Prediction of Aerosol Deposition in the Human Respiratory Tract via Computational Models: A Review with Recent Updates
Abstract
:1. Introduction
2. Aerosol Deposition Mechanisms
2.1. Impaction
2.2. Sedimentation
2.3. Diffusion
3. Lung-Geometry Models
4. Semi-Empirical Models
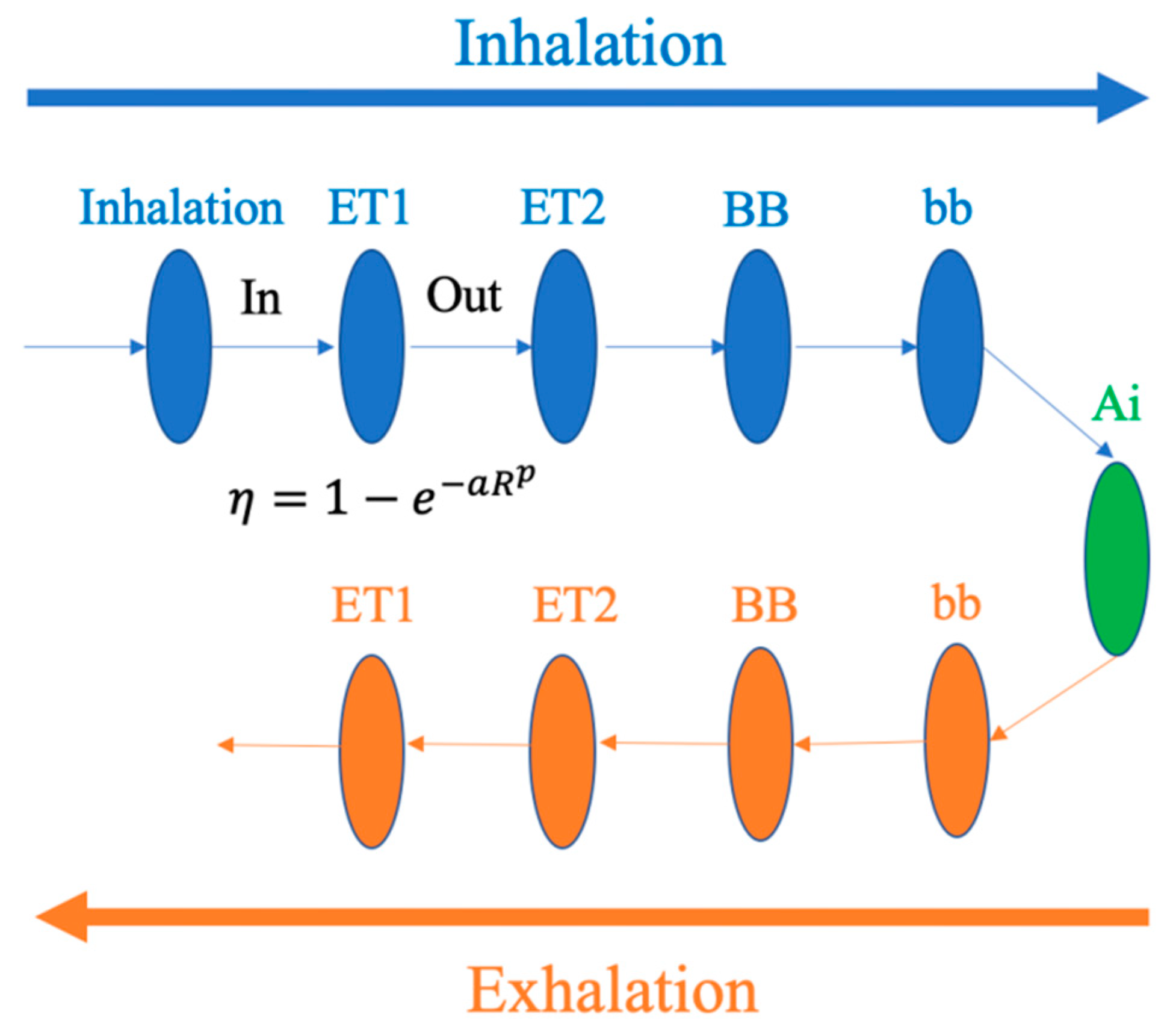
4.1. ICRP Model
4.1.1. Introduction
4.1.2. Applications
4.2. Exposure Dose Model (ExDoM)
4.2.1. Introduction
4.2.2. Applications
4.3. Exposure Dose Model 2 (ExDoM2)
4.3.1. Introduction
4.3.2. Applications
5. One-Dimensional (1D) Whole-Lung Deposition Models
5.1. Trumpet Model
5.1.1. Introduction
5.1.2. Applications
5.2. Multiple-Path Particle Dosimetry Model (MPPD)
5.2.1. Introduction
5.2.2. Applications
5.3. Stochastic Model
5.3.1. Introduction
5.3.2. Applications
6. Three-Dimensional (3D) Computational Fluid Dynamics (CFD) Models
6.1. Introduction
6.2. Applications
7. Artificial Neural Networks
7.1. Introduction
7.2. Applications
8. Conclusios
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | One dimensional |
| 2D | Two dimensional |
| 3D | Three dimensional |
| AL | Alveolar |
| ANN | Artificial neural networks |
| BB | Trachea and bronchi |
| bb | Bronchiolar |
| CFD | Computational fluid dynamics |
| COPD | Chronic obstructive pulmonary disease |
| DPI | Dry Powder Inhaler |
| ET | Extrathoracic |
| GI | Gastrointestinal |
| ICRP | International Commission on Radiological Protection |
| LUDEP | Lung Dose Evaluation Program |
| MLP | Multilayer perceptron |
| PM | Particulate matter |
| RT | Respiratory tract |
| SIPs | Stochastic individual pathways |
| TB | Tracheobronchial |
| WLAM | Whole-lung-airway model |
References
- Olivieri, D.; Scoditti, E. Impact of environmental factors on lung defences. Eur. Respir. Rev. 2005, 14, 51 LP-56. [Google Scholar] [CrossRef]
- Shang, Y.; Dong, J.; Tian, L.; Inthavong, K.; Tu, J. Detailed computational analysis of flow dynamics in an extended respiratory airway model. Clin. Biomech. 2019, 61, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J.; Blasi, F.; Ward, B.; Reeves, E.; Rabe, K.F. Respiratory diseases in the world: One voice “united for lung health”. Eur. Respir. J. 2014, 43, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, A.A. Computational modeling of aerosol deposition in respiratory tract: A review. Inhal. Toxicol. 2009, 21, 262–290. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S. Air Quality Criteria for Particulate Matter (Final Report, 1996); Vol. EPA 600/P-95/001; U.S. Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- IARC. Outdoor Air Pollution a Leading Environmental Cause of Cancer Deaths; IARC Scientific Publication: Lyon, France, 2013. [Google Scholar]
- IARC. Outdoor air pollution. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2016; Volume 109. [Google Scholar]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Chalvatzaki, E.; Chatoutsidou, E.S.; Mammi-Galani, E.; Almeida, M.S.; Gini, I.M.; Eleftheriadis, K.; Diapouli, E.; Lazaridis, M. Estimation of the personal deposited dose of particulate matter and particle-bound metals using data from selected european cities. Atmosphere 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Kolanjivil, A.V.; Kleinstreuer, C. Computational analysis of aerosol-dynamics in a human whole-lung airway model. J. Aerosol Sci. 2017, 114, 301–316. [Google Scholar] [CrossRef]
