Pollution Characteristics of Atmospheric Carbonyls in Urban Linfen in Winter
Abstract
:1. Introduction
2. Materials and Methods
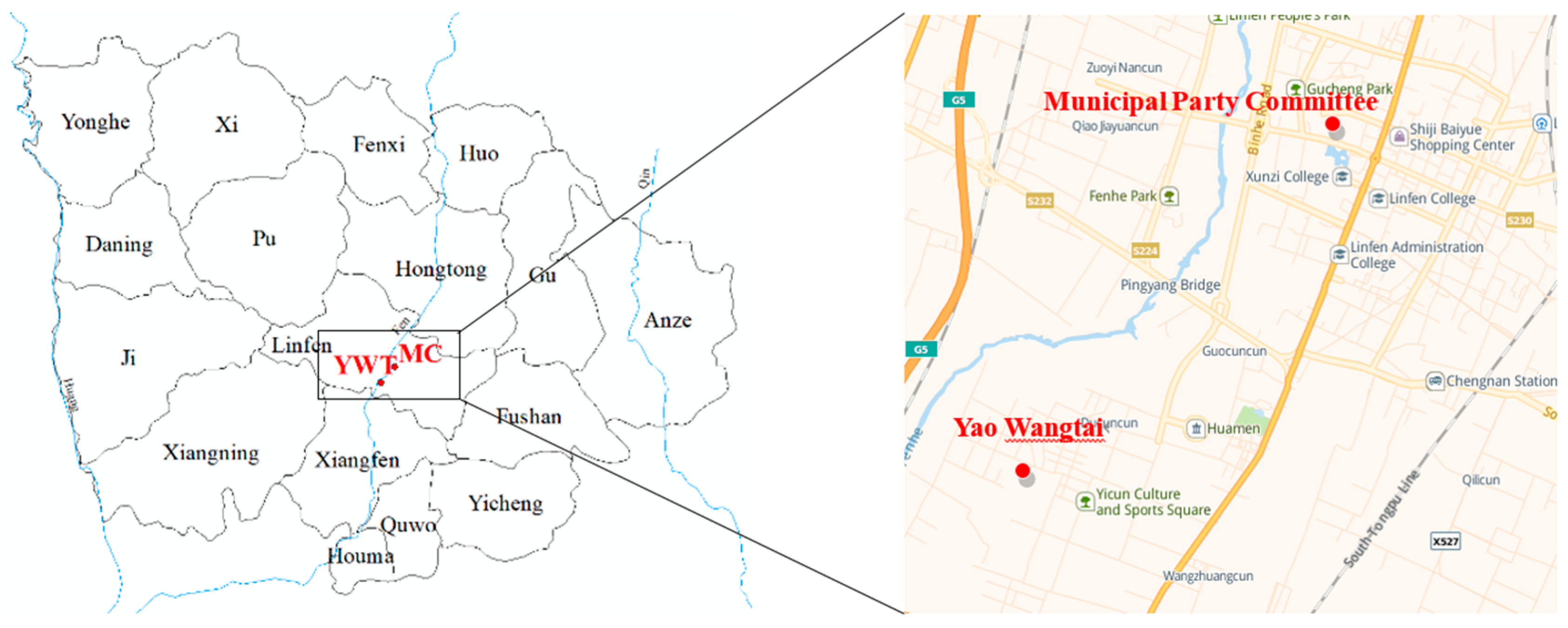
2.1. Sampling Sites and Times
2.2. Monitored Parameters
2.3. Sample Collection and Analysis
2.4. Quality Assurance and Quality Control
2.5. Methods of Contaminated Air Mass Transport Research
3. Results and Discussion
3.1. Levels of Atmospheric Carbonyls in Urban Linfen in Winter
3.2. Diurnal Variations of Same Key Carbonyls at the Two Sites
3.3. Diurnal Variations of Different Carbonyls at the Same Sampling Site
3.4. Influence of Wind Speed and Wind Direction on the Atmospheric Concentration of Key Carbonyls in Urban Linfen in Winter
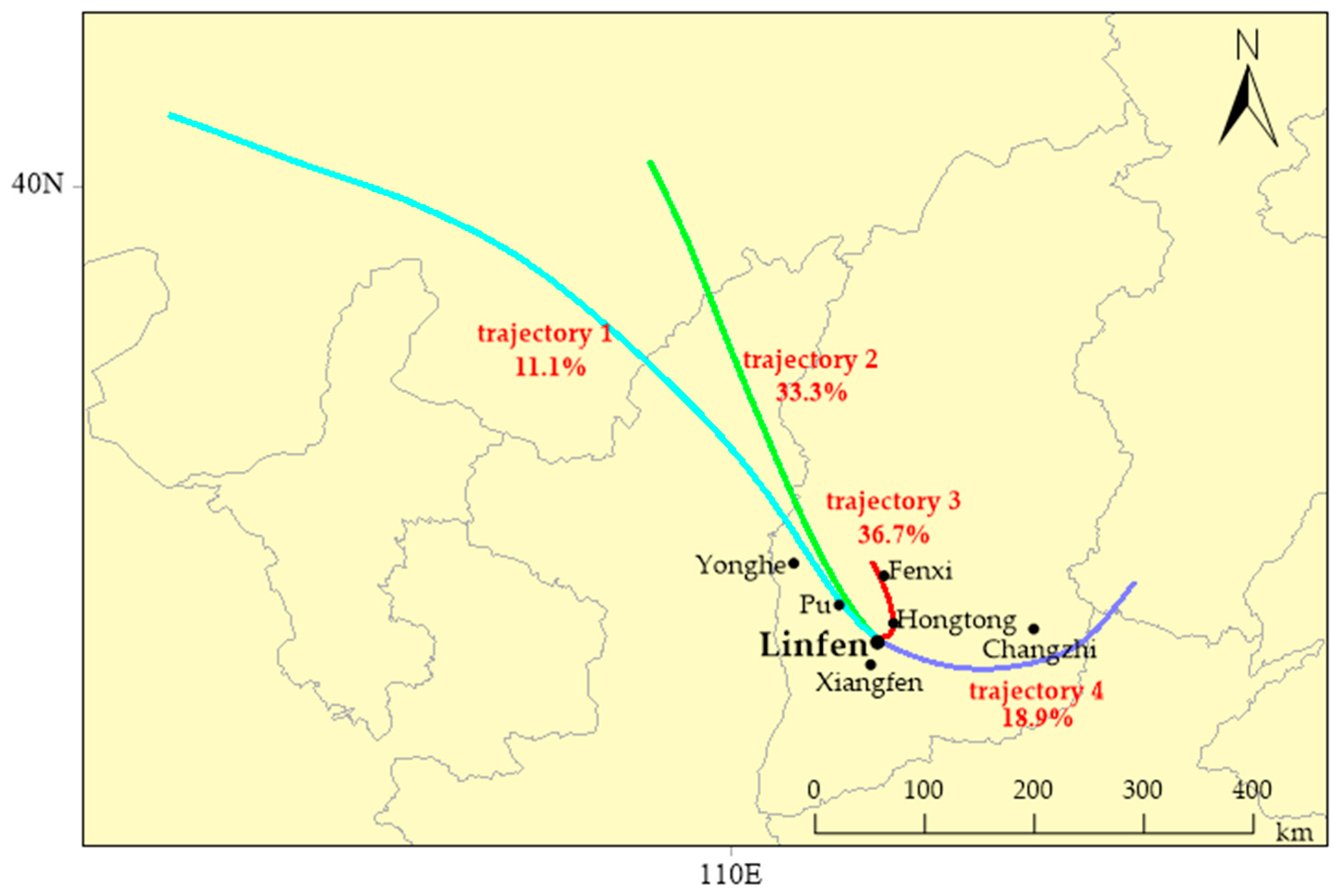
3.5. Analysis of the Transport Paths and Potential Source Areas of Atmospheric Carbonyls in Urban Linfen
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Li, X. Study Advance on Oxygenated Volatile Organic Compounds in Atmosphere. J. Cap. Norm. Univ. (Nat. Sci. Ed.) 2018, 039, 45–55. [Google Scholar]
