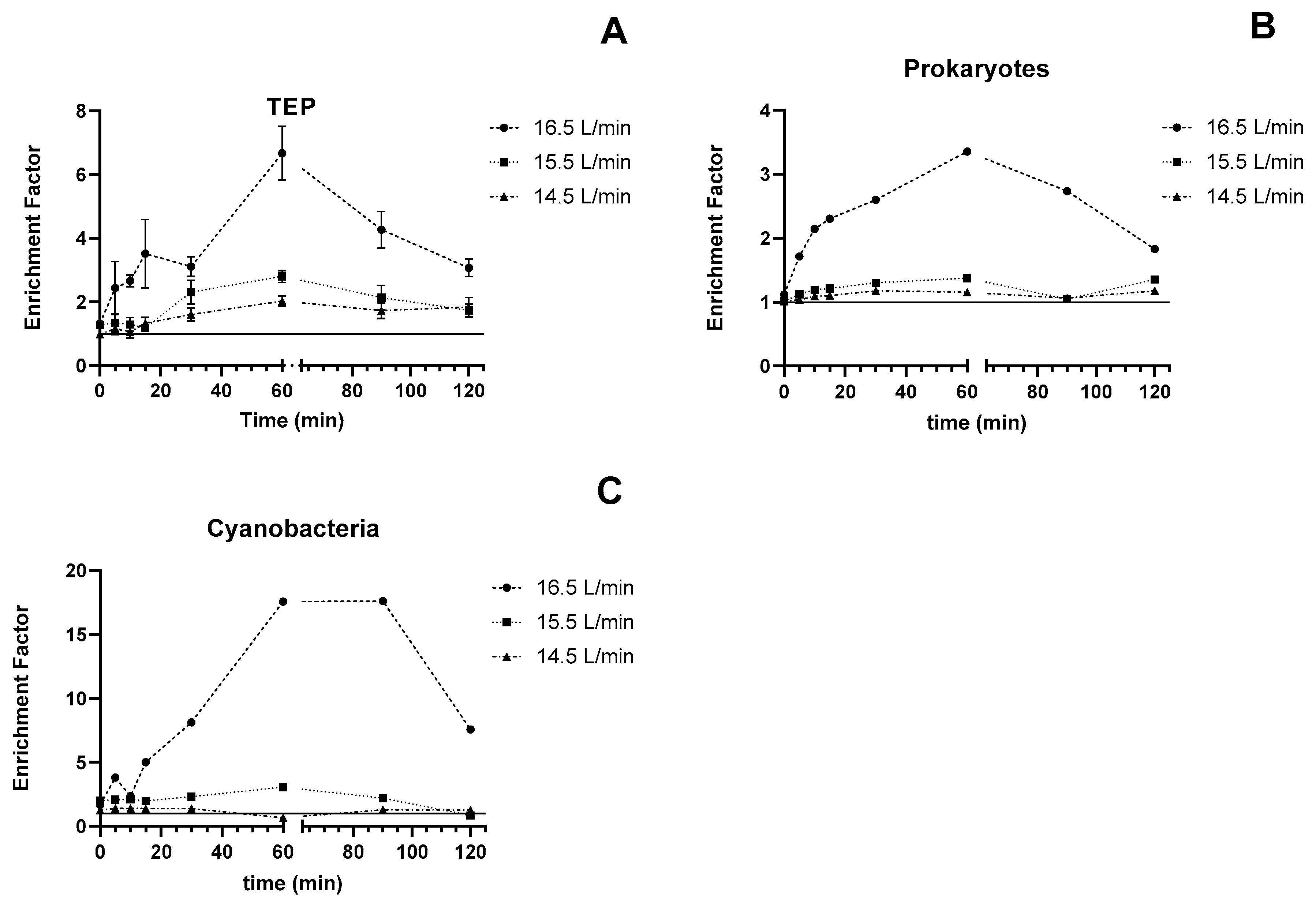
3.1. Experiment 1—Effect of Bubble Concentration and Time on TEP
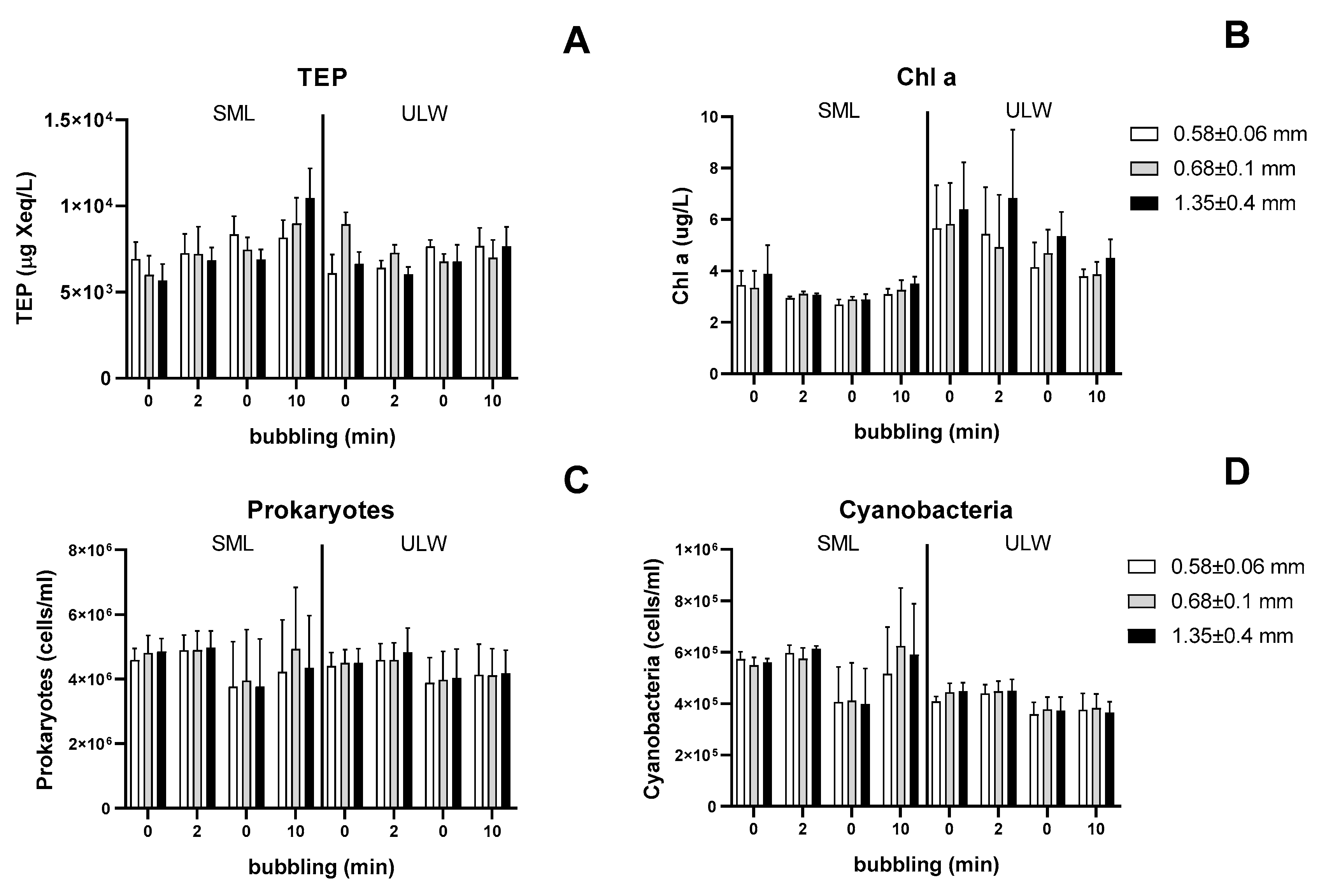
3.2. Experiment 2—Effect of Bubble Size on TEP
3.3. Experiment 3—Effect of Bubbling on Depth Profiles of TEP and Chl a
3.4. Experiment 4—Effect of Different Species of Phytoplankton on Bubbling Enrichment of TEP
4. Discussion
4.1. How Bubble Size Affects TEP Enrichment
4.2. Effect of Bubbling Rate on TEP Enrichment
4.3. TEP Enrichment over Time with and without Bubbling
4.4. Effect of Phytoplankton Presence and Species Difference on TEP Enrichment
4.5. Enrichments of Organic Materials in the SML and Its Air–Sea Transfer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunliffe, M.; Engel, A.; Frka, S.; Gasparovic, B.; Guitart, C.; Murrell, C.; Salter, M.; Stolle, C.; Upstill-Goddard, R.; Wurl, O. Sea surface microlayers: A unified physicochemical and biological perspective of the air–ocean interface. Prog. Oceanogr. 2013, 109, 104–116. [Google Scholar] [CrossRef]
- Sieburth, J. Microbiological and organic-chemical processes in the surface and mixed layers. In Air-Sea Exchange of Gases and Particles; Liss, P., Slinn, W., Eds.; Reidel Publishers Co: Hingham, MA, USA, 1983. [Google Scholar]
- Wurl, O.; Stolle, C.; Van Thuoc, C.; The Thu, P.; Mari, X. Biofilm-like properties of the sea surface and predicted effects on air–sea co2 exchange. Prog. Oceanogr. 2016, 144, 15–24. [Google Scholar] [CrossRef]
- Gustafsson, M.E.; Franzén, L.G. Inland transport of marine aerosols in southern sweden. Atmos. Environ. 2000, 34, 313–325. [Google Scholar] [CrossRef]
- Grammatika, M.; Zimmerman, W.B. Microhydrodynamics of flotation processes in the sea surface layer. Dyn. Atmos. Ocean. 2001, 34, 327–348. [Google Scholar] [CrossRef]
- Hardy, J.T. The sea surface microlayer: Biology, chemistry and anthropogenic enrichment. Prog. Oceanogr. 1982, 11, 21. [Google Scholar] [CrossRef]
- Liss, P.; Duce, R. The Sea Surface and Global Change; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Stolle, C.; Labrenz, M.; Meeske, C.; Jurgens, K. Bacterioneuston community structure in the southern baltic sea and its dependence on meteorological conditions. Appl. Environ. Microbiol. 2011, 77, 3726–3733. [Google Scholar] [CrossRef] [PubMed]
- Wurl, O.; Miller, L.; Vagle, S. Production and fate of transparent exopolymer particles in the ocean. J. Geophys. Res. 2011, 116, 16. [Google Scholar] [CrossRef]
- Passow, U.; Alldredge, A. A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (tep). Limnol. Oceanogr. 1995, 40, 10. [Google Scholar] [CrossRef]