- Martins, V.; Cruz Minguillón, M.; Moreno, T.; Querol, X.; de Miguel, E.; Capdevila, M.; Centelles, S.; Lazaridis, M. Deposition of aerosol particles from a subway microenvironment in the human respiratory tract. J. Aerosol Sci. 2015, 90, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Madl, P.; Khan, A. Lung deposition predictions of airborne particles and the emergence of contemporary diseases - part I. theHealth 2011, 2, 51–59. [Google Scholar]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999; p. 504. [Google Scholar]
- Finlay, W.H. Chapter 3 - Motion of a single aerosol particle in a fluid. In The Mechanics of Inhaled Pharmaceutical Aerosols (Second Edition); Finlay, W.H., Ed.; Academic Press: London, UK, 2019; pp. 21–52. [Google Scholar]
- Weibel, E.E. Morphometry of the Human Lung; Springer: Berlin/Heidelberg, Germany, 1963. [Google Scholar]
- Baron, P.A.; Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Bi, X.; Sheng, G.; Peng, P.a.; Chen, Y.; Fu, J. Size distribution of n-alkanes and polycyclic aromatic hydrocarbons (PAHs) in urban and rural atmospheres of Guangzhou, China. Atmos. Environ. 2005, 39, 477–487. [Google Scholar] [CrossRef]
- Oberdörster, G. Effects and fate of inhaled ultrafine particles. In Nanotechnology and the Environment; American Chemical Society: Washington, DC, USA, 2004; Volume 890, pp. 37–59. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Di Vaio, P.; Magli, E.; Caliendo, G.; Corvino, A.; Fiorino, F.; Frecentese, F.; Saccone, I.; Santagada, V.; Severino, B.; Onorati, G.; et al. Heavy metals size distribution in PM10 and environmental-sanitary risk analysis in Acerra (Italy). Atmosphere 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Horsfield, K.; Cumming, G. Morphology of the bronchial tree in man. J. Appl. Physiol. 1968, 24, 373–383. [Google Scholar] [CrossRef]
- Takano, H.; Nishida, N.; Itoh, M.; Hyo, N.; Majima, Y. Inhaled particle deposition in unsteady-state respiratory flow at a numerically constructed model of the human larynx. J. Aerosol Med. 2006, 19, 314–328. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P.W. Transport and deposition of micro-aerosols in realistic and simplified models of the oral airway. Ann. Biomed. Eng. 2007, 35, 560–581. [Google Scholar] [CrossRef]
- Luo, H.Y.; Liu, Y. Modeling the bifurcating flow in a CT-scanned human lung airway. J. Biomech. 2008, 41, 2681–2688. [Google Scholar] [CrossRef]
- Imai, Y.; Miki, T.; Ishikawa, T.; Aoki, T.; Yamaguchi, T. Deposition of micrometer particles in pulmonary airways during inhalation and breath holding. J. Biomech. 2012, 45, 1809–1815. [Google Scholar] [CrossRef]
- Ebert, M.; Grossmann, T.; Heil, W.; Otten, E.W.; Surkau, R.; Thelen, M.; Leduc, M.; Bachert, P.; Knopp, M.V.; Schad, L.R. Nuclear magnetic resonance imaging with hyperpolarised helium-3. The Lancet 1996, 347, 1297–1299. [Google Scholar] [CrossRef]
- Musch, G.; Layfield, J.D.H.; Harris, R.S.; Melo, M.F.V.; Winkler, T.; Callahan, R.J.; Fischman, A.J.; Venegas, J.G. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J. Appl. Physiol. 2002, 93, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, K.; Missbach-Guentner, J.; Alves, F. Using in vivo imaging for asthma. Drug Discov. Today Dis. Models 2009, 6, 129–135. [Google Scholar] [CrossRef]
- Kitaoka, H.; Koc, S.; Tetsumoto, S.; Koumo, S.; Hirata, H.; Kijima, T. 4D model generator of the human lung, “Lung4Cer”. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 453–456. [Google Scholar]
