Characteristics, Secondary Formation and Regional Contributions of PM2.5 Pollution in Jinan during Winter
Abstract
:1. Introduction
2. Experiments
2.1. Site Location and Data Collection
2.2. Weighing and Chemical Analysis
3. Results
3.1. General Characteristics of PM2.5 Pollution in Winter
3.2. Chemical Compositions of Different Pollution Levels
4. Discussion
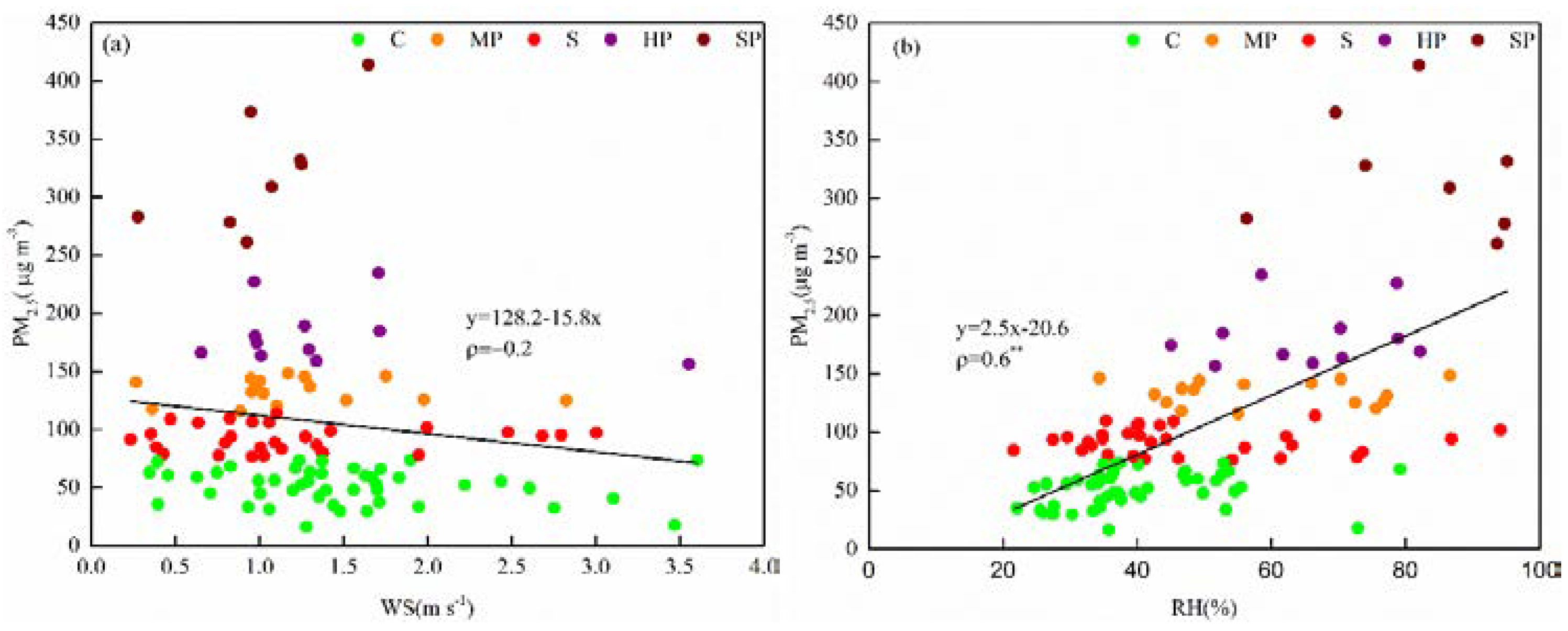
4.1. Meteorological Conditions
4.2. Secondary Formation
4.3. Regional Transport
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, H.; Chen, J. Formation, features and controlling strategies of severe haze-fog pollutions in China. Sci. Total Environ. 2017, 578, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Dui, W. Hazy weather research in China in the last decade: A review. Acta Sci. Circumstantiae 2012, 32, 257–269. [Google Scholar] [CrossRef]
- Han, S.-Q.; Wu, J.-H.; Zhang, Y.-F.; Cai, Z.-Y.; Feng, Y.-C.; Yao, Q.; Li, X.-J.; Liu, Y.-W.; Zhang, M. Characteristics and formation mechanism of a winter haze–fog episode in Tianjin, China. Atmos. Environ. 2014, 98, 323–330. [Google Scholar] [CrossRef]
- Hu, Q.; Fu, H.; Wang, Z.; Kong, L.; Chen, M.; Chen, J. The variation of characteristics of individual particles during the haze evolution in the urban Shanghai atmosphere. Atmos. Res. 2016, 181, 95–105. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Zhang, J.; Wen, T.; Ji, D.; Wang, Y. Seasonal variation and secondary formation of size-segregated aerosol water-soluble inorganic ions during pollution episodes in Beijing. Atmos. Res. 2016, 168, 70–79. [Google Scholar] [CrossRef]
- Lai, S.C.; Zhao, Y.; Ding, A.J.; Zhang, Y.Y.; Song, T.L.; Zheng, J.Y.; Ho, K.F.; Lee, S.C.; Zhong, L.J. Characterization of PM2.5 and the major chemical components during a 1-year campaign in rural Guangzhou, Southern China. Atmos. Res. 2016, 167, 208–215. [Google Scholar] [CrossRef]
- Wu, Y.; Ge, X.; Wang, J.; Shen, Y.; Ye, Z.; Ge, S.; Wu, Y.; Yu, H.; Chen, M. Responses of secondary aerosols to relative humidity and photochemical activities in an industrialized environment during late winter. Atmos. Environ. 2018, 193, 66–78. [Google Scholar] [CrossRef]
- Sun, Z.; Mu, Y.; Liu, Y.; Shao, L. A comparison study on airborne particles during haze days and non-haze days in Beijing. Sci. Total Environ. 2013, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, Y.L.; Duan, F.K.; He, K.B.; Zhu, L.D.; Huang, T.; Kimoto, T.; Ma, X.; Ma, T.; Xu, L.L.; et al. Typical winter haze pollution in Zibo, an industrial city in China: Characteristics, secondary formation, and regional contribution. Environ. Pollut. 2017, 229, 339–349. [Google Scholar] [CrossRef]