- Alldredge, A.; Passow, U.; Logan, B. The abundance and significance of a class of large, transparent organic particles in the ocean. Deep Sea Res. Part I: Oceanogr. Res. Pap. 1993, 40, 9. [Google Scholar] [CrossRef]
- Passow, U. Transparent exopolymer particles (tep) in aquatic environments. Prog. Oceanogr. 2002, 55, 287–333. [Google Scholar] [CrossRef]
- Bartual, A.; Vicenta-Cera, I.; Flecha, S.; Prieto, L. Effect of dissolved polyunsaturated aldehydes on the size distribution of transparent exopolymeric particles in an experimental diatom bloom. Mar. Biol. 2017, 164, 11. [Google Scholar] [CrossRef]
- Passow, U. Production of transparent exopolymer particles (tep) by phyto- and bacterioplankton. Mar. Ecol. Prog. Ser. 2002, 236, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Thornton, D.C. Transparent exopolymer particle production and aggregation by a marine planktonic diatom (thalassiosira weissflogii) at different growth rates. J. Phycol. 2015, 51, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Claquin, P.; Probert, I.; Lefebvre, S.; Veron, B. Effects of temperature on photosynthetic parameters and tep production in eight species of marine microalgae. Aquat. Microb. Ecol. 2008, 51, 1–11. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.; Lyklema, J.; Norde, W.; Zehnder, A. Bacterial adhesion: A physicochemical approach. Microb. Ecol. 1989, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Grossart, H.; Schweitzer, B.; Ploug, H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 2002, 28, 175–211. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.D.; Cunliffe, M. Coastal bacterioplankton community response to diatom-derived polysaccharide microgels. Environ. Microbiol. Rep. 2017, 9, 151–157. [Google Scholar] [CrossRef]
- Passow, U.; Shipe, R.; Murray, A.; Pak, D.; Brzezinski, M.; Alldredge, A. The origin of transparent exopolymer particles (tep) and their role in the sedimentation of particulate matter. Cont. Shelf Res. 2001, 21, 327–346. [Google Scholar] [CrossRef]
- Mari, X.; Kiorboe, T. Abundance, size distribution and bacterial colonization of transparent exopolymeric particles (tep) during spring in the kattegat. J. Plankton Res. 1996, 18, 17. [Google Scholar] [CrossRef]
- Zhou, J.; Mopper, K.; Passow, U. The role of surface-active carbohydrates in the formation of transparent exopolymer particles by bubble adsorption of seawater. Limnol. Oceanogr. 1998, 43, 12. [Google Scholar] [CrossRef]