- Roth, C.J.; Ismail, M.; Yoshihara, L.; Wall, W.A. A comprehensive computational human lung model incorporating inter-acinar dependencies: Application to spontaneous breathing and mechanical ventilation. Int. J. Numer. Meth. Bio. 2017, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, C.J.; Becher, T.; Frerichs, I.; Weiler, N.; Wall, W.A. Coupling of EIT with computational lung modeling for predicting patient-specific ventilatory responses. J. Appl. Physiol. 2016, 122, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Tong, Z.B.; Chan, H.K.; Yang, R.Y. CFD modelling of air and particle flows in different airway models. J. Aerosol Sci. 2019, 134, 14–28. [Google Scholar] [CrossRef]
- Augusto, L.L.X.; Goncalves, J.A.S.; Lopes, G.C. CFD evaluation of the influence of physical mechanisms, particle size, and breathing condition on the deposition of particulates in a triple bifurcation airway. Water Air Soil Poll. 2016, 227, 56. [Google Scholar] [CrossRef]
- Kim, C.S.; Fisher, D.M. Deposition characteristics of aerosol particles in sequentially bifurcating airway models. Aerosol Sci. Tech. 1999, 31, 198–220. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, H. A 4D model generator of the human lung. Forma 2011, 26, 19–24. [Google Scholar]
- Guha, S.; Hariharan, P.; Myers, M.R. Enhancement of ICRP’s lung deposition model for pathogenic bioaerosols. Aerosol Sci. Tech. 2014, 48, 1226–1235. [Google Scholar] [CrossRef]
- Aleksandropoulou, V.; Lazaridis, M. Development and application of a model (ExDoM) for calculating the respiratory tract dose and retention of particles under variable exposure conditions. Air Qual. Atmos. Hlth. 2013, 6, 13–26. [Google Scholar] [CrossRef]
- Watanabe, T.; Bartrand, T.A.; Weir, M.H.; Omura, T.; Haas, C.N. Development of a dose-response model for SARs coronavirus. Risk Anal. 2010, 30, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, P.S.; Koontz, M.; Wilkes, C.; Ryan, B.; Macintosh, D.; Georgopoulos, P. Construction of a Comprehensive Chemical Exposure Framework Using Person Oriented Modeling; American Chemistry Council: Washington, DC, USA, 2003; Contract Number 1338. [Google Scholar]
- Querol, X.; Moreno, T.; Karanasiou, A.; Reche, C.; Alastuey, A.; Viana, M.; Font, O.; Gil, J.; Miguel, E.d.; Capdevila, M. Variability of levels and composition of PM10 and PM2.5 in the Barcelona metro system. Atmos. Chem. Phys. 2012, 12, 5055–5076. [Google Scholar] [CrossRef] [Green Version]
- Chalvatzaki, E.; Lazaridis, M. Development and application of a dosimetry model (ExDoM2) for calculating internal dose of specific particle-bound metals in the human body. Inhal. Toxicol. 2015, 27, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Chio, C.-P.; Liao, C.-M. Assessing airborne PM-bound arsenic exposure risk in semiconductor manufacturing facilities. J. Hazard. Mater. 2009, 167, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Maheshwari, M.; Morisawa, S. Dietary and inhalation intake of lead and estimation of blood lead levels in adults and children in kanpur, india. Risk Anal. 2005, 25, 1573–1588. [Google Scholar] [CrossRef]
- O’Flaherty, E.J.; Kerger, B.D.; Hays, S.M.; Paustenbach, D.J. A physiologically based model for the ingestion of chromium(III) and chromium(VI) by humans. Toxicol. Sci. 2001, 60, 196–213. [Google Scholar] [CrossRef] [Green Version]