- Xu, L.; Duan, F.; He, K.; Ma, Y.; Zhu, L.; Zheng, Y.; Huang, T.; Kimoto, T.; Ma, T.; Li, H.; et al. Characteristics of the secondary water-soluble ions in a typical autumn haze in Beijing. Environ. Pollut. 2017, 227, 296–305. [Google Scholar] [CrossRef]
- Tian, S.L.; Pan, Y.P.; Liu, Z.R.; Wen, T.X.; Wang, Y.S. Size-resolved aerosol chemical analysis of extreme haze pollution events during early 2013 in urban Beijing, China. J. Hazard. Mater. 2014, 279, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Yang, L.; Gao, X.; Nie, W.; Yu, Y.; Xu, P.; Zhou, Y.; Wang, Z. The secondary formation of inorganic aerosols in the droplet mode through heterogeneous aqueous reactions under haze conditions. Atmos. Environ. 2012, 63, 68–76. [Google Scholar] [CrossRef]
- Lv, C.; Wang, X.; Pang, N.; Wang, L.; Wang, Y.; Xu, T.; Zhang, Y.; Zhou, T.; Li, W. The impact of airborne particulate matter on pediatric hospital admissions for pneumonia among children in Jinan, China: A case-crossover study. J. Air Waste Manag. Assoc. 2017, 67, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, M.; Sun, H.; Wang, X.; Wang, Y.; Li, Y.; Zhang, L.; Mu, Z. Enhanced health risks from exposure to environmentally persistent free radicals and the oxidative stress of PM2.5 from Asian dust storms in Erenhot, Zhangbei and Jinan, China. Environ. Int. 2018, 121, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yang, L.; Yan, C.; Yuan, Q.; Yu, Y.; Wang, W. Particle size distributions, PM2.5 concentrations and water-soluble inorganic ions in different public indoor environments: A case study in Jinan, China. Front. Environ. Sci. Eng. 2013, 7, 55–65. [Google Scholar] [CrossRef]
- Gu, J.; Du, S.; Han, D.; Hou, L.; Yi, J.; Xu, J.; Liu, G.; Han, B.; Yang, G.; Bai, Z.-P. Major chemical compositions, possible sources, and mass closure analysis of PM2.5 in Jinan, China. Air Qual. Atmos. Health 2014, 7, 251–262. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, L.; Zhou, X.; Wang, Z.; Zhou, Y.; Gao, X.; Nie, W.; Wang, X.; Xu, P.; Wang, W. Evaluating PM2.5 ionic components and source apportionment in Jinan, China from 2004 to 2008 using trajectory statistical methods. J. Environ. Monit. 2011, 13, 1662–1671. [Google Scholar] [CrossRef]
- Jinan Environmental Quality Brief of 2017; Jinan Environmental Protection Bureau: Jiana, China, 2017.