- Robinson, T.B.; Wurl, O.; Bahlmann, E.; Jürgens, K.; Stolle, C. Rising bubbles enhance the gelatinous nature of the air–sea interface. Limnol. Oceanogr. 2019, 1–15. [Google Scholar] [CrossRef]
- Calleja, M. The Metabolic Significance of the Surface Layer of the Ocean; Universitat de les Illes Balears: Palma, Spain, 2007. [Google Scholar]
- Aller, J.Y.; Kuznetsova, M.R.; Jahns, C.J.; Kemp, P.F. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 2005, 36, 801–812. [Google Scholar] [CrossRef]
- Kuznetsova, M.; Lee, C.; Aller, J. Characterization of the proteinaceous matter in marine aerosols. Mar. Chem. 2005, 96, 359–377. [Google Scholar] [CrossRef]
- Bigg, K.; Leck, C. The composition of fragments of bubbles bursting at the ocean surface. J. Geophys. Res. 2008, 113, 7. [Google Scholar] [CrossRef]
- Rastelli, E.; Corinaldesi, C.; Dell’Anno, A.; Lo Martire, M.; Greco, S.; Cristina Facchini, M.; Rinaldi, M.; O’Dowd, C.; Ceburnis, D.; Danovaro, R. Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: An experimental approach. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Leck, C.; Bigg, K. Biogenic particles in the surface microlayer and overlaying atmosphere in the central arctic ocean during summer. Tellus 2005, 57, 9. [Google Scholar]
- Wilson, T.W.; Ladino, L.A.; Alpert, P.A.; Breckels, M.N.; Brooks, I.M.; Browse, J.; Burrows, S.M.; Carslaw, K.S.; Huffman, J.A.; Judd, C. A marine biogenic source of atmospheric ice-nucleating particles. Nature 2015, 525, 234. [Google Scholar] [CrossRef]
- Orellana, M.V.; Matrai, P.A.; Leck, C.; Rauschenberg, C.D.; Lee, A.M.; Coz, E. Marine microgels as a source of cloud condensation nuclei in the high arctic. Proc. Natl. Acad. Sci. USA 2011, 108, 13612–13617. [Google Scholar] [CrossRef]
- Bowyer, P.A. Video measurements of near-surface bubble spectra. J. Geophys. Res. Ocean. 2001, 106, 14179–14190. [Google Scholar] [CrossRef]
- Norris, S.; Brooks, I.; Moat, B.; Yelland, M.; De Leeuw, G.; Pascal, R.; Brooks, B. Near-surface measurements of sea spray aerosol production over whitecaps in the open ocean. Ocean Sci. 2013, 9, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Cunliffe, M.; Wurl, O. Guide to the Best Practices to Study the Ocean’s S; Marine Biological Association of the United Kingdom: Plymouth, UK, 2014; p. 118. [Google Scholar]
- Harvey, G.; Burzell, L. A simple microlayer method for small samples. Limnol. Oceanogr. 1972. [Google Scholar] [CrossRef]
- Engel, A. Determination of marine gel particles. In Practical Guidelines for the Analysis of Seawater; Wurl, O., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 125–142. [Google Scholar]