- Gali, N.K.; Jiang, S.Y.; Yang, F.; Sun, L.; Ning, Z. Redox characteristics of size-segregated PM from different public transport microenvironments inHong Kong. Air Qual. Atmos. Hlth. 2017, 10, 833–844. [Google Scholar] [CrossRef]
- Johansson, C.; Johansson, P.-Å. Particulate matter in the underground of Stockholm. Atmos. Environ. 2003, 37, 3–9. [Google Scholar] [CrossRef]
- Moreno, T.; Martins, V.; Querol, X.; Jones, T.; BéruBé, K.; Minguillón, M.C.; Amato, F.; Capdevila, M.; de Miguel, E.; Centelles, S.; et al. A new look at inhalable metalliferous airborne particles on rail subway platforms. Sci. Total Environ. 2015, 505, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, H.L.; Nilsson, L.; Möller, L. Subway particles are more genotoxic than street particles and induce oxidative stress in cultured human lung cells. Chem. Res. Toxicol. 2005, 18, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Mammi-Galani, E.; Eleftheriadis, K.; Mendes, L.; Lazaridis, M. Exposure and dose to particulate matter inside the subway system of Athens, Greece. Air Qual. Atmos. Hlth. 2017, 10, 1015–1028. [Google Scholar] [CrossRef]
- Longest, P.W.; Holbrook, L.T. In silico models of aerosol delivery to the respiratory tract—Development and applications. Adv. Drug Deliver. Rev. 2012, 64, 296–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byron, P.R.; Hindle, M.; Lange, C.F.; Longest, P.W.; McRobbie, D.; Oldham, M.J.; Olsson, B.; Thiel, C.G.; Wachtel, H.; Finlay, W.H. In vivo–in vitro correlations: Predicting pulmonary drug deposition from pharmaceutical aerosols. J. Aerosol Med. Pulm. D. 2010, 23, S-59–S-69. [Google Scholar] [CrossRef]
- Tian, G.; Hindle, M.; Lee, S.; Longest, P.W. Validating CFD predictions of pharmaceutical aerosol deposition with in vivo data. Pharm. Res. 2015, 32, 3170–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.P. Exact analysis of aerosol deposition during steady breathing. Powder Technol. 1978, 21, 55–62. [Google Scholar] [CrossRef]
- Choi, J.-I.; Kim, C.S. Mathematical analysis of particle deposition in human lungs: An improved single path transport model. Inhal. Toxicol. 2007, 19, 925–939. [Google Scholar] [CrossRef]
- Deng, Q.; Deng, L.; Miao, Y.; Guo, X.; Li, Y. Particle deposition in the human lung: Health implications of particulate matter from different sources. Environ. Res. 2019, 169, 237–245. [Google Scholar] [CrossRef]
- Karthiga Devi, S.G.; Panchagnula, M.V.; Alladi, M. Designing aerosol size distribution to minimize inter-subject variability of alveolar deposition. J. Aerosol Sci. 2016, 101, 144–155. [Google Scholar] [CrossRef]
- Anjilvel, S.; Asgharian, B. A multiple-path model of particle deposition in the rat lung. Fund. Appl. Toxicol. 1995, 28, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Manigrasso, M.; Buonanno, G.; Fuoco, F.C.; Stabile, L.; Avino, P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ. Pollut. 2015, 196, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Manojikumar, N.; Srimuruganandam, B.; Nagendra, S.M.S. Application of multiple-path particle dosimetry model for quantifying age specified deposition of particulate matter in human airway. Ecotox. Environ. Safe. 2019, 168, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.; Koblinger, L. Monte carlo modeling of aerosol deposition in human lungs. Part II: Deposition fractions and their sensitivity to parameter variations. J. Aerosol Sci. 1990, 21, 675–688. [Google Scholar] [CrossRef]
