Iron Speciation in Different Saharan Dust Advections and Effect of the Procedural Blank on the Results From X-ray Absorption Spectroscopy and Selective Leaching Experiments
Abstract
:1. Introduction
2. Results
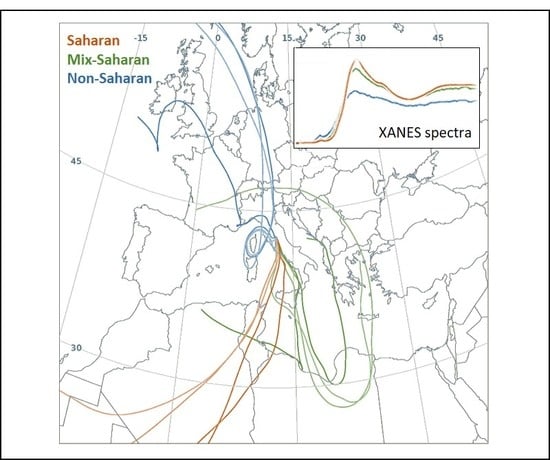
2.1. Aerosol Samples Characterization
2.2. Total Iron Concentrations
2.3. Iron Speciation
2.3.1. X-ray Absorption Spectroscopy
2.3.2. Selective Leaching Experiments
3. Discussion
3.1. Iron Speciation in the Different Advections
3.2. Effect of the Procedural Blank on Fe Speciation Measurements
4. Materials and Methods
4.1. Sampling
4.2. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
4.3. Sequential Leaching Extraction
4.4. X-ray Absorption Spectroscopy (XAS)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCR | European Community Bureau of Reference |
| BTs | Back Trajectories |
| BVM | Bond Valence Method |
| DMS | Dimethyl Sulfate |
| EMEP | European Monitoring and Evaluation Programme |
| ESRF | European Synchrotron Radiation Facility |
| EXAFS | Extended X-ray Absorption Fine Structure |
| HNLC | High Nutrient Low Chlorophyll |
| HVS | High Volume Sampler |
| ICP-OES | Inductively Coupled Plasma - Optical Emission Spectroscopy |
| LISA | Linea Italiana per la Spettroscopia d’Assorbimento x |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MM | Monte Martano |
| NCEP-GFS | National Centers for Environmental Prediction - Global Forecast System |
| SWAM | Model of particulate matter sampling instrument (FAI instruments) |
| WMO SDS-WAS | World Meteorological Organization Sand and Dust Storms–Warning Advisory and Assessment System |
| XAS | X-ray Absorption Spectroscopy |
| XANES | X-ray Absorption Near Edge Structure |
References
- Prospero, J.; Ginoux, P.; Torres, O.; Nicholson, S.; Gill, T. Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev. Geophys. 2002, 40, 1–31. [Google Scholar] [CrossRef]
- Luo, C.; Mahowald, N.; Bond, T.; Chuang, P.; Artaxo, P.; Siefert, R.; Chen, Y.; Schauer, J. Combustion iron distribution and deposition. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Mahowald, N.; Engelstaedter, S.; Luo, C.; Sealy, A.; Artaxo, P.; Benitez-Nelson, C.; Bonnet, S.; Chen, Y.; Chuang, P.; Cohen, D.; et al. Atmospheric iron deposition: Global distribution, variability and human perturbations. Annu. Rev. Mar. Sci. 2009, 1, 245–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkikas, A.; Hatzianastassiou, N.; Mihalopoulos, N.; Katsoulis, V.; Kazadzis, S.; Pey, J.; Querol, X.; Torres, O. The regime of intense desert dust episodes in the Mediterranean based on contemporary satellite observations and ground measurements. Atmos. Chem. Phys. 2013, 13, 12135–12154. [Google Scholar] [CrossRef] [Green Version]
- Prospero, J. Saharan dust transport over the North Atlantic Ocean and Mediterranean: An overview. In The Impact of Desert Dust Across the Mediterranean; Guerzoni, S., Chester, R., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 133–151. [Google Scholar]
- Prospero, J.; Glaccum, R.; Nees, R. Atmospheric transport of soil dust from Africa to South America. Nature 1981, 289, 570–572. [Google Scholar] [CrossRef]
- Zhuang, G.; Yi, Z.; Duce, R.; Brown, P. Link between iron and sulphur cycles suggested by detection of Fe(II) in remote marine aerosols. Nature 1992, 355, 537–539. [Google Scholar] [CrossRef]
- Smetacek, V.; Klaas, C.; Strass, V.; Assmy, P.; Montresor, M.; Cisewski, B.; Savoye, N.; Webb, A.; d’Ovidio, F.; Arrieta, J.; et al. Deep carbon export from a Southern Ocean iron-fertilized diatom bloom. Nature 2012, 487, 313–319. [Google Scholar] [CrossRef]
- Meskhidize, N.; Volker, C.; Al-Abadleh, H.; Barbeau, K.; Bressac, M.; Buck, C.; Bundy, R.; Croot, P.; Feng, Y.; Ito, A.; et al. Perspective on identifying and characterizing the processes controlling iron speciation and residence time at the atmosphere-ocean interface. Mar. Chem. 2019, 217, 103704. [Google Scholar] [CrossRef] [Green Version]