- Zhao, X.; Gu, C.; Yang, H.; Wang, J.; Yang, W.; Wang, X.; Bai, Z. Chemical composition and source apportionment of PM2.5 during a winter air pollution episode in the KuiDu-Wu Area of Xinjiang Uygur Autonomous Region. Res. Environ. Sci. 2017, 30, 1515–1523. [Google Scholar]
- Feng, J.; Yu, H.; Su, X.; Liu, S.; Li, Y.; Pan, Y.; Sun, J.-H. Chemical composition and source apportionment of PM2.5 during Chinese Spring Festival at Xinxiang, a heavily polluted city in North China: Fireworks and health risks. Atmos. Res. 2016, 182, 176–188. [Google Scholar] [CrossRef]
- Zhang, N.; Han, B.; Zhao, R.; Zhao, X.; Xu, J.; Zhang, Y.; Bai, Z. Source profile and excess cancer risk evaluation of environmental tobacco smoking under real conditions, China. Atmos. Pollut. Res. 2019, 10, 1994–1999. [Google Scholar] [CrossRef]
- Sillanpää, M.; Hillamo, R.; Saarikoski, S.; Frey, A.; Pennanen, A.; Makkonen, U.; Spolnik, Z.; Van Grieken, R.; Braniš, M.; Brunekreef, B.; et al. Chemical composition and mass closure of particulate matter at six urban sites in Europe. Atmos. Environ. 2006, 40, 212–223. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.J. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Koulouri, E.; Saarikoski, S.; Theodosi, C.; Markaki, Z.; Gerasopoulos, E.; Kouvarakis, G.; Makela, T.; Hillamo, R.; Mihalopoulos, N. Chemical composition and sources of fine and coarse aerosol particles in the Eastern Mediterranean. Atmos. Environ. 2008, 42, 6542–6550. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Wang, Z.; Zhou, Y.; Cheng, S.; Xu, P.; Gao, X.; Nie, W.; Wang, X.; Wang, W. Airborne fine particulate pollution in Jinan, China: Concentrations, chemical compositions and influence on visibility impairment. Atmos. Environ. 2012, 55, 506–514. [Google Scholar] [CrossRef]
- Chow, J.C.; Lowenthal, D.H.; Chen, L.W.; Wang, X.; Watson, J.G. Mass reconstruction methods for PM2.5: A review. Air Qual. Atmos. Health 2015, 8, 243–263. [Google Scholar] [CrossRef] [Green Version]
- Tai, A.P.K.; Mickley, L.J.; Jacob, D.J. Correlations between fine particulate matter (PM2.5) and meteorological variables in the United States: Implications for the sensitivity of PM2.5 to climate change. Atmos. Environ. 2010, 44, 3976–3984. [Google Scholar] [CrossRef]
- Quan, J.N.; Tie, X.X.; Zhang, Q.; Liu, Q.; Li, X.; Gao, Y.; Zhao, D.L. Characteristics of heavy aerosol pollution during the 2012–2013 winter in Beijing, China. Atmos. Environ. 2014, 88, 83–89. [Google Scholar] [CrossRef]
- Zheng, G.J.; Duan, F.K.; Su, H.; Ma, Y.L.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D.; et al. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef] [Green Version]
- Tang, I.N.; Munkelwitz, H.R. Aerosol Phase Transformation and Growth in the Atmosphere. J. Appl. Meteorol. 1994, 33, 791–796. [Google Scholar] [CrossRef]
- Tianxue, Q.; Chen, J.; Yin, L.; Niu, Z.; Zhang, F.; Xu, L. Study on the characteristics of water-soluble inorganic ions in PM2.5 in spring in the key cities of sourthern Fujian province. J. Environ. Sci. 2013, 22, 512–516. [Google Scholar] [CrossRef]
- Cheng, Y.; He, K.B.; Du, Z.Y.; Zheng, M.; Duan, F.K.; Ma, Y.L. Humidity plays an important role in the PM2.5 pollution in Beijing. Environ. Pollut. 2015, 197, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Tian, H.Z.; Cheng, K.; Lu, L.; Zheng, M.; Wang, S.X.; Hao, J.M.; Wang, K.; Hua, S.B.; Zhu, C.Y.; et al. The variation of chemical characteristics of PM2.5 and PM10 and formation causes during two haze pollution events in urban Beijing, China. Atmos. Environ. 2015, 107. [Google Scholar] [CrossRef]
