Classification of Clouds Sampled at the Puy de Dôme Station (France) Based on Chemical Measurements and Air Mass History Matrices
Abstract
1. Introduction
2. Experiments
2.1. Cloud Sampling
2.2. PuyCloud Database
2.3. Dynamical Analysis
2.4. Statistical Analysis
3. Results and Discussion
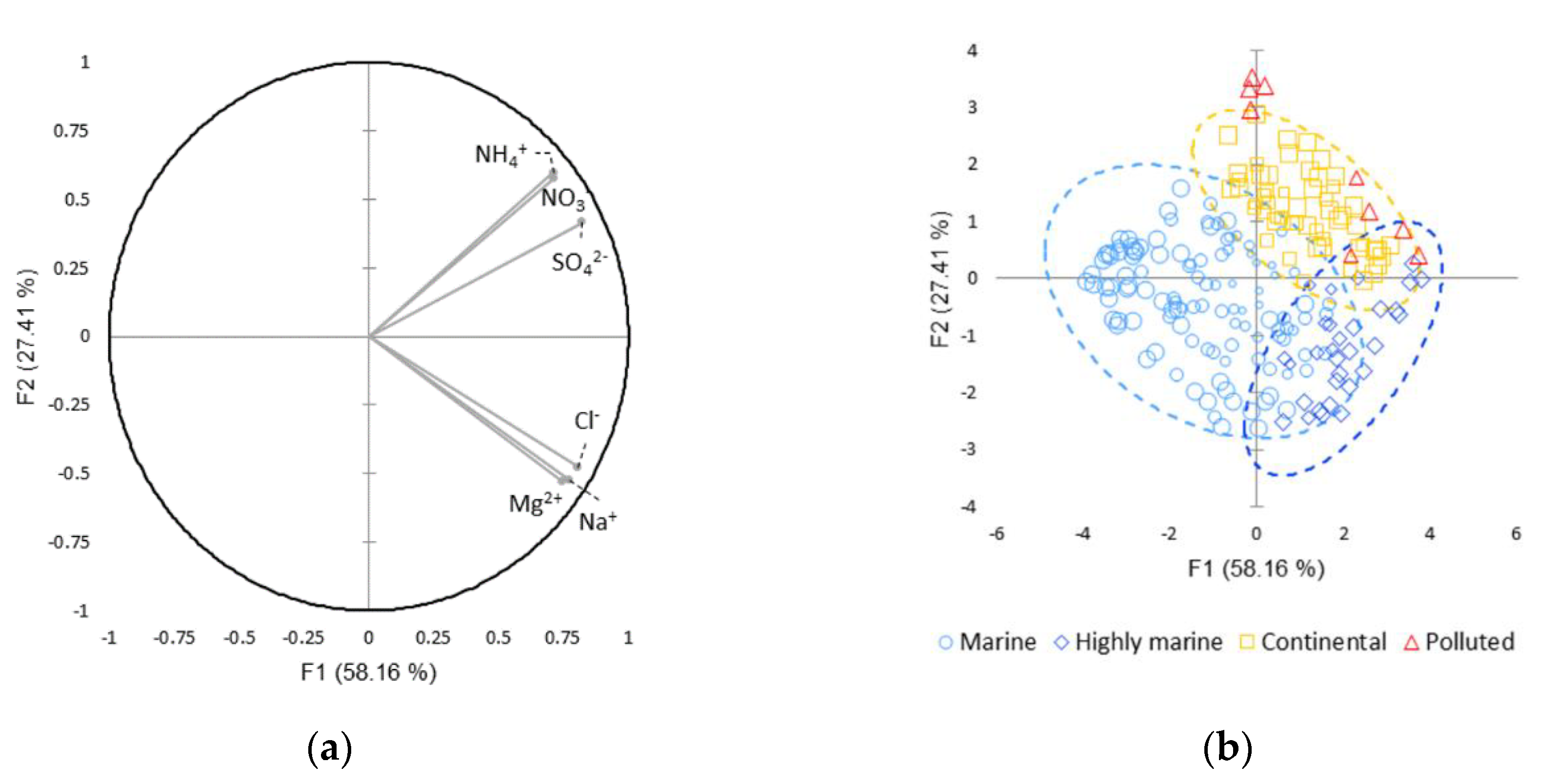
3.1. Clusterization of Cloud Waters at PUY
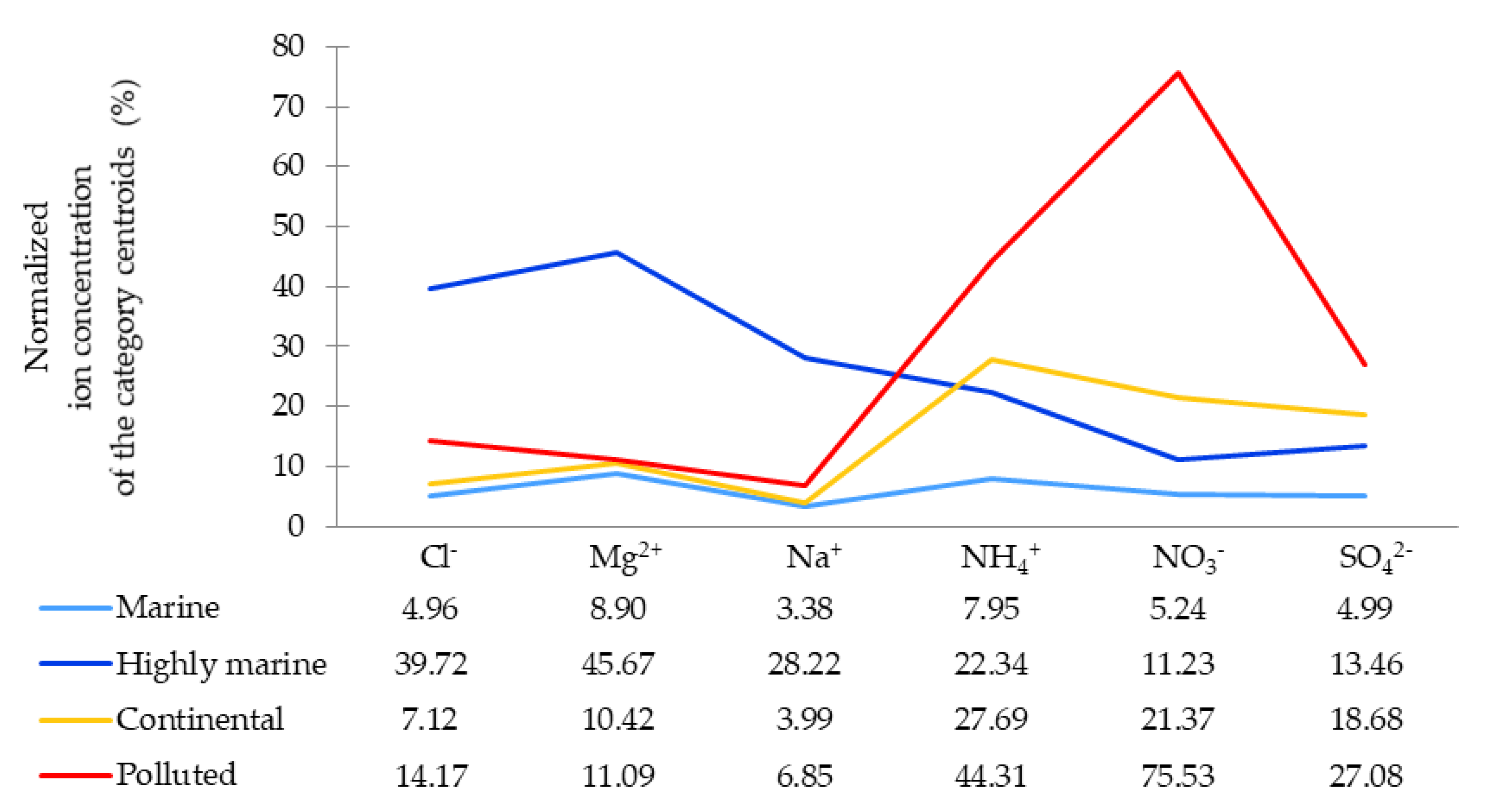
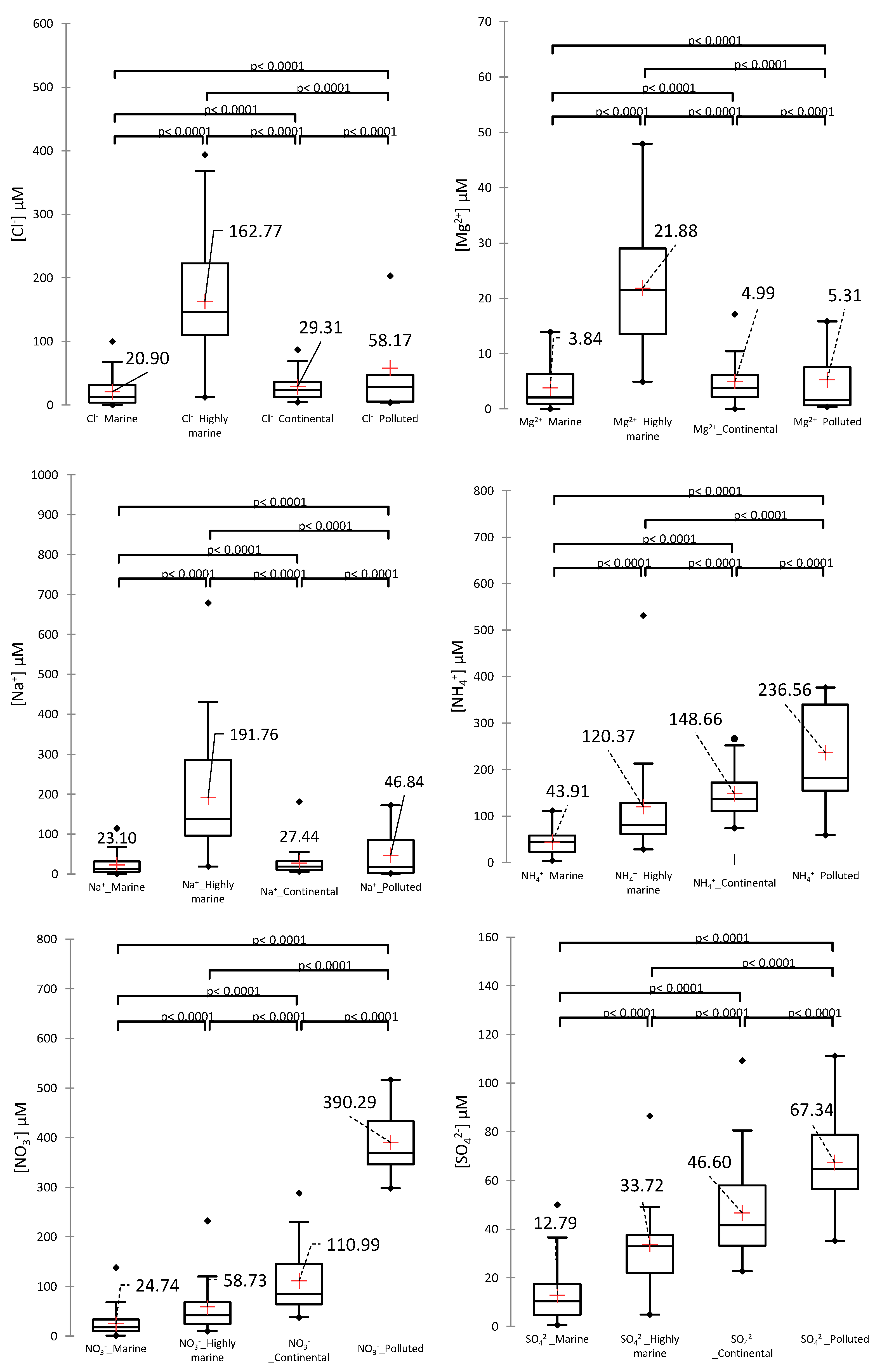
3.1.1. Chemical Categories
3.1.2. Variable Validation
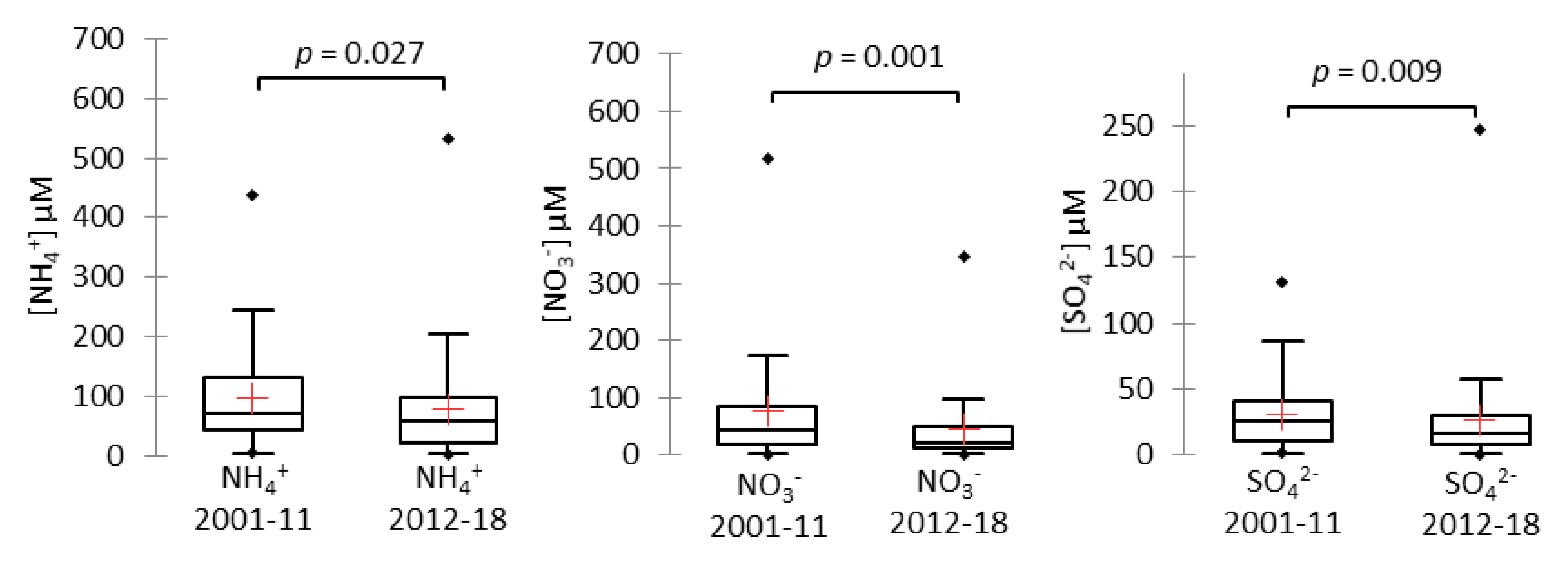
3.1.3. Evolution Since the 2001–2011 Study
3.2. Influence of Air Mass History at PUY
3.3. Influence of Cloud Microphysics at PUY
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herckes, P.; Valsaraj, K.T.; Collett, J.L. A review of observations of organic matter in fogs and clouds: Origin, processing and fate. Atmos. Res. 2013, 132–133, 434–449. [Google Scholar] [CrossRef]
- Herrmann, H.; Schaefer, T.; Tilgner, A.; Styler, S.A.; Weller, C.; Teich, M.; Otto, T. Tropospheric aqueous-phase chemistry: Kinetics, mechanisms, and its coupling to a changing gas phase. Chem. Rev. 2015, 115, 4259–4334. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Riva, M.; Baray, J.-L.; Ribeiro, M.; Chaumerliac, N.; George, C.; Bridoux, M.; Deguillaume, L. Chemical characterization of cloud water collected at puy de Dôme by FT-ICR MS reveals the presence of SOA components. ACS Earth Space Chem. 2019, 3, 2076–2087. [Google Scholar] [CrossRef]
- Vaïtilingom, M.; Deguillaume, L.; Vinatier, V.; Sancelme, M.; Amato, P.; Chaumerliac, N.; Delort, A.-M. Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds. Proc. Natl. Acad. Sci. USA 2013, 110, 559–564. [Google Scholar] [CrossRef]
- Wei, M.; Xu, C.; Chen, J.; Zhu, C.; Li, J.; Lv, G. Characteristics of bacterial community in cloud water at Mt Tai: Similarity and disparity under polluted and non-polluted cloud episodes. Atmos. Chem. Phys. 2017, 17, 5253–5270. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M.; Perroux, H.; Voyard, G.; Mouchel-Vallon, C.; Chaumerliac, N.; Mailhot, G.; Deguillaume, L.; Brigante, M. A better understanding of hydroxyl radical photochemical sources in cloud waters collected at the puy de Dôme station—Experimental versus modelled formation rates. Atmos. Chem. Phys. 2015, 15, 9191–9202. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Zhu, C.; Li, W.; Li, C.; Liu, L.; Xu, C.; Wen, L.; Xue, L.; et al. Chemical composition and droplet size distribution of cloud at the summit of Mount Tai, China. Atmos. Chem. Phys. 2017, 17, 9885–9896. [Google Scholar] [CrossRef]
- Chen, J.; Griffin, R.J.; Grini, A.; Tulet, P. Modeling secondary organic aerosol formation through cloud processing of organic compounds. Atmos. Chem. Phys. 2007, 7, 5343–5355. [Google Scholar] [CrossRef]
- Schurman, M.I.; Boris, A.; Desyaterik, Y.; Jeffrey, L.; Collett, J. Aqueous secondary organic aerosol formation in ambient cloud water photo-oxidations. Aerosol Air Qual. Res. 2017, 18, 15–25. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L., Jr.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H.; et al. The acidity of atmospheric particles and clouds. Atmos. Chem. Phys. 2020, 20, 4809–4888. [Google Scholar] [CrossRef]