- Johnson, B.; Cooke, R. Bubble populations and spectra in coastal waters: A photographic approach. J. Geophys. Res. Ocean. 1979, 84, 3761–3766. [Google Scholar] [CrossRef]
- Crawford, G.; Farmer, D. On the spatial distribution of ocean bubbles. J. Geophys. Res. Ocean. 1987, 92, 8231–8243. [Google Scholar] [CrossRef]
- Vagle, S.; Farmer, D.M. The measurement of bubble-size distributions by acoustical backscatter. J. Atmos. Ocean. Technol. 1992, 9, 630–644. [Google Scholar] [CrossRef]
- Zhang, X.; Lewis, M.; Johnson, B. Influence of bubbles on scattering of light in the ocean. Appl. Opt. 1998, 37, 6525–6536. [Google Scholar] [CrossRef] [PubMed]
- Deane, G.; Stokes, M. Scale dependence of bubble creation mechanisms in breaking waves. Nature 2002, 418, 6. [Google Scholar] [CrossRef] [PubMed]
- Bittar, T.B.; Passow, U.; Hamaraty, L.; Bidle, K.D.; Harvey, E.L. An updated method for the calibration of transparent exopolymer particle measurements. Limnol. Oceanogr. Methods 2018, 16, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Giebel, H.-A.; Wolterink, M.; Brinkhoff, T.; Simon, M. Complementary energy acquisition via aerobic anoxygenic photosynthesis and carbon monoxide oxidation by planktomarina temperata of the roseobacter group. Fems Microbiol. Ecol. 2019, 95, fiz050. [Google Scholar] [CrossRef] [PubMed]
- Mari, X.; Passow, U.; Migon, C.; Burd, A.B.; Legendre, L. Transparent exopolymer particles: Effects on carbon cycling in the ocean. Prog. Oceanogr. 2017, 151, 13–37. [Google Scholar] [CrossRef]
- Wurl, O.; Holmes, M. The gelatinous nature of the sea-surface microlayer. Mar. Chem. 2008, 110, 89–97. [Google Scholar] [CrossRef]
- Cunliffe, M.; Murrell, C. The sea-surface microlayer is a gelatinous biofilm. Isme J. 2009, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Bange, H.W.; Cunliffe, M.; Burrows, S.M.; Friedrichs, G.; Galgani, L.; Herrmann, H.; Hertkorn, N.; Johnson, M.; Liss, P.S. The ocean’s vital skin: Toward an integrated understanding of the sea surface microlayer. Front. Mar. Sci. 2017, 4, 165. [Google Scholar] [CrossRef]
- Wurl, O.; Wurl, E.; Miller, L.; Johnson, K.; Vagle, S. Formation and global distribution of sea-surface microlayers. Biogeosciences 2011, 8, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.; Cooke, R. Organic particle and aggregate formation resulting from the dissolution of bubbles in seawater. Limnol. Oceanogr. 1980, 25, 8. [Google Scholar] [CrossRef]
- Gao, Q.; Leck, C.; Rauschenburg, C.; Matrai, P. On the chemical dynamics of extracellular polysaccharides in the high arctic surface microlayer. Ocean Sci. Discuss. 2012, 9, 45. [Google Scholar] [CrossRef]