- Fittschen, U.; Meirer, F.; Streli, C.; Wobrauschek, P.; Thiele, J.; Falkenberg, G.; Pepponi, G. Characterization of atmospheric aerosols using Synchrotron radiation total reflection X-ray fluorescence and Fe K-edge total reflection X-ray fluorescence-X-ray absorption near edge structure. Spectrochim. Acta Part B 2008, 63, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Mahowald, N.; Baker, A.; Bergametti, G.; Brooks, N.; Duce, R.; Jickells, T.; Kybilay, N.; Prospero, J.; Tegen, I. Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Shi, Z.; Krom, M.; Jickells, T.; Bonneville, S.; Carslaw, K.; Mihalopoulos, N.; Baker, A.; Benning, L. Impacts on iron solubility in the mineral dust by processes in the source region and the atmosphere: A review. Aeolian Res. 2012, 5, 21–42. [Google Scholar] [CrossRef]
- Longo, A.; Feng, Y.; Lai, B.; Landing, W.; Shelley, R.; Nenes, A.; Mihalopoulos, N.; Violaki, K.; Ingall, E. Influence of atmospheric processes on the solubility and composition of iron in Saharan dust. Environ. Sci. Technol. 2016, 50, 6912–6920. [Google Scholar] [CrossRef] [PubMed]
- Takahama, S.; Gilardoni, S.; Russel, L. Single-particle oxidation state and morphology of atmospheric iron. J. Geophys. Res. Atmos. 2008, 113, D22. [Google Scholar] [CrossRef]
- Ohta, A.; Tsuno, H.; Kagi, H.; Kanai, Y.; Nomura, M.; Zhang, R.; Terashima, S.; Imai, N. Chemical compositions and XANES speciations of Fe, Mn and Zn from aerosols collected in China and Japan during dust events. Geochem. J. 2006, 40, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Jacob, D.; Waldman, J.; Munger, J.; Hoffmann, M. Chemical composition of fogwater collected along the California coast. Environ. Sci. Technol. 1985, 19, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Kieber, R.; Skrabal, S.; Smith, B.; Willey, J. Organic complexation of Fe(II) and its impact on the redox cycling of iron in rain. Environ. Sci. Technol. 2005, 39, 1576–1583. [Google Scholar] [CrossRef]
- Shi, Z.; Krom, M.; Bonneville, S.; Baker, A.; Bristow, C.; Drake, N.; Mann, G.; Carslaw, K.; McQuaid, J.; Jickells, T.; et al. Influence of chemical weathering and aging of iron oxides on the potential iron solubility of Saharan dust during simulated atmospheric processing. Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U.; Fechter, H.; Alferez, F. Quantitative relationships between soil color and hematite content. Soil Sci. 1983, 136, 354–358. [Google Scholar] [CrossRef]
- Formenti, P.; Caquineau, S.; Chevaillier, S.; Klaver, A.; Desboeufs, K.; Rajot, J.; Belin, S.; Briois, V. Dominance of goethite over hematite in iron oxides of mineral dust from Western Africa: Quantitative partitioning by X-ray absorption spectroscopy. J. Geophys. Res. Atmos. 2014, 119, 12740–12754. [Google Scholar] [CrossRef]
- Majestic, B.; Schauer, J.; Shafer, M. Application of synchrotron radiation for measurements of iron red-ox speciation in atmospherically processed aerosols. Atmos. Chem. Phys. 2007, 7, 2475–2487. [Google Scholar] [CrossRef] [Green Version]
- Stafoggia, M.; Zauli-Sajani, S.; Pey, J.; Samoli, E.; Alessandrini, E.; Basagana, X.; Cernigliaro, A.; Chiusolo, M.; Demaria, M.; Dìaz, J.; et al. Desert dust outbreaks in Southern Europe: Contribution to daily PM10 concentrations and short-term associations with mortality and hospital admissions. Environ. Health Perspect. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, P.; Dedik, A.; Ensling, J.; Weinbruch, S.; Weber, S.; Sinner, T.; Gulich, P.; Ortner, H. Speciation of iron in atmospheric aerosol samples. J. Aerosol Sci. 1996, 27, 325–337. [Google Scholar] [CrossRef]