- Hegg, D.A.; Hobbs, P.V. Cloud water chemistry and the production of sulfates in clouds. Atmos. Environ. 1967 1981, 15, 1597–1604. [Google Scholar] [CrossRef]
- Vong, R.J.; Hansson, H.-C.; Covert, D.S.; Charlson, R.J. Acid rain: Simultaneous observations of a natural marine background and its acidic sulfate aerosol precursor. Geophys. Res. Lett. 1988, 15, 338–341. [Google Scholar] [CrossRef]
- Ervens, B.; Turpin, B.J.; Weber, R.J. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos. Chem. Phys. 2011, 11, 11069–11102. [Google Scholar] [CrossRef]
- Ervens, B.; Sorooshian, A.; Aldhaif, A.M.; Shingler, T.; Crosbie, E.; Ziemba, L.; Campuzano-Jost, P.; Jimenez, J.L.; Wisthaler, A. Is there an aerosol signature of chemical cloud processing? Atmos. Chem. Phys. 2018, 18, 16099–16119. [Google Scholar] [CrossRef]
- Quinn, P.K.; Bates, T.S.; Coffman, D.J.; Covert, D.S. Influence of particle size and chemistry on the cloud nucleating properties of aerosols. Atmos. Chem. Phys. 2008, 8, 1029–1042. [Google Scholar] [CrossRef]
- Bator, A.; Collett, J.L. Cloud chemistry varies with drop size. J. Geophys. Res. Atmos. 1997, 102, 28071–28078. [Google Scholar] [CrossRef]
- Moore, K.F.; Sherman, D.E.; Reilly, J.E.; Collett, J.L. Drop size-dependent chemical composition in clouds and fogs. Part I. Observations. Atmos. Environ. 2004, 38, 1389–1402. [Google Scholar] [CrossRef]
- Hao, L.; Romakkaniemi, S.; Kortelainen, A.; Jaatinen, A.; Portin, H.; Miettinen, P.; Komppula, M.; Leskinen, A.; Virtanen, A.; Smith, J.N.; et al. Aerosol chemical composition in cloud events by high resolution time-of-flight aerosol mass spectrometry. Environ. Sci. Technol. 2013, 47, 2645–2653. [Google Scholar] [CrossRef]
- Van Pinxteren, D.; Fomba, K.W.; Mertes, S.; Müller, K.; Spindler, G.; Schneider, J.; Lee, T.; Collett, J.L.; Herrmann, H. Cloud water composition during HCCT-2010: Scavenging efficiencies, solute concentrations, and droplet size dependence of inorganic ions and dissolved organic carbon. Atmos. Chem. Phys. 2016, 16, 3185–3205. [Google Scholar] [CrossRef]
- Triesch, N.; van Pinxteren, M.; Engel, A.; Herrmann, H. Concerted measurements of free amino acids at the Cape Verde Islands: High enrichments in submicron sea spray aerosol particles and cloud droplets. Atmos. Chem. Phys. Discuss. 2020, 1–24. [Google Scholar] [CrossRef]
- Van Pinxteren, D.; Plewka, A.; Hofmann, D.; Müller, K.; Kramberger, H.; Svrcina, B.; Bächmann, K.; Jaeschke, W.; Mertes, S.; Collett, J.L.; et al. Schmücke hill cap cloud and valley stations aerosol characterisation during FEBUKO (II): Organic compounds. Atmos. Environ. 2005, 39, 4305–4320. [Google Scholar] [CrossRef]