- Tseng, R.-S.; Viechnicki, J.T.; Skop, R.A.; Brown, J.W. Sea-to-air transfer of surface-active organic compounds by bursting bubbles. J. Geophys. Res. 1992, 97, 5201. [Google Scholar] [CrossRef]
- Stefan, R.L.; Szeri, A.J. Surfactant scavenging and surface deposition by rising bubbles. J. Colloid Interface Sci. 1999, 212, 1–13. [Google Scholar] [CrossRef]
- Lewis, E.R.; Lewis, E.R.; Lewis, R.; Karlstrom, K.E.; Schwartz, S.E. Sea Salt Aerosol Production: Mechanisms, Methods, Measurements, and Models; American Geophysical Union: Washington, DC, USA, 2004; Volume 152. [Google Scholar]
- Stokes, M.; Deane, G.; Prather, K.; Bertram, T.; Ruppel, M.; Ryder, O.; Brady, J.; Zhao, D. A marine aerosol reference tank system as a breaking wave analogue for the production of foam and sea-spray aerosols. Atmos. Meas. Tech. 2013, 6, 1085–1094. [Google Scholar] [CrossRef]
- Azetsu-Scott, K.; Passow, U. Ascending marine particles: Significance of transparent exopolymer particles (tep) in the upper ocean. Limnol. Oceanogr. 2004, 49, 7. [Google Scholar] [CrossRef]
- Ramaiah, N.; Yoshikawa, T.; Furuya, K. Temporal variations in transparent exopolymer particles (tep) associated with a diatom spring bloom in a subarctic ria in japan. Mar. Ecol. Prog. Ser. 2001, 212, 79–88. [Google Scholar] [CrossRef]
- Galgani, L.; Engel, A. Accumulation of gel particles in the sea-surface microlayer during an experimental study with the diatom thalassiosira weissflogii. Int. J. Geosci. 2013, 04, 129–145. [Google Scholar] [CrossRef]
- Fukao, T.; Kimoto, K.; Kotani, Y. Production of transparent exopolymer particles by four diatom species. Jpn. Soc. Fish. Sci. 2010, 76, 6. [Google Scholar] [CrossRef]
- Mari, X.; Rassoulzadegan, F.; Brussaard, C.; Wassmann, P. Dynamics of transparent exopolymeric particles (tep) production by phaeocystis globosa under n- or p-limitation: A controlling factor of the retention/export balance. Elsevier 2004, 4, 19. [Google Scholar] [CrossRef]
- Lewis, J.; Hallett, R. Lingulodinium polyedrum (gonyaulux polyedra) a blooming dinoflagellate. Oceanogr. Mar. Biol. 1997, 35, 97–162. [Google Scholar]
- Marks, R.; Górecka, E.; McCartney, K.; Borkowski, W. Rising bubbles as mechanism for scavenging and aerosolization of diatoms. J. Aerosol Sci. 2019, 128, 79–88. [Google Scholar] [CrossRef]
- Blanchard, D.; Syzdek, L. Mechanism for the water-to-air transfer and concentration of bacteria. Science 1970, 170, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Bigg, E.K.; Leck, C.; Tranvik, L. Particulates of the surface microlayer of open water in the central arctic ocean in summer. Mar. Chem. 2004, 91, 131–141. [Google Scholar] [CrossRef]