- Petroselli, C.; Crocchianti, S.; Moroni, B.; Castellini, S.; Selvaggi, R.; Nava, S.; Calzolai, G.; Lucarelli, F.; Cappelletti, D. Disentangling the major source areas for an intense aerosol advection in the Central Mediterranean on the basis of Potential Source Contribution Function modeling of chemical and size distribution measurements. Atmos. Res. 2018, 204, 67–77. [Google Scholar] [CrossRef]
- McDaniel, M.; Ingall, E.; Morton, P.; Castorina, E.; Weber, R.; Shelley, R.; Landing, W.; Longo, A.; Feng, Y.; Lai, B. Relationship between atmospheric aerosol mineral surface area and iron solubility. ACS Earth Space Chem. 2019, 3, 2443–2451. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, M.; Feng, L.; Li, X. An EXAFS study on the local structure around iron in atmospheric aerosols collected in the Qingdao area. Molecules 2003, 8, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Oakes, M.; Weber, R.; Lai, B.; Russel, A.; Ingall, E. Characterization of iron speciation in urban and rural single particles using XANES spectroscopy and micro X-ray fluorescence measurements: Investigating the relationship between speciation and fractional iron solubility. Atmos. Chem. Phys. 2012, 12, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Escudero, M.; Querol, X.; Pey, J.; Alastuey, A.; Perez, N.; Ferreira, F.; Alonso, S.; Rodriguez, S.; Cuevas, E. A methodology for the quantification of the net African dust load in air quality monitoring networks. Atmos. Environ. 2007, 41, 5516–5524. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.; Brown, G.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Giuli, G.; Pratesi, G.; Cipriani, C.; Paris, E. Iron local structure in tektites and impact glasses by extended X-ray absorption fine structure and high-resolution X-ray absorption near-edge structure spectroscopy. Geochim. Cosmochim. Acta 2002, 66, 4347–4353. [Google Scholar] [CrossRef]
- Altermatt, D.; Brown, I. The automatic searching for chemical bonds in inorganic crystal structures. Acta Crystallogr. 1985, B41, 240–244. [Google Scholar] [CrossRef]
- Newville, M. Using Bond Valence sums as restrains in XAFS analysis. Phys. Scr. 2005, T115, 159–161. [Google Scholar] [CrossRef]
- Suda, A.; Makino, T.; Higashi, T. Extractability of manganese and iron oxides in typical Japanese soils by 0.5 mol/L hydroxylamine hydrochloride (pH 1.5). Soil Sci. Plant Nutr. 2012, 58, 684–695. [Google Scholar] [CrossRef]
- Suda, A.; Makino, T.; Higashi, T. An improved selective extraction method for Mn oxides and occluded metals with emphasis on applicability to Andisols. Soil Sci. Plant Nutr. 2013, 59, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Petroselli, C.; Moroni, B.; Crocchianti, S.; Selvaggi, R.; Vivani, R.; Soggia, F.; Grotti, M.; d’Acapito, F.; Cappelletti, D. Iron Speciation of Natural and Anthropogenic Dust by Spectroscopic and Chemical Methods. Atmosphere 2019, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Federici, E.; Petroselli, C.; Montalbani, E.; Casagrande, C.; Ceci, E.; Moroni, B.; Porta, G.L.; Castellini, S.; Selvaggi, R.; Sebastiani, B.; et al. Airborne bacteria and persistent organic pollutants associated with an intense Saharan dust event in the Central Mediterranean. Sci. Total Environ. 2018, 645, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Moroni, B.; Castellini, S.; Crocchianti, S.; Piazzalunga, A.; Fermo, P.; Scardazza, F.; Cappelletti, D. Ground-based measurements of long-range transported aerosol at the rural regional background site of Monte Martano (Central Italy). Atmos. Res. 2015, 155, 26–36. [Google Scholar] [CrossRef]
- Draxler, R.; Rolph, G. HYSPLIT. (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model access via NOAA ARL READY; US Department of Commerce: Washington, DC, USA, 2012.