- Wang, M.; Perroux, H.; Fleuret, J.; Bianco, A.; Bouvier, L.; Colomb, A.; Borbon, A.; Deguillaume, L. Anthropogenic and biogenic hydrophobic VOCs detected in clouds at the puy de Dôme station using Stir Bar Sorptive Extraction: Deviation from the Henry’s law prediction. Atmos. Res. 2020, 237, 104844. [Google Scholar] [CrossRef]
- Atlas, E.; Giam, C.S. Ambient concentration and precipitation scavenging of atmospheric organic pollutants. Water. Air. Soil Pollut. 1988, 38, 19–36. [Google Scholar] [CrossRef]
- Levsen, K.; Behnert, S.; Prieß, B.; Svoboda, M.; Winkeler, H.-D.; Zietlow, J. Organic compounds in precipitation. Chemosphere 1990, 21, 1037–1061. [Google Scholar] [CrossRef]
- Bianco, A.; Deguillaume, L.; Vaïtilingom, M.; Nicol, E.; Baray, J.-L.; Chaumerliac, N.; Bridoux, M. Molecular characterization of cloud water samples collected at the puy de Dôme (France) by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Environ. Sci. Technol. 2018, 52, 10275–10285. [Google Scholar] [CrossRef] [PubMed]
- Löflund, M.; Kasper-Giebl, A.; Schuster, B.; Giebl, H.; Hitzenberger, R.; Puxbaum, H. Formic, acetic, oxalic, malonic and succinic acid concentrations and their contribution to organic carbon in cloud water. Atmos. Environ. 2002, 36, 1553–1558. [Google Scholar] [CrossRef]
- Petrenchuk, O.P.; Drozdova, V.M. On the chemical composition of cloud water. Tellus 1966, 18, 280–286. [Google Scholar] [CrossRef]
- Fuzzi, S.; Facchini, M.C.; Decesari, S.; Matta, E.; Mircea, M. Soluble organic compounds in fog and cloud droplets: What have we learned over the past few years? Atmos. Res. 2002, 64, 89–98. [Google Scholar] [CrossRef]
- Ghauri, B.M.; Ishaq Mirza, M.; Richter, R.; Dutkiewicz, V.A.; Rusheed, A.; Khan, A.R.; Husain, L. Composition of aerosols and cloud water at a remote mountain site (2.8 kms) in Pakistan. Chemosphere Glob. Chang. Sci. 2001, 3, 51–63. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Shen, X.; Wang, Z.; Lee, T.; Wang, X.; Li, P.; Sun, M.; Collett, J.L.; Wang, W.; et al. Characterization of cloud water chemistry at Mount Tai, China: Seasonal variation, anthropogenic impact, and cloud processing. Atmos. Environ. 2012, 60, 467–476. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Gao, R.; Xue, L.; Guo, J.; Zhou, Y.; Nie, W.; Wang, X.; Xu, P.; Gao, J.; et al. Source and variation of carbonaceous aerosols at Mount Tai, North China: Results from a semi-continuous instrument. Atmos. Environ. 2011, 45, 1655–1667. [Google Scholar] [CrossRef]
- Aleksic, N.; Roy, K.; Sistla, G.; Dukett, J.; Houck, N.; Casson, P. Analysis of cloud and precipitation chemistry at Whiteface Mountain, NY. Atmos. Environ. 2009, 43, 2709–2716. [Google Scholar] [CrossRef]
- Gioda, A.; Mayol-Bracero, O.L.; Scatena, F.N.; Weathers, K.C.; Mateus, V.L.; McDowell, W.H. Chemical constituents in clouds and rainwater in the Puerto Rican rainforest: Potential sources and seasonal drivers. Atmos. Environ. 2013, 68, 208–220. [Google Scholar] [CrossRef]
- Weathers, K.C.; Likens, G.E.; Bormann, F.H.; Bicknell, S.H.; Bormann, B.T.; Daube, B.C.; Eaton, J.S.; Galloway, J.N.; Keene, W.C. Cloudwater chemistry from ten sites in North America. Environ. Sci. Technol. 1988, 22, 1018–1026. [Google Scholar] [CrossRef]