- Ortega-Retuerta, E.; Passow, U.; Duarte Quesada, C.M.; Reche Cañabate, I. Effects of ultraviolet b radiation on (not so) transparent exopolymer particles. Biogeosciences 2009, 6, 3071–3080. [Google Scholar] [CrossRef]
- O’Dowd, C.; Ceburnis, D.; Ovadnevaite, J.; Bialek, J.; Stengel, D.B.; Zacharias, M.; Nitschke, U.; Connan, S.; Rinaldi, M.; Fuzzi, S. Connecting marine productivity to sea-spray via nanoscale biological processes: Phytoplankton dance or death disco? Sci. Rep. 2015, 5, 14883. [Google Scholar] [CrossRef] [PubMed]
- Prather, K.A.; Bertram, T.H.; Grassian, V.H.; Deane, G.B.; Stokes, M.D.; DeMott, P.J.; Aluwihare, L.I.; Palenik, B.P.; Azam, F.; Seinfeld, J.H. Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol. Proc. Natl. Acad. Sci. USA 2013, 110, 7550–7555. [Google Scholar] [CrossRef] [Green Version]

| Experiment 1 | ||||||
| EF (A/B 60 min bubbling) ± SD | Test | Parameter (bubbling flow rate) | n | mean diff ± SE | p-value | |
| 2-way ANOVA | 16.5 L/min, 15.5 L/min, 14.5 L/min | 9 | 0.0361 | |||
| 16.5 L/min | 5.03 ± 0.33 | Post-hoc Tukey | 16.5 L/min vs. 15.5 L/min | 24 | 1.619 ± 0.30 | <0.0001 |
| 15.5 L/min | 2.21 ± 0.27 | 16.5 L/min vs. 14.5 L/min | 24 | 1.915 ± 0.30 | <0.0001 | |
| 14.5 L/min | 2.12 ± 0.42 | 15.5 L/min vs. 14.5 L/min | 24 | 0.296 ± 0.13 | 0.0744 | |
| Experiment 2 | ||||||
| EF(A/B) ± SD | Parameter (bubbling time–bubble size) | n | mean diff ± SE | p-value | ||
| 0.58 mm−2 min | 0.97 ± 0.07 | Post-hoc Tukey | 2 min–0.58 mm vs. 0.68 mm | 6 | −0.55 ± 0.27 | 0.1504 |
| 0.68 mm−2 min | 1.52 ± 0.50 | 2 min–0.58 mm vs. 1.35 mm | 6 | −0.43 ± 0.27 | 0.2979 | |
| 1.35 mm−2 min | 1.39 ± 0.36 | 2 min–0.68 mm vs. 1.35 mm | 6 | 1.23 ± 0.27 | 0.8938 | |
| 0.58 mm−10 min | 0.99 ± 0.08 | 10 min–0.58 mm vs. 0.68 mm | 6 | −0.18 ± 0.27 | 0.7828 | |
| 0.68 mm−10 min | 1.18 ± 0.14 | 10 min–0.58 mm vs. 1.35 mm | 6 | −0.33 ± 0.27 | 0.4559 | |
| 1.35 mm−10 min | 1.33 ± 0.20 | 10 min–0.68 mm vs. 1.35 mm | 6 | −0.15 ± 0.27 | 0.8423 | |
| Experiment 3 | ||||||
| Without Bubbling | With Bubbling | |||||
| Depth (cm) | ΔTEP (µg XG eq L−1) 2 min | ΔTEP (µg XG eq L−1) 10 min | ΔTEP (µg XG eq L−1) 2 min | ΔTEP (µg XG eq L−1) 10 min | ||
| 0 | −125 ± 151 | −122 ± 134 | 44 ± 481 | 1941 ± 1344 | Statistical tests not applied | |
| 40 | −113 ± 357 | 4 ± 124 | −235 ± 70 | 121 ± 88 | ||
| 85 | 199 ± 309 | 87 ± 55 | −61 ± 128 | 114 ± 157 | ||
| 135 | 75 ± 190 | 221 ± 325 | 118 ± 107 | 46 ± 62 | ||