- Tan, P.-G.; Yu, Y.B.; Jiang, H.W.; Liu, Z. Analysis and concentration variability of carbonyl compounds in Qingdao atmosphere. China Environ. Sci. 2002, 22, 451–455. [Google Scholar]
- Xue, Y. Charanteristics and Source of Carbonyls and Effects on Photochemistry Pollution in North China Plain; Shandong University: Jinan, China, 2018. [Google Scholar]
- Liu, Y. Study on Atmospheric Methane in Beijing: Emission from Rice Paddies and Consumption by Oxidizing; Chinese Rwsearch Academy of Environmental Sciences: Beijing, China, 1995. [Google Scholar]
- Lv, W. The Study on the Spatial-Temporal Distribution of Volatile Carbonyls in Atmosphere of the Pearl River Delta; Jinan University: Guangzhou, China, 2007. [Google Scholar]
- Xu, J.-Y.; Gao, Y. Seasonal Variations of Carbonyl Compounds in Urban Atmosphere of Beijing. Environ. Sci. 2009, 30, 625–630. [Google Scholar]
- Wang, Q.; Shao, M.; Wei, Q.; Chen, W.T.; Lu, S.H.; Zhao, Y. Spatial and Temporal Variations of Ambient Carbonyl Compounds in Beijingand Its Surrounding Areas. Environ. Sci. 2011, 32, 3522–3530. [Google Scholar]