- Crosbie, E.; Brown, M.D.; Shook, M.; Ziemba, L.; Moore, R.H.; Shingler, T.; Winstead, E.; Thornhill, K.L.; Robinson, C.; MacDonald, A.B.; et al. Development and characterization of a high-efficiency, aircraft-based axial cyclone cloud water collector. Atmos. Meas. Tech. 2018, 11, 5025–5048. [Google Scholar] [CrossRef]
- Skarżyńska, K.; Polkowska, Ż.; Namieśnik, J. Sample handling and determination of physico-chemical parameters in rime, hoarfrost, dew, fog and cloud water samples—A review. Pol. J. Environ. Stud. 2005, 15, 185–209. [Google Scholar]
- Wieprecht, W.; Acker, K.; Mertes, S.; Collett, J.; Jaeschke, W.; Brüggemann, E.; Möller, D.; Herrmann, H. Cloud physics and cloud water sampler comparison during FEBUKO. Atmos. Environ. 2005, 39, 4267–4277. [Google Scholar] [CrossRef]
- Zhao, Y.; Hallar, A.G.; Mazzoleni, L.R. Atmospheric organic matter in clouds: Exact masses and molecular formula identification using ultrahigh-resolution FT-ICR mass spectrometry. Atmos. Chem. Phys. 2013, 13, 12343–12362. [Google Scholar] [CrossRef]
- Baray, J.-L.; Deguillaume, L.; Colomb, A.; Sellegri, K.; Freney, E.; Rose, C.; Baelen, J.V.; Pichon, J.-M.; Picard, D.; Fréville, P.; et al. Cézeaux-Aulnat-Opme-Puy De Dôme: A multi-site for the long term survey of the tropospheric composition and climate change. Atmos. Meas. Tech. 2020, 13, 3413–3445. [Google Scholar] [CrossRef]
- Acker, K. Mt. Brocken, a site for a cloud chemistry measurement programme in Central Europe. Water Air Soil Pollut. 1995, 4, 6. [Google Scholar]
- Takeuchi, M.; Okochi, H.; Igawa, M. Characteristics of water-soluble components of atmospheric aerosols in Yokohama and Mt. Oyama, Japan from 1990 to 2001. Atmos. Environ. 2004, 38, 4701–4708. [Google Scholar] [CrossRef]
- Science, G. Experimental evidence of the feeding of the free troposphere with aerosol particles from the mixing layer. Aerosol Air Qual. Res. 2016, 16, 702–716. [Google Scholar] [CrossRef]
- Putaud, J.-P.; Raes, F.; Van Dingenen, R.; Brüggemann, E.; Facchini, M.-C.; Decesari, S.; Fuzzi, S.; Gehrig, R.; Hüglin, C.; Laj, P.; et al. A European aerosol phenomenology—2: Chemical characteristics of particulate matter at kerbside, urban, rural and background sites in Europe. Atmos. Environ. 2004, 38, 2579–2595. [Google Scholar] [CrossRef]
- Clain, G.; Baray, J.-L.; Delmas, R.; Keckhut, P.; Cammas, J.-P. A lagrangian approach to analyse the tropospheric ozone climatology in the tropics: Climatology of stratosphere–troposphere exchange at Reunion Island. Atmos. Environ. 2010, 44, 968–975. [Google Scholar] [CrossRef]
- Hondula, D.M.; Sitka, L.; Davis, R.E.; Knight, D.B.; Gawtry, S.D.; Deaton, M.L.; Lee, T.R.; Normile, C.P.; Stenger, P.J. A back-trajectory and air mass climatology for the Northern Shenandoah Valley, USA. Int. J. Climatol. 2010, 30, 569–581. [Google Scholar] [CrossRef]