- Majestic, B.; Schaufer, J.; Shater, M.; Turner, J.; Fine, P.; Singh, M.; Sioutas, C. Development of a wet-chemical method for the speciation of iron in atmospheric aerosols. Environ. Sci. Technol. 2006, 40, 2346–2351. [Google Scholar] [CrossRef]
- Pueyo, M.; Rauret, G.; Lück, D.; Yli-Halla, M.; Muntau, H.; Quevauviller, P.; Lopez-Sanchez, J. Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimised three-step sequential extraction procedure. J. Environ. Monit. 2001, 3, 243–250. [Google Scholar] [CrossRef]
- Smichowski, P.; Polla, G.; Gomez, D. Metal fractionation of atmospheric aerosols via sequential chemical extraction: A review. Anal. Bioanal. Chem. 2005, 381, 302–316. [Google Scholar] [CrossRef]
- d’Acapito, F.; Trapananti, A.; Puri, A. LISA: The Italian CRG beamline for X-ray Absorption Spectroscopy at ESRF. J. Phys. Conf. Ser. 2016, 712, 012021. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Date | Code | PM | PM | PM/PM | Fe [g m] | Fe [atoms cm] |
|---|---|---|---|---|---|---|
| 30 November 2014 | SH_Dec_PM | 83.9 | 0.38 | 2.36(1) | 7.64(2) × 10 | |
| SH_Dec_PM | 32.3 | 0.38 | 0.39(2) | 3.2(1) × 10 | ||
| 01 December 2014 | SH_Dec_PM | 86.9 | 0.36 | 7.81(1) | 2.564(3) × 10 | |
| SH_Dec_PM | 31.2 | 0.36 | 0.39(1) | 3.30(5) × 10 | ||
| 19 February 2014 | mix-SH_Feb_PM | 18.9 | 7.7 | 0.41 | 1.174(1) | 2.701(3) × 10 |
| 23 April 2014 | mix-SH_Apr_PM | 12.4 | 8.5 | 0.68 | 0.990(1) | 1.622(2) × 10 |
| 31 October 2014 | non-SH_Oct_PM | 18.9 | 16.9 | 0.89 | 0.099(3) | 8.2(2) × 10 |
| 07 January 2015 | non-SH_Jan_PM | 22.0 | 18.5 | 0.84 | 0.071(1) | 5.91(4) × 10 |
| blank | blank_SWAM | – | – | – | 0.026(4) | 2.2(3) × 10 |
| blank | blank_HVS | – | – | – | 0.055(1) | 1.44(3) × 10 |
| Sample (PM) | Centroid | Intensity |
|---|---|---|
| (±0.2) | (±0.02) | |
| SH_Dec-3011 | 7114.9 | 0.05 |
| SH_Dec-0112 | 7115.0 | 0.05 |
| mix-SH_Feb | 7114.7 | 0.11 |
| mix-SH_Apr | 7114.5 | 0.17 |
| Sample (PM) | Centroid | Intensity |
| (±0.2) | (±0.02) | |
| SH_Dec-3011 | 7115.0 | 0.05 |
| SH_Dec-0112 | 7114.9 | 0.05 |
| non-SH_Oct | 7113.9 | 0.16 |
| non-SH_Jan | 7114.4 | 0.20 |
| SWAM-blank | 7114.4 | 0.20 |
| Sample | Fe-O | Fe-Fe | ||||
|---|---|---|---|---|---|---|
| r [Å] | N | [Å] | r [Å] | N | [Å] | |
| PM | ||||||
| SH_Dec-3011 | 1.98 ± 0.01 | 5.4 ± 0.5 | 0.009 ± 0.005 | 2.96 ± 0.02 | 0.8 ± 0.5 | 0.02 ± 0.02 |
| SH_Dec-0112 | 1.98 ± 0.01 | 5.5 ± 0.4 | 0.009 ± 0.003 | 2.95 ± 0.01 | 0.8 ± 0.3 | 0.01 ± 0.01 |
| mix-SH_Feb | 1.96 ± 0.01 | 5.2 ± 0.4 | 0.010 ± 0.004 | 2.93 ± 0.01 | 0.6 ± 0.3 | 0.02 ± 0.02 |