- Rao, Z.; Chen, Z.; Liang, H.; Huang, L.; Huang, D. Carbonyl compounds over urban Beijing: Concentrations on haze and non-haze days and effects on radical chemistry. Atmos. Environ. 2016, 124, 207–216. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Y.; Li, J.; Xiong, B.; Feng, J.; Wen, S.; Sheng, G.; Fu, J.; Wu, M. Characteristics of carbonyl compounds in ambient air of Shanghai, China. J. Atmos. Chem. 2008, 61, 1–20. [Google Scholar] [CrossRef]
- Jing, S. Study on the level of ambient carbonyl compounds in typical regions of Shanghai. Environ. Pollut. Control 2017, 39, 713–716. [Google Scholar]
- Shi, J.-W.; Pang, X.-B.; Bai, Z.-P.; Jin, L.-M.; Li, W.-F.; Kong, S.-F. Measurement of Carbonyl Compounds in Ambient Air of Tianjin City and Bohai Sea. J. Tianjin Univ. 2011, 3, 49–57. [Google Scholar]
- Jiang, Z.; Wang, Y.; Zheng, X.; Liu, X.; Xia, C.; Xiao, X.; Li, S. Variation Characteristics of Atmospheric Carbonyl Compounds in Zhangjiajie Forest. Res. Environ. Sci. 2016, 29, 1272–1278. [Google Scholar]
- US EPA. Compendium Method TO-11A Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC): Active Sampling Methodology; Center for Environmental Research Information Office of Research and Development: Cincinnati, OH, USA, 1999.