- Deguillaume, L.; Charbouillot, T.; Joly, M.; Vaïtilingom, M.; Parazols, M.; Marinoni, A.; Amato, P.; Delort, A.-M.; Vinatier, V.; Flossmann, A.; et al. Classification of clouds sampled at the puy de Dôme (France) based on 10 yr of monitoring of their physicochemical properties. Atmos. Chem. Phys. 2014, 14, 1485–1506. [Google Scholar] [CrossRef]
- Deininger, C.K.; Saxena, V.K. A validation of back trajectories of air masses by principal component analysis of ion concentrations in cloud water. Atmos. Environ. 1997, 31, 295–300. [Google Scholar] [CrossRef]
- Kim, M.-G.; Lee, B.-K.; Kim, H.-J. Cloud/fog water chemistry at a high elevation site in South Korea. J. Atmos. Chem. 2006, 55, 13–29. [Google Scholar] [CrossRef]
- Yue, Y.; Niu, S.; Zhao, L.; Zhang, Y.; Xu, F. The influences of macro- and microphysical characteristics of sea-fog on fog-water chemical composition. Adv. Atmos. Sci. 2014, 31, 624–636. [Google Scholar] [CrossRef]
- Baray, J.-L.; Bah, A.; Cacault, P.; Sellegri, K.; Pichon, J.-M.; Deguillaume, L.; Montoux, N.; Noel, V.; Seze, G.; Gabarrot, F.; et al. Cloud occurrence frequency at puy de Dôme (France) deduced from an automatic camera image analysis: Method, validation, and comparisons with larger scale parameters. Atmosphere 2019, 10, 808. [Google Scholar] [CrossRef]
- Brantner, B.; Fierlinger, H.; Puxbaum, H. Cloudwater chemistry in the subcooled droplet regime at Mount Sonnblick (3106 M A.S.L., Salzburg, Austria). Water Air Soil Pollut. 1993, 74, 362–384. [Google Scholar]
- Lebedev, A.T.; Polyakova, O.V.; Mazur, D.M.; Artaev, V.B.; Canet, I.; Lallement, A.; Vaïtilingom, M.; Deguillaume, L.; Delort, A.-M. Detection of semi-volatile compounds in cloud waters by GC×GC-TOF-MS. Evidence of phenols and phthalates as priority pollutants. Environ. Pollut. 2018, 241, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Wirgot, N.; Vinatier, V.; Deguillaume, L.; Sancelme, M.; Delort, A.-M. H2O2 modulates the energetic metabolism of the cloud microbiome. Atmos. Chem. Phys. 2017, 17, 14841–14851. [Google Scholar] [CrossRef]
- Renard, P.; Canet, I.; Sancelme, M.; Wirgot, N.; Deguillaume, L.; Delort, A.-M. Screening of cloud microorganisms isolated at the Puy de Dôme (France) station for the production of biosurfactants. Atmos. Chem. Phys. 2016, 16, 12347–12358. [Google Scholar] [CrossRef]
- Lallement, A.; Besaury, L.; Tixier, E.; Sancelme, M.; Amato, P.; Vinatier, V.; Canet, I.; Polyakova, O.V.; Artaev, V.B.; Lebedev, A.T.; et al. Potential for phenol biodegradation in cloud waters. Biogeosciences 2018, 15, 5733–5744. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Amato, P.; Joly, M.; Besaury, L.; Oudart, A.; Taib, N.; Moné, A.I.; Deguillaume, L.; Delort, A.-M.; Debroas, D. Active microorganisms thrive among extremely diverse communities in cloud water. PLoS ONE 2017, 12, e0182869. [Google Scholar] [CrossRef]
- Amato, P.; Besaury, L.; Joly, M.; Penaud, B.; Deguillaume, L.; Delort, A.-M. Metatranscriptomic exploration of microbial functioning in clouds. Sci. Rep. 2019, 9, 4383. [Google Scholar] [CrossRef]