| mix-SH_Apr | 1.95 ± 0.01 | 5.2 ± 0.2 | 0.006 ± 0.005 | 3.03 ± 0.01 | 2.2 ± 0.9 | 0.02 ± 0.02 |
| PM | ||||||
| SH_Dec-3011 | 1.98 ± 0.01 | 5.4 ± 0.3 | 0.009 ± 0.003 | 2.95 ± 0.01 | 0.9 ± 0.3 | 0.01 ± 0.01 |
| SH_Dec-0112 | 1.98 ± 0.01 | 5.5 ± 0.6 | 0.010 ± 0.005 | 2.95 ± 0.02 | 0.8 ± 0.5 | 0.02 ± 0.02 |
| non-SH_Oct | 1.93 ± 0.01 | 4.8 ± 0.3 | 0.009 ± 0.004 | 2.94 ± 0.01 | 0.9 ± 0.3 | 0.01 ± 0.01 |
| non-SH_Jan | 1.92 ± 0.01 | 4.9 ± 0.1 | 0.004 ± 0.002 | 2.98 ± 0.01 | 4.8 ± 0.6 | 0.02 ± 0.01 |
| SWAM-blank | 1.84 ± 0.01 | 4.4 ± 0.1 | 0.008 ± 0.004 | 2.84 ± 0.01 | 1.1 ± 0.1 | 0.01 ± 0.01 |
| Date | Sample | I (%) | II (%) | III (%) | IV (%) | tot Fe (g) |
|---|---|---|---|---|---|---|
| 24 April 2014 | mix-SH_Apr_PM | 2 | 5 | 23 | 69 | 2.0 |
| 19 February 2014 | mix-SH_Feb_PM | 10 | 11 | 8 | 70 | 11.1 |
| 30 November 2014 | SH_Dec_PM | 14 | 6 | 8 | 72 | 29.6 |
| 01 December 2014 | SH_Dec_PM | 12 | 7 | 8 | 73 | 100.6 |
| 30 November 2014 | SH_Dec_PM | 16 | 7 | 8 | 69 | 9.6 |
| 01 December 2014 | SH_Dec_PM | 18 | 8 | 13 | 61 | 11.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroselli, C.; Moroni, B.; Crocchianti, S.; Selvaggi, R.; Soggia, F.; Grotti, M.; d’Acapito, F.; Cappelletti, D. Iron Speciation in Different Saharan Dust Advections and Effect of the Procedural Blank on the Results From X-ray Absorption Spectroscopy and Selective Leaching Experiments. Atmosphere 2020, 11, 735. https://doi.org/10.3390/atmos11070735
Petroselli C, Moroni B, Crocchianti S, Selvaggi R, Soggia F, Grotti M, d’Acapito F, Cappelletti D. Iron Speciation in Different Saharan Dust Advections and Effect of the Procedural Blank on the Results From X-ray Absorption Spectroscopy and Selective Leaching Experiments. Atmosphere. 2020; 11(7):735. https://doi.org/10.3390/atmos11070735
Chicago/Turabian StylePetroselli, Chiara, Beatrice Moroni, Stefano Crocchianti, Roberta Selvaggi, Francesco Soggia, Marco Grotti, Francesco d’Acapito, and David Cappelletti. 2020. "Iron Speciation in Different Saharan Dust Advections and Effect of the Procedural Blank on the Results From X-ray Absorption Spectroscopy and Selective Leaching Experiments" Atmosphere 11, no. 7: 735. https://doi.org/10.3390/atmos11070735
APA StylePetroselli, C., Moroni, B., Crocchianti, S., Selvaggi, R., Soggia, F., Grotti, M., d’Acapito, F., & Cappelletti, D. (2020). Iron Speciation in Different Saharan Dust Advections and Effect of the Procedural Blank on the Results From X-ray Absorption Spectroscopy and Selective Leaching Experiments. Atmosphere, 11(7), 735. https://doi.org/10.3390/atmos11070735