- Ren, C.B.; Wu, L.X.; Zhang, Y.Y. Analyze to the seasonal differences of transport pathways and potential source-zones of Beijing Urban PM2.5. China Environ. Sci. 2016, 36, 2591–2598. [Google Scholar]
- Wang, G.; Wang, D.; Chen, Z. Characteristics and transportation pathways and potential sources of a severe pm2.5 episodes during winter in beijing. China Environ. Sci. 2016, 36, 1931–1937. [Google Scholar]
- Begum, B.A.; Kim, E.; Jeong, C.H.; Lee, D.W.; Hopke, P.K. Evaluation of the potential source contribution function using the 2002 Quebec forest fire episode. Atmos. Environ. 2005, 39, 3719–3724. [Google Scholar] [CrossRef]
- Xu, X.; Akhtar, U.S. Identification of potential regional sources of atmospheric total gaseous mercury in Windsor, Ontario, Canada using hybrid receptor modeling. Atmos. Chem. Phys. 2010, 10, 7073–7083. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Zhang, X.Y.; Draxler, R.R. TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environ. Model. Softw. 2009, 24, 938–939. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, B.; Yin, Y.; Jin, L.; Zhang, L. Aerosol number concentration properties and potential sources areas transporting to the top of mountain Huangshan in summer. China Environ. Sci. 2014, 34, 852–861. [Google Scholar]
- Hsu, Y.K.; Holsen, T.M.; Hopke, P.K. Comparison of hybrid receptor models to locate PCB sources in Chicago. Atmos. Environ. 2003, 37, 545–562. [Google Scholar] [CrossRef]
- Pongkiatkul, P.; Oanh, N.T.K. Assessment of potential long-range transport of particulate air pollution using trajectory modeling and monitoring data. Atmos. Res. 2007, 85, 3–17. [Google Scholar] [CrossRef]
- Byčenkienė, S.; Plauškaitė, K.; Dudoitis, V.; Ulevicius, V. Urban background levels of particle number concentration and sources in Vilnius, Lithuania. Atmos. Res. 2014, 143, 279–292. [Google Scholar] [CrossRef]
- Liu, N.; Ye, Y.; He, J.; Zhao, S. Integrated modeling ofurban-scale pollutant transport: Application in a semi-arid urban valley, Northwestern China. Atmos. Pollut. Res. 2013, 4, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Riuttanen, L.; Hulkkonen, M.; Dal Maso, M.; Junninen, H.; Kulmala, M. Trajectory analysis of atmospheric transport of fine particles, SO2,NOxand O3 to the SMEAR II station in Finland in 1996–2008. Atmos. Chem. Phys. 2013, 13, 2153–2164. [Google Scholar] [CrossRef] [Green Version]
- Jeong, U.; Kim, J.; Lee, H.; Jung, J.; Kim, Y.J.; Song, C.H.; Koo, J.-H. Estimation of the contributions of long range transported aerosol in EastAsia to carbonaceous aerosol and PM concentrations in Seoul, Koreausing highly time resolved measurements: A PSCF model approach. J. Environ. Monit. 2011, 13, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Kabashnikov, V.P.; Chaikovsky, A.P.; Kucsera, T.L.; Metelskaya, N.S. Estimated accuracy of three common trajectory statistical methods. Atmos. Environ. 2011, 45, 5425–5430. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Tan, J.-H.; Xiang, P.; He, X.L.; Guo, S.J.; Duan, J.C.; He, K.B.; Ma, Y.L.; Deng, S.X.; Situ, S.P. Chemical characteristics of atmospheric carbonyls in winter and summer in Foshan City. China Environ. Sci. 2017, 37, 844–850. [Google Scholar]
- Wang, L.-L.; Wang, W.-S.; Zhao, X.-N.; Xu, X.-L. Pollution Characteristics and Sources of Carbonyl Compounds in Zhengzhou Ambient Air of Winter and Summer; Environmental Monitoring in China: Henan, China, 2014. [Google Scholar]