- Bianco, A.; Voyard, G.; Deguillaume, L.; Mailhot, G.; Brigante, M. Improving the characterization of dissolved organic carbon in cloud water: Amino acids and their impact on the oxidant capacity. Sci. Rep. 2016, 6, 37420. [Google Scholar] [CrossRef]
- Bianco, A.; Vaïtilingom, M.; Bridoux, M.; Chaumerliac, N.; Pichon, J.-M.; Piro, J.-L.; Deguillaume, L. Trace metals in cloud water sampled at the puy de Dôme station. Atmosphere 2017, 8, 225. [Google Scholar] [CrossRef]
- Bianco, A.; Deguillaume, L.; Chaumerliac, N.; Vaïtilingom, M.; Wang, M.; Delort, A.-M.; Bridoux, M.C. Effect of endogenous microbiota on the molecular composition of cloud water: A study by Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR MS). Sci. Rep. 2019, 9, 7663. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, J.; Monteil, G.; Sporre, M.K.; Kaldal Hansen, A.M.; Kristensson, A.; Eriksson Stenström, K.; Swietlicki, E.; Glasius, M. Exploring sources of biogenic secondary organic aerosol compounds using chemical analysis and the FLEXPART model. Atmos. Chem. Phys. 2017, 17, 11025–11040. [Google Scholar] [CrossRef]
- Van Pinxteren, D.; Brüggemann, E.; Gnauk, T.; Müller, K.; Thiel, C.; Herrmann, H. A GIS based approach to back trajectory analysis for the source apportionment of aerosol constituents and its first application. J. Atmos. Chem. 2010, 67, 1–28. [Google Scholar] [CrossRef]
- Hoffmann, L.; Günther, G.; Li, D.; Stein, O.; Wu, X.; Griessbach, S.; Heng, Y.; Konopka, P.; Müller, R.; Vogel, B.; et al. From ERA-Interim to ERA5: The considerable impact of ECMWF’s next-generation reanalysis on Lagrangian transport simulations. Atmos. Chem. Phys. 2019, 19, 3097–3124. [Google Scholar] [CrossRef]
- Bezdek, A.; Sebera, J. MATLAB script for visualizing geoid height and other elevation data on rotating 3D globe. Comput. Geosci. 2013, 56, 127–130. [Google Scholar] [CrossRef]
- Ilin, A.; Raiko, T. Practical Approaches to principal component analysis in the presence of missing values. J. Mach. Learn. Res. 2010, 11, 1957–2000. [Google Scholar]
- Addinsoft Addinsoft. XLSTAT Statistical and Data Analysis Solution. New York, NY, USA, 2020. Available online: https://www.xlstat.com (accessed on 23 May 2020).
- Błaś, M.; Polkowska, Ż.; Sobik, M.; Klimaszewska, K.; Nowiński, K.; Namieśnik, J. Fog water chemical composition in different geographic regions of Poland. Atmos. Res. 2010, 95, 455–469. [Google Scholar] [CrossRef]
- Collett, J.L.; Bator, A.; Sherman, D.E.; Moore, K.F.; Hoag, K.J.; Demoz, B.B.; Rao, X.; Reilly, J.E. The chemical composition of fogs and intercepted clouds in the United States. Atmos. Res. 2002, 64, 29–40. [Google Scholar] [CrossRef]
- Nieberding, F.; Breuer, B.; Braeckevelt, E.; Klemm, O.; Song, Q.; Zhang, Y. Fog Water Chemical Composition on Ailaoshan Mountain, Yunnan Province, SW China. Aerosol Air Qual. Res. 2017, 18, 37–48. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Marcotte, A.R.; Karl, M.; Dye, C.; Herckes, P. Fog chemistry at three sites in Norway. Atmos. Res. 2015, 151, 72–81. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhu, C.; Li, J.; Sui, X.; Liu, L.; Sun, J. Fog composition along the Yangtze River basin: Detecting emission sources of pollutants in fog water. J. Environ. Sci. 2018, 71, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Möller, D.; Acker, K.; Wieprecht, W. A relationship between liquid water content and chemical composition in clouds. Atmos. Res. 1996, 41, 321–335. [Google Scholar] [CrossRef]