- Ji, D.S.; Wang, Y.S.; Wang, L.L.; Chen, L.F.; Hu, B.; Tang, G.Q.; Xin, J.Y.; Song, T.; Wen, T.X.; Sun, Y.; et al. Analysis of heavy pollution episodes in selected cities of northern China. Atmos. Environ. 2012, 50, 338–348. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, G.; Tang, A.; Wang, Y.; An, Z. Chemical Characteristics of PM2.5 and PM10 in Haze−Fog Episodes in Beijing. Environ. Sci. Technol. 2006, 40, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, R.; Gomez, M.E.; Yang, L.; Levy Zamora, M.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.J.; Zhao, P.S.; Xu, J.; Meng, W.; Pu, W.W.; Dong, F.; He, D.; Shi, Q.F. Analysis of a winter regional haze event and its formation mechanism in the North China Plain. Atmos. Chem. Phys. 2013, 13, 5685–5696. [Google Scholar] [CrossRef] [Green Version]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd ed.; John Wiley: New York, NY, USA, 2006. [Google Scholar]
- Cheng, Y.; Zheng, G.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.; Gao, M.; Zhang, Q.; He, K.; Carmichael, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, e1601530. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Zhang, X.Y.; Draxler, R.R. TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environ. Model. Softw. 2009, 24, 938–939. [Google Scholar] [CrossRef]
- Kong, S.; Han, B.; Bai, Z.; Chen, L.; Shi, J.; Xu, Z. Receptor modeling of PM2.5, PM10 and TSP in different seasons and long-range transport analysis at a coastal site of Tianjin, China. Sci. Total Environ. 2010, 408, 4681–4694. [Google Scholar] [CrossRef]
- Hu, J.; Chai, F.; Duan, J.; Yi, P.; Chu, Y.; Xie, D. Explosive Growth of PM2.5 during the Autumn and Winter Seasons in the Jing-Jin-Ji and Surrounding Area and Its Control Measures with Emergency Response. Res. Environ. Sci. 2019, 32, 1704–1712. [Google Scholar] [CrossRef]








| PM2.5 | PM2.5/PM10 | |||
|---|---|---|---|---|
| Average | SDa | Median | ||
| μg m−3 | ||||
| mass | 107.1 | 75.4 | 86.8 | 0.6 |
| MDb | 8.5 | 5.2 | 7 | 0.2 |
| TEb | 1.1 | 0.7 | 1 | 0.5 |
| OMb | 19 | 11.4 | 15.8 | 0.8 |
| EC | 4.9 | 3.4 | 4 | 0.7 |
| SO42− | 19 | 21.2 | 12.4 | 0.8 |
| NO3− | 21.9 | 15.6 | 17.8 | 0.8 |
| NH4+ | 11.6 | 10.7 | 8.8 | 0.8 |
| Cl− | 4.1 | 3 | 3.3 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, J.; Zhao, X.; Wang, J.; Wang, X.; Hou, L.; Yang, W.; Han, B.; Bai, Z. Characteristics, Secondary Formation and Regional Contributions of PM2.5 Pollution in Jinan during Winter. Atmosphere 2020, 11, 273. https://doi.org/10.3390/atmos11030273
Liu Y, Wang J, Zhao X, Wang J, Wang X, Hou L, Yang W, Han B, Bai Z. Characteristics, Secondary Formation and Regional Contributions of PM2.5 Pollution in Jinan during Winter. Atmosphere. 2020; 11(3):273. https://doi.org/10.3390/atmos11030273
Chicago/Turabian StyleLiu, Yingying, Jing Wang, Xueyan Zhao, Jian Wang, Xinhua Wang, Lujian Hou, Wen Yang, Bin Han, and Zhipeng Bai. 2020. "Characteristics, Secondary Formation and Regional Contributions of PM2.5 Pollution in Jinan during Winter" Atmosphere 11, no. 3: 273. https://doi.org/10.3390/atmos11030273