- Fan, Z.; Ma, W.; He, Y.; Wang, F. Pollution characterizations of aldehyde and ketones in haze air during winter domestic heating season of Xi’an. Environ. Pollut. Control. 2019, 41, 88–91. [Google Scholar]
- Possanzini, M.; Tagliacozzo, G.; Cecinato, A. Ambient Levels and Sources of Lower Carbonyls at Montelibretti, Rome (Italy). Water Air Soil Pollut. 2007, 183, 447–454. [Google Scholar] [CrossRef]
- Cavalcante, R.M.; Campelo, C.S.; Barbosa, M.J.; Silveira, E.R.; Carvalho, T.V.; Nascimento, R.F. Determination of carbonyl compounds in air and cancer risk assessment in an academic institute in Fortaleza, Brazil. Atmos. Environ. 2006, 40, 5701–5711. [Google Scholar] [CrossRef]
- Jiang, Z.; Grosselin, B.; Daële, V.; Mellouki, A.; Mu, Y. Seasonal, diurnal and nocturnal variations of carbonyl compounds in the semi-urban environment of Orléans, France. J. Environ. Sci. 2016, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, Y.; Gao, X.; Jia, S.; Xu, X.; Cheng, H.; Meng, Z. Characteristics of Ambient Formaldehyde at Two Rural Sites in the North China Plain in Summer. Res. Environ. Sci. 2016, 29, 1119–1127. [Google Scholar]
- Shi, Y.-Z.; Wang, G.-C.; Xu, Y.-F. Simulation Study of Photochemical Smog in Beijing Urban and Suburban Areas. Clim. Environ. Res. 2008, 13, 84–92. [Google Scholar]
- Liu, J.; Chu, B.; He, H. Diurnal Variation of SOA Formation Potential from Ambient Air at an Urban Site in Beijing. Environ. Sci. 2018, 39, 2505–2511. [Google Scholar]
- Anderson, L.G.; Lanning, J.A.; Barrell, R.; Miyagishima, J.; Jones, R.H.; Wolfe, P. Sources and sinks of formaldehyde and acetaldehyde: An analysis of Denver’s ambient concentration data. Atmos. Environ. 1996, 30, 2113–2123. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Louie, P.K.; Zou, S.C. Seasonal variation of carbonyl compounds concentrations in urban area of Hong Kong. Atmos. Environ. 2002, 36, 1259–1265. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, H.R.; Wang, Z.W.; Peng, J.; Zhu, J.X.; Lyu, X.P.; Guo, H. Chemical characteristics of atmospheric carbonyl compounds and source identification of formaldehyde in Wuhan, Central China. Atmos. Res. 2019, 228, 95–106. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Kuster, W.C.; Goldan, P.D.; Li, X.; Lu, S.; Gouw, J.A.D. Source identification of reactive hydrocarbons and oxygenated VOCs in the summertime in Beijing. Environ. Sci. Technol. 2009, 43, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Biesenthal, T.A.; Shepson, P.B. Observations of anthropogenic inputs of the isoprene oxidation products methyl vinyl ketone and methacrolein to the atmosphere. Geophys. Res. Lett. 1997, 24, 1375–1378. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kawai, S.; Igawa, M. Dominant factors controlling concentrations of aldehydes in rain, fog, dew water, and in the gas phase. Atmos. Environ. 2005, 39, 7321–7329. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Koppmann, R. (Ed.) Volatile Organic Compounds in the Atmosphere; Blackwell Publishing: Oxford, UK, 2007; p. 512. ISBN 978-1-4051-3115-5. [Google Scholar]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Grosjean, D. Formaldehyde and Other Carbonyls in Ambient Air. Los Angeles Environ. Sci. Technol. 1982, 16, 254–262. [Google Scholar] [CrossRef]