- Collett, J.L.; Daube, B.C.; Gunz, D.; Hoffmann, M.R. Intensive studies of Sierra Nevada cloudwater chemistry and its relationship to precursor aerosol and gas concentrations. Atmos. Environ. Part Gen. Top. 1990, 24, 1741–1757. [Google Scholar] [CrossRef]
- Watanabe, K.; Ishizaka, Y.; Takenaka, C. Chemical characteristics of cloud water over the Japan Sea and the Northwestern Pacific Ocean near the central part of Japan: Airborne measurements. Atmos. Environ. 2001, 35, 645–655. [Google Scholar] [CrossRef]
- Bourcier, L.; Sellegri, K.; Chausse, P.; Pichon, J.M.; Laj, P. Seasonal variation of water-soluble inorganic components in aerosol size-segregated at the puy de Dôme station (1465 m a.s.l.), France. J. Atmos. Chem. 2012, 69, 47–66. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K.; Kobayashi, M.; Simoneit, B.R.T. Seasonal variations of sugars in atmospheric particulate matter from Gosan, Jeju Island: Significant contributions of airborne pollen and Asian dust in spring. Atmos. Environ. 2012, 55, 234–239. [Google Scholar] [CrossRef]
- Shapiro, J.B.; Simpson, H.J.; Griffin, K.L.; Schuster, W.S.F. Precipitation chloride at West Point, NY: Seasonal patterns and possible contributions from non-seawater sources. Atmos. Environ. 2007, 41, 2240–2254. [Google Scholar] [CrossRef]
- Kundu, S.; Kawamura, K.; Andreae, T.W.; Hoffer, A.; Andreae, M.O. Diurnal variation in the water-soluble inorganic ions, organic carbon and isotopic compositions of total carbon and nitrogen in biomass burning aerosols from the LBA-SMOCC campaign in Rondônia, Brazil. J. Aerosol Sci. 2010, 41, 118–133. [Google Scholar] [CrossRef]
- Elbert, W.; Krämer, M.; Andreae, M.O. Reply to discussion on “Control of solute concentrations in cloud and fog water by liquid water content”. Atmos. Environ. 2002, 11, 1909–1910. [Google Scholar] [CrossRef]
- Fahey, K.M.; Pandis, S.N.; Collett, J.L.; Herckes, P. The influence of size-dependent droplet composition on pollutant processing by fogs. Atmos. Environ. 2005, 39, 4561–4574. [Google Scholar] [CrossRef]
- Reilly, J.E.; Rattigan, O.V.; Moore, K.F.; Judd, C.; Eli Sherman, D.; Dutkiewicz, V.A.; Kreidenweis, S.M.; Husain, L.; Collett, J.L. Drop size-dependent S(IV) oxidation in chemically heterogeneous radiation fogs. Atmos. Environ. 2001, 35, 5717–5728. [Google Scholar] [CrossRef]
- Herrmann, H.; Exner, M.; Zellner, R. Reactivity trends in reactions of the nitrate radical (NO3) with inorganic and organic cloudwater constituents. Geochim. Cosmochim. Acta 1994, 58, 3239–3244. [Google Scholar] [CrossRef]
- Heuvel, A.P.V.D.; Mason, B.J. The formation of ammonium sulphate in water droplets exposed to gaseous sulphur dioxide and ammonia. Q. J. R. Meteorol. Soc. 1963, 89, 271–275. [Google Scholar] [CrossRef]