- Hofmann, W.; Asgharian, B.; Winkler-Heil, R. Modeling intersubject variability of particle deposition in human lungs. J. Aerosol Sci. 2002, 33, 219–235. [Google Scholar] [CrossRef]
- Hofmann, W.; Bergmann, R.; Koblinger, L. Characterization of local particle deposition patterns in human and rat lungs by different morphometric parameters. J. Aerosol Sci. 1999, 30, 651–667. [Google Scholar] [CrossRef]
- Hofmann, W.; Bolt, L.; Sturm, R.; Fleming, J.S.; Conway, J.H. Simulation of three-dimensional particle deposition patterns in human lungs and comparison with experimental spect data. Aerosol Sci. Tech. 2005, 39, 771–781. [Google Scholar] [CrossRef]
- Hofmann, W.; Winkler-Heil, R.; Balásházy, I. The effect of morphological variability on surface deposition densities of inhaled particles in human bronchial and acinar airways. Inhal. Toxicol. 2006, 18, 809–819. [Google Scholar] [CrossRef]
- Sturm, R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal. Toxicol. 2016, 28, 49–60. [Google Scholar] [CrossRef]
- Sturm, R. Theoretical models of carcinogenic particle deposition and clearence in children’s lung. J. Thorac. Dis. 2012, 4, 368–376. [Google Scholar]
- Kolanjiyil, A.V.; Kleinstreuer, C. Nanoparticle mass transfer from lung airways to systemic regions—part I: Whole-lung aerosol dynamics. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z. Airflow and particle transport in the human respiratory system. Annu. Rev. Fluid Mech. 2009, 42, 301–334. [Google Scholar] [CrossRef]
- Kolanjiyil, A.V.; Kleinstreuer, C. Nanoparticle mass transfer from lung airways to systemic regions—part II: Multi-compartmental modeling. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef] [PubMed]
- Kolanjiyil, A.V.; Kleinstreuer, C.; Sadikot, R.T. Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part II: Dry powder inhaler application. Comput. Biol. Med. 2017, 84, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kolanjiyil, A.V.; Kleinstreuer, C. Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part I: Theory and model validation. Comput. Biol. Med. 2016, 79, 193–204. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—a review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Kleven, M.; Melaaen, M.C.; Djupesland, P.E.R.G. Computational fluid dynamics (CFD) applied in the drug delivery design process to the nasal passages: A review. J. Mech. Med. Biol. 2012, 12, 1230002. [Google Scholar] [CrossRef]
- Aasgrav, E.; Johnsen, S.G.; Simonsen, A.J.; Muller, B. CFD simulations of turbulent flow in the human upper airways. In Proceedings of the 12th International Conference on CFD in Oil & Gas, Metallurgical and Process Industries, Trondheim, Norway, 2017. [Google Scholar]
- Sharma, A. Introduction to Computational Fluid Dynamics: Development, Application, and Analysis; Wiley: West Sussex, UK, 2017. [Google Scholar]
- Surana, K.S.; Allu, S.; Tenpas, P.W.; Reddy, J.N. K-version of finite element method in gas dynamics: Higher-order global differentiability numerical solutions. Int. J. Numer. Meth. Eng. 2007, 69, 1109–1157. [Google Scholar] [CrossRef]
- Huebner, K.H.; Dewhrist, D.L.; Smith, D.E.; Byrom, T.G. The Finite Element Method for Engineers, 4th ed.; Wiley: West Sussex, UK, 2001; p. 720. [Google Scholar]