| Compounds | MC (μg/m3) | YWT (μg/m3) | LOD (μg/m3) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | Mean | Min. | Max. | ||
| Formaldehyde | 4.59 ± 2.64 | 0.44 | 10.22 | 3.66 ± 2.11 | 0.35 | 8.35 | 0.056 |
| Acetaldehyde | 4.47 ± 2.22 | 0.99 | 9.22 | 4.89 ± 2.42 | 0.00 | 10.41 | 0.083 |
| Acrolein | 0.29 ± 0.23 | 0.00 | 0.91 | 0.19 ± 0.17 | 0.00 | 0.81 | 0.110 |
| Acetone | 3.24 ± 1.82 | 0.00 | 7.29 | 4.83 ± 2.74 | 0.00 | 10.87 | 0.128 |
| Propionaldehyde | 0.68 ± 0.34 | 0.00 | 3.61 | 0.77 ± 0.41 | 0.00 | 1.55 | 0.137 |
| Butenal | 0.38 ± 0.24 | 0.00 | 1.05 | 0.53 ± 0.40 | 0.00 | 1.74 | 0.184 |
| Butyraldehyde | 1.32 ± 0.60 | 0.29 | 2.60 | 1.49 ± 0.76 | 0.27 | 3.25 | 0.214 |
| Benzaldehyde | 0.39 ± 0.27 | 0.00 | 1.05 | 0.51 ± 0.37 | 0.00 | 2.58 | 0.329 |
| Isovaleraldehyde | 0.02 ± 0.07 | 0.00 | 0.32 | 0.08 ± 0.17 | 0.00 | 0.79 | 0.363 |
| Valeraldehyde | 0.11 ± 0.14 | 0.00 | 0.49 | 0.14 ± 0.22 | 0.00 | 0.94 | 0.353 |
| O-methylbenzaldehyde | 0.01 ± 0.07 | 0.00 | 0.49 | 0.01 ± 0.08 | 0.00 | 0.73 | 0.513 |
| M-methylbenzaldehyde | 0.20 ± 0.33 | 0.00 | 1.78 | 0.15 ± 0.40 | 0.00 | 2.41 | 0.512 |
| P-methylbenzaldehyde | - | - | - | 0.02 ± 0.14 | 0.00 | 1.26 | 0.557 |
| Hexanal | 0.28 ± 0.29 | 0.00 | 1.03 | 0.14 ± 0.23 | 0.00 | 1.20 | 0.293 |
| 2,5-dimethylbenzaldehyde | 0.10 ± 0.22 | 0.00 | 1.19 | 0.27 ± 0.39 | 0.00 | 2.25 | 0.351 |
| MACR | 0.50 ± 0.39 | 0.00 | 1.35 | 0.64 ± 0.42 | 0.00 | 1.66 | 0.2 |
| Total | 16.9 ± 7.65 | 4.30 | 33.6 | 18.3 ± 7.90 | 4.86 | 38.5 | - |
| City | Type | Formaldehyde | Acetaldehyde | Acetone | Sampling Time | Reference |
|---|---|---|---|---|---|---|
| (μg/m3) | ||||||
| Linfen | urban | 4.14 | 4.82 | 4.00 | 2019/01 | Present study |
| Beijing | urban | 7.1 | 10.3 | 9.5 | 2007/01 | [6] |
| urban | 4.01 | 2.96 | 2.01 | 2011/01 | [7] | |
| urban | 5.51 | 4.07 | 4.22 | 2014/01 | [8] | |
| Foshan | urban | 8.55 | 5.8 | 8.29 | 2014/12 | [27] |
| Zhengzhou | urban | 8.23 | 7.67 | 13.9 | 2012/01 | [28] |
| Xi’an | urban | 3.3 | 2.89 | 7.37 | 2015–2016 winter | [29] |
| We Rome, Italy | semi-rural area | 1.36 | 0.92 | 4.57 | 2006/12 | [30] |
| Fortaleza, Brazil | urban | 12.42 | 2.9 | 52.48 | 2004/11–12 | [31] |
| Orleans, France | semi-urban area | 1.46 | 0.67 | 1.06 | 2011/01 | [32] |
| Formaldehyde | Acetaldehyde | Acetone | |
|---|---|---|---|
| CO | 0.44 | 0.82 | 0.77 |
| Temperature | 0.58 | 0.32 | 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, H.; Wang, X.; Xue, Z.; Duan, L.; Kou, Y.; Zhang, Y.; Chen, X. Pollution Characteristics of Atmospheric Carbonyls in Urban Linfen in Winter. Atmosphere 2020, 11, 685. https://doi.org/10.3390/atmos11070685
Li F, Wang H, Wang X, Xue Z, Duan L, Kou Y, Zhang Y, Chen X. Pollution Characteristics of Atmospheric Carbonyls in Urban Linfen in Winter. Atmosphere. 2020; 11(7):685. https://doi.org/10.3390/atmos11070685
Chicago/Turabian StyleLi, Fanxiu, Hengyuan Wang, Xuezhong Wang, Zhigang Xue, Liqin Duan, Yongjun Kou, Yujie Zhang, and Xuan Chen. 2020. "Pollution Characteristics of Atmospheric Carbonyls in Urban Linfen in Winter" Atmosphere 11, no. 7: 685. https://doi.org/10.3390/atmos11070685
APA StyleLi, F., Wang, H., Wang, X., Xue, Z., Duan, L., Kou, Y., Zhang, Y., & Chen, X. (2020). Pollution Characteristics of Atmospheric Carbonyls in Urban Linfen in Winter. Atmosphere, 11(7), 685. https://doi.org/10.3390/atmos11070685