- Hung, H.-M.; Hoffmann, M.R. Oxidation of Gas-Phase SO2 on the Surfaces of Acidic Microdroplets: Implications for Sulfate and Sulfate Radical Anion Formation in the Atmospheric Liquid Phase. Environ. Sci. Technol. 2015, 49, 13768–13776. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.C.; Chim, M.M.; Davies, J.F.; Wilson, K.R.; Chan, M.N. Importance of sulfate radical anion formation and chemistry in heterogeneous OH oxidation of sodium methyl sulfate, the smallest organosulfate. Atmos. Chem. Phys. Online 2018, 18. [Google Scholar] [CrossRef]
- Rose, C.; Chaumerliac, N.; Deguillaume, L.; Perroux, H.; Mouchel-Vallon, C.; Leriche, M.; Patryl, L.; Armand, P. Modeling the partitioning of organic chemical species in cloud phases with CLEPS (1.1). Atmos. Chem. Phys. 2018, 18, 2225–2242. [Google Scholar] [CrossRef]


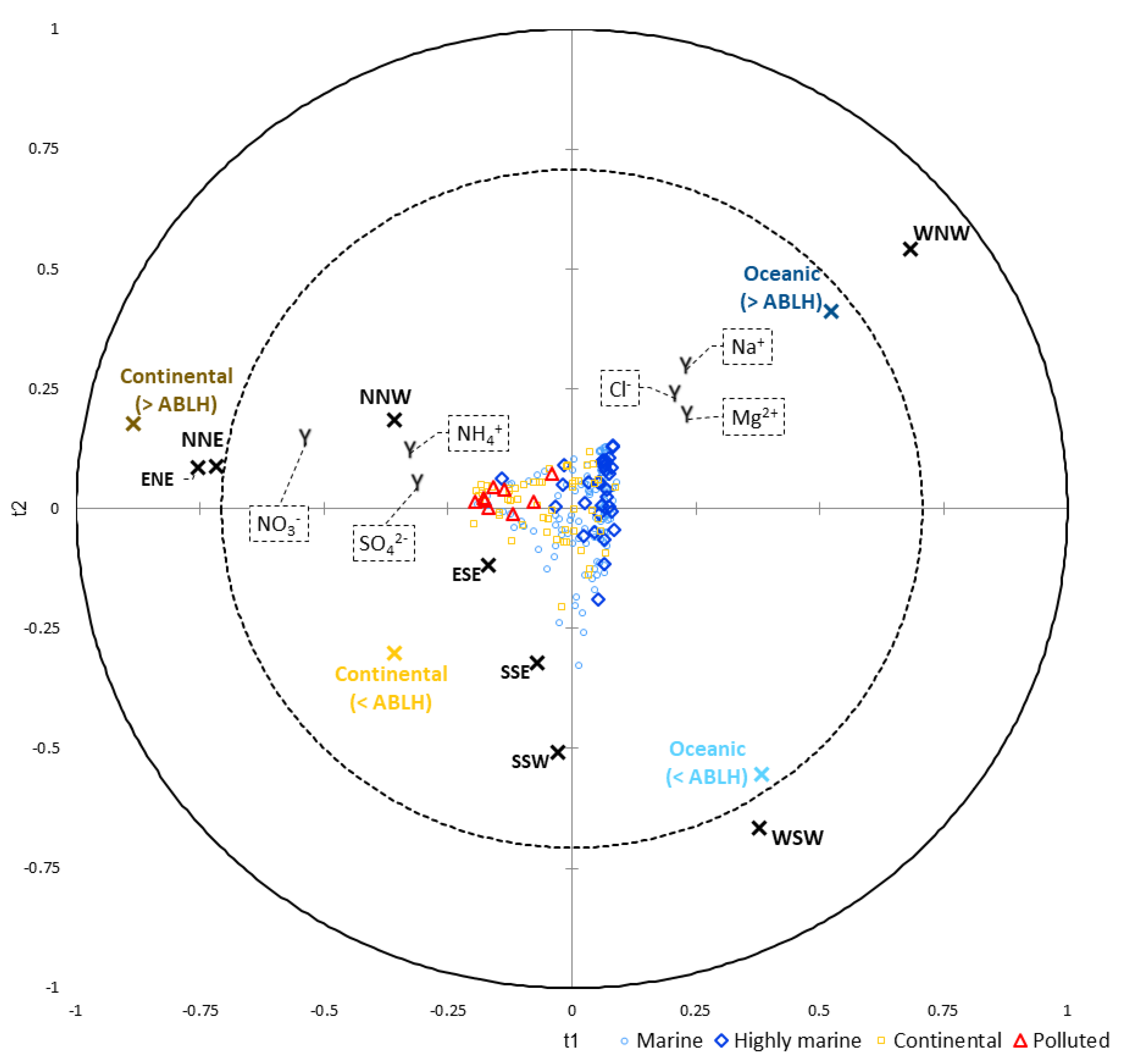
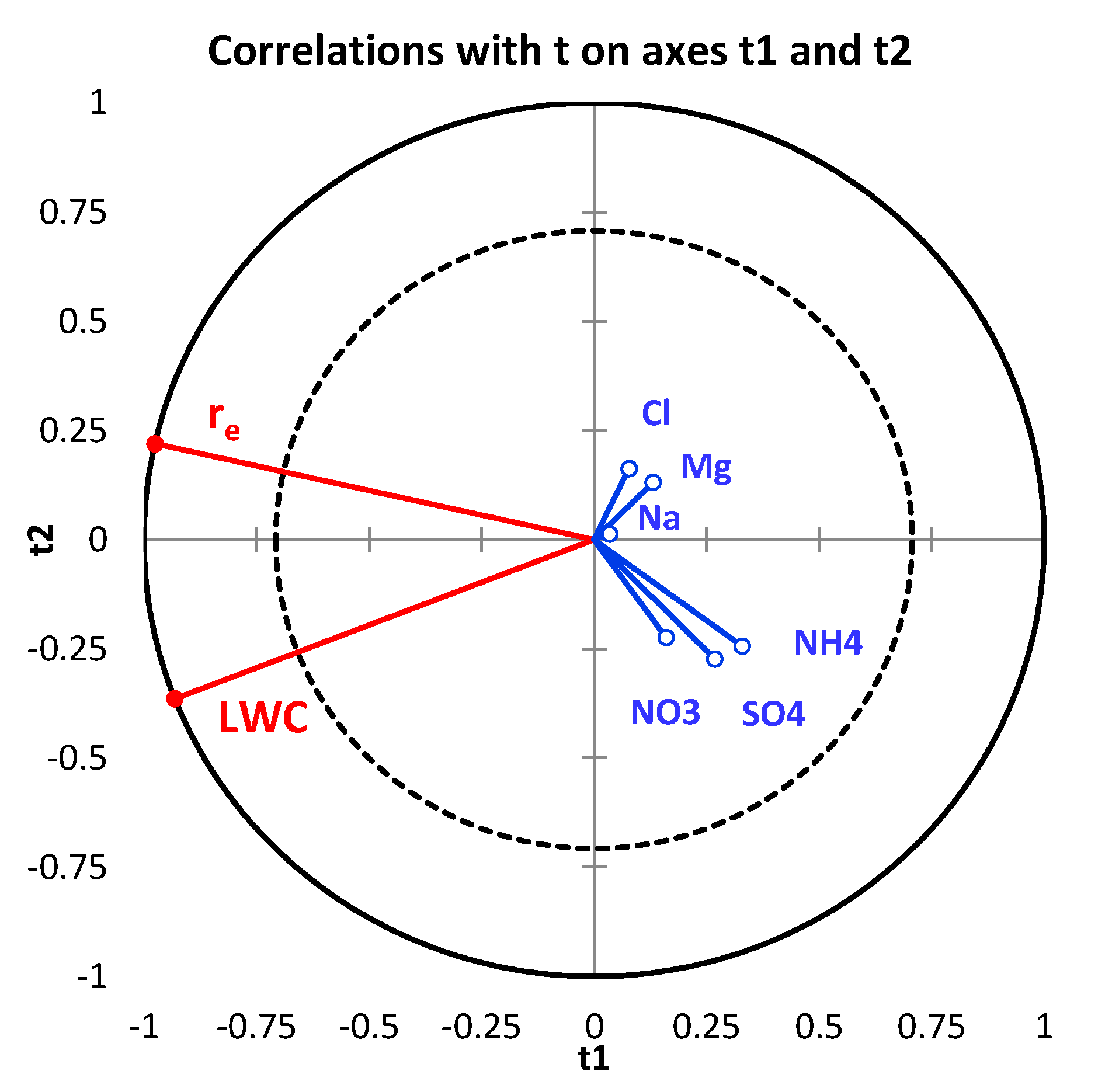
| Variables | “Sea Surface” (<ABLH) | “Sea Surface” (>ABLH) | “Continental Surface” (<ABLH) | “Continental Surface” (>ABLH) |
|---|---|---|---|---|
| WSW_WNW | 0.35 | 0.38 | −0.27 | −0.72 |
| NNW_NNE_ENE | −0.33 | −0.44 | 0.31 | 0.76 |
| ESE_SSE_SSW | −0.03 | −0.02 | −0.04 | 0.07 |
| Variables | “Sea Surface” (<ABLH) | “Sea Surface” (>ABLH) | “Continental Surface” (<ABLH) | “Continental Surface” (>ABLH) | WSW WNW | NNW NNE ENE | ESE SSE SSW |
|---|---|---|---|---|---|---|---|
| Cl− | 0.03 | 0.14 | −0.12 | −0.16 | 0.16 | −0.13 | −0.10 |
| Mg2+ | 0.06 | 0.14 | −0.13 | −0.19 | 0.18 | −0.15 | −0.10 |
| Na+ | −0.09 | 0.25 | −0.16 | −0.16 | 0.20 | −0.18 | −0.08 |
| NH4+ | −0.28 | −0.03 | 0.03 | 0.31 | −0.30 | 0.30 | 0.01 |
| NO3− | −0.25 | −0.26 | 0.15 | 0.51 | −0.46 | 0.51 | −0.04 |
| SO42− | −0.18 | −0.06 | 0.12 | 0.21 | −0.35 | 0.30 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renard, P.; Bianco, A.; Baray, J.-L.; Bridoux, M.; Delort, A.-M.; Deguillaume, L. Classification of Clouds Sampled at the Puy de Dôme Station (France) Based on Chemical Measurements and Air Mass History Matrices. Atmosphere 2020, 11, 732. https://doi.org/10.3390/atmos11070732
Renard P, Bianco A, Baray J-L, Bridoux M, Delort A-M, Deguillaume L. Classification of Clouds Sampled at the Puy de Dôme Station (France) Based on Chemical Measurements and Air Mass History Matrices. Atmosphere. 2020; 11(7):732. https://doi.org/10.3390/atmos11070732
Chicago/Turabian StyleRenard, Pascal, Angelica Bianco, Jean-Luc Baray, Maxime Bridoux, Anne-Marie Delort, and Laurent Deguillaume. 2020. "Classification of Clouds Sampled at the Puy de Dôme Station (France) Based on Chemical Measurements and Air Mass History Matrices" Atmosphere 11, no. 7: 732. https://doi.org/10.3390/atmos11070732
APA StyleRenard, P., Bianco, A., Baray, J.-L., Bridoux, M., Delort, A.-M., & Deguillaume, L. (2020). Classification of Clouds Sampled at the Puy de Dôme Station (France) Based on Chemical Measurements and Air Mass History Matrices. Atmosphere, 11(7), 732. https://doi.org/10.3390/atmos11070732