| 185 | 70 ± 79 | 102 ± 185 | −4 ± 94 | −171 ± 119 | ||
| Experiment 4 | ||||||
| EF(A/B) ± SD | Test | Parameter (bubbling time–species) | n | mean diff ± SE | p-value | |
| 2 min—Control | 1.19 ± 0.10 | Post-hoc Tukey | 2 min—Control vs. T. rotula | 6 | −0.5787 ± 0.48 | 0.7469 |
| 2 min—T. rotula | 1.77 ± 0.49 | 2 min—Control vs. Pheaocistus spp. | 6 | −0.7784 ± 0.48 | 0.5591 | |
| 2 min—Phaeocystis spp. | 1.97 ± 0.72 | 2 min—Control vs. L. polydra | 6 | 0.2523 ± 0.48 | 0.9684 | |
| 2 min—L. polyedra | 0.94 ± 0.10 | 10 min—Control vs. T. rotula | 6 | −1.869 ± 0.48 | 0.0424 | |
| 10 min—Control | 1.25 ± 0.33 | 10 min—Control vs. Pheaocistus spp. | 6 | −0.9975 ± 0.48 | 0.3726 | |
| 10 min—T. rotula | 3.12 ± 1.27 | 10 min—Control vs. L. polydra | 6 | −2.276 ± 0.48 | 0.0131 | |
| 10 min—Phaeocystis spp. | 2.25 ± 0.28 | |||||
| 10 min—L. polyedra | 3.52 ± 1.15 | |||||
| Species | Culture # | Species concn. in Culture (Cell/mL) | Species concn. in Tower (Cell/mL) | Species | Culture # | Species concn. in Culture (Cell/mL) | Species concn. in Tower (Cell/mL) |
|---|---|---|---|---|---|---|---|
| Lingulodinium polyedra | 1 | 10,472 | 34 | Phaeocystis spp. (free cells) | 1 | 21,201 | 30 |
| 2 | 8586 | 28 | 2 | 14,322 | 20 | ||
| 3 | 7970 | 26 | 3 | 23,459 | 34 | ||
| Thalassiosira rotula | 1 | 15,708 | 50 | Phaeocystis spp. (colonies) | 1 | 1848 | 3 |
| 2 | 19,507 | 63 | 2 | 2772 | 4 | ||
| 3 | 16,863 | 54 | 3 | 2618 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, T.-B.; Giebel, H.-A.; Wurl, O. Riding the Plumes: Characterizing Bubble Scavenging Conditions for the Enrichment of the Sea-Surface Microlayer by Transparent Exopolymer Particles. Atmosphere 2019, 10, 454. https://doi.org/10.3390/atmos10080454
Robinson T-B, Giebel H-A, Wurl O. Riding the Plumes: Characterizing Bubble Scavenging Conditions for the Enrichment of the Sea-Surface Microlayer by Transparent Exopolymer Particles. Atmosphere. 2019; 10(8):454. https://doi.org/10.3390/atmos10080454
Chicago/Turabian StyleRobinson, Tiera-Brandy, Helge-Ansgar Giebel, and Oliver Wurl. 2019. "Riding the Plumes: Characterizing Bubble Scavenging Conditions for the Enrichment of the Sea-Surface Microlayer by Transparent Exopolymer Particles" Atmosphere 10, no. 8: 454. https://doi.org/10.3390/atmos10080454
APA StyleRobinson, T.-B., Giebel, H.-A., & Wurl, O. (2019). Riding the Plumes: Characterizing Bubble Scavenging Conditions for the Enrichment of the Sea-Surface Microlayer by Transparent Exopolymer Particles. Atmosphere, 10(8), 454. https://doi.org/10.3390/atmos10080454