- Tang, Y.; Guo, B. Computational fluid dynamics simulation of aerosol transport and deposition. Front. Env. Sci. Eng. China 2011, 5, 362–377. [Google Scholar] [CrossRef]
- Zhang, Z.; Kleinstreuer, C. Laminar-to-turbulent fluid–nanoparticle dynamics simulations: Model comparisons and nanoparticle-deposition applications. Int. J. Numer. Meth. Bio. 2011, 27, 1930–1950. [Google Scholar] [CrossRef]
- Houzeaux, G.; Borrell, R.; Fournier, Y.; Garcia-Gasulla, M.; Gobbert, J.H.; Hachem, E.; Mehta, V.; Mesri, Y.; Owen, H.; Vazquez, M. High-Performance Computing: Dos and Don’ts; Ionescu, A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Lintermann, A. Efficient parallel geometry distribution for the simulation of complex flows. In ECCOMAS Congress; ECCOMAS: Crete, Greece, 2016. [Google Scholar]
- Longest, P.W.; Tian, G.; Khajeh-Hosseini-Dalasm, N.; Hindle, M. Validating whole-airway CFD predictions of DPI aerosol deposition at multiple flow rates. J. Aerosol Med. Pulm. D. 2016, 29, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, C.; Shi, H.; Zhang, Z. Computational analyses of a pressurized metered dose inhaler and a new drug–aerosol targeting methodology. J. Aerosol Med. 2007, 20, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Vinchurkar, S.; De Backer, L.; Vos, W.; Van Holsbeke, C.; De Backer, J.; De Backer, W. A case series on lung deposition analysis of inhaled medication using functional imaging based computational fluid dynamics in asthmatic patients: Effect of upper airway morphology and comparison with in vivo data. Inhal. Toxicol. 2012, 24, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lintermann, A.; Schröder, W. Simulation of aerosol particle deposition in the upper human tracheobronchial tract. Eur. J. Mech. B-Fluid. 2017, 63, 73–89. [Google Scholar] [CrossRef]
- Calmet, H.; Yamamoto, T.; Eguzkitza, B.; Lehmkuhl, O.; Olivares, E.; Kobayashi, Y.; Tomoda, K.; Houzeaux, G.; Vázquez, M. Numerical evaluation of aerosol exhalation through nose treatment. J. Aerosol Sci. 2019, 128, 1–13. [Google Scholar] [CrossRef]
- Calmet, H.; Gambaruto, A.M.; Bates, A.J.; Vázquez, M.; Houzeaux, G.; Doorly, D.J. Large-scale CFD simulations of the transitional and turbulent regime for the large human airways during rapid inhalation. Comput. Biol. Med. 2016, 69, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-L.; Tawhai, M.H.; McLennan, G.; Hoffman, E.A. Multiscale simulation of gas flow in subject-specific models of the human lung. IEEE Eng. Med. Biol. 2009, 28, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Kleinstreuer, C.; Zhang, Z. An adjustable triple-bifurcation unit model for air-particle flow simulations in human tracheobronchial airways. J. Biomech. Eng. 2008, 131. [Google Scholar] [CrossRef]
- Walters, D.K.; Luke, W.H. A method for three-dimensional Navier–Stokes simulations of large-scale regions of the human lung airway. J. Fluid. Eng. 2010, 132. [Google Scholar] [CrossRef]
- Walters, D.K.; Luke, W.H. Computational fluid dynamics simulations of particle deposition in large-scale, multigenerational lung models. J. Biomech. Eng. 2010, 133. [Google Scholar] [CrossRef]
- Paiva, M.; Engel, L.A. Pulmonary interdependence of gas transport. J. Appl. Physiol. 1979, 47, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Biancofiore, F.; Busilacchio, M.; Verdecchia, M.; Tomassetti, B.; Aruffo, E.; Bianco, S.; Di Tommaso, S.; Colangeli, C.; Rosatelli, G.; Di Carlo, P. Recursive neural network model for analysis and forecast of PM10 and PM2.5. Atmos. Pollut. Res. 2017, 8, 652–659. [Google Scholar] [CrossRef]
- Grivas, G.; Chaloulakou, A. Artificial neural network models for prediction of PM10 hourly concentrations, in the greater area of Athens, Greece. Atmos. Environ. 2006, 40, 1216–1229. [Google Scholar] [CrossRef]
- Perez, P.; Reyes, J. An integrated neural network model for PM10 forecasting. Atmos. Environ. 2006, 40, 2845–2851. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Kim, M.; Bui, V.K.H.; Park, D.; Lee, Y.-C. Particulate matter exposure of passengers at bus stations: A review. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [Green Version]
- Nazir, J.; Barlow, D.J.; Lawrence, M.J.; Richardson, C.J.; Shrubb, I. Artificial neural network prediction of aerosol deposition in human lungs. Pharm. Res. 2002, 19, 1130–1136. [Google Scholar] [CrossRef]
- Nazir, J.; Barlow, D.J.; Lawrence, M.J.; Shrubb, I. Artificial neural network prediction of the patterns of deposition of polydisperse aerosols within human lungs. J. Pharm. Sci. 2005, 94, 1986–1997. [Google Scholar] [CrossRef]
- Emami, J. In vitro - in vivo Correlation: From Theory to Applications. J. Pharm. Pharm. Sci. 2006; Volume 9, 169–189. [Google Scholar]
- Matas, M.d.; Shao, Q.; Richardson, C.H.; Chrystyn, H. Evaluation of in vitro in vivo correlations for dry powder inhaler delivery using artificial neural networks. Eur. J. Pharm. Sci. 2008, 33, 80–90. [Google Scholar] [CrossRef]
- Muddle, J.; Kirton, S.B.; Muddle, I.P.A.; Murnane, D.; Ali, J.; Brown, M.; Page, C.; Forbes, B. Predicting the fine particle fraction of dry powder inhalers using artificial neural networks. J. Pharm. Sci. 2017, 106, 313–321. [Google Scholar] [CrossRef] [Green Version]







| RT Region | Monodisperse 5 μm | Polydisperse 5 μm (σg = 2.5) | ||||
|---|---|---|---|---|---|---|
| ExDoM | LUDEP | MPPD | ExDoM | LUDEP | MPPD | |
| ET | 92.76 (43.14) | 89.78 (41.76) | 91.00 | 85.41 (39.56) | 75.73 (34.79) | 84.10 |
| TB | 3.72 (2.57) | 3.59 (2.48) | 2.60 | 2.78 (1.88) | 2.68 (1.80) | 2.80 |
| AL | 2.77 | 2.68 | 5.80 | 4.45 | 4.46 | 5.60 |
| Total | 99.25 | 96.05 | 99.40 | 92.64 | 82.87 | 92.50 |
| Type of Models | Remarkable Models | Advantages | Disadvantages | Remarkable Recent Updates | Availability | References |
|---|---|---|---|---|---|---|
| Semi-empirical models |
|
|
|
| [12,38,51] | |
| 1-D whole-lung models |
|
|
|
|
| [57,61,67] |
| 3-D CFD models |
|
|
|
|
| [72,73] |
| Artificial neural networks |
|
|
|
| [99,100,102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, V.K.H.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Prediction of Aerosol Deposition in the Human Respiratory Tract via Computational Models: A Review with Recent Updates. Atmosphere 2020, 11, 137. https://doi.org/10.3390/atmos11020137
Bui VKH, Moon J-Y, Chae M, Park D, Lee Y-C. Prediction of Aerosol Deposition in the Human Respiratory Tract via Computational Models: A Review with Recent Updates. Atmosphere. 2020; 11(2):137. https://doi.org/10.3390/atmos11020137
Chicago/Turabian StyleBui, Vu Khac Hoang, Ju-Young Moon, Minhe Chae, Duckshin Park, and Young-Chul Lee. 2020. "Prediction of Aerosol Deposition in the Human Respiratory Tract via Computational Models: A Review with Recent Updates" Atmosphere 11, no. 2: 137. https://doi.org/10.3390/atmos11020137





