Characterization of Atmospheric PM2.5 Inorganic Aerosols Using the Semi-Continuous PPWD-PILS-IC System and the ISORROPIA-II
Abstract
:1. Introduction
2. Experimental Method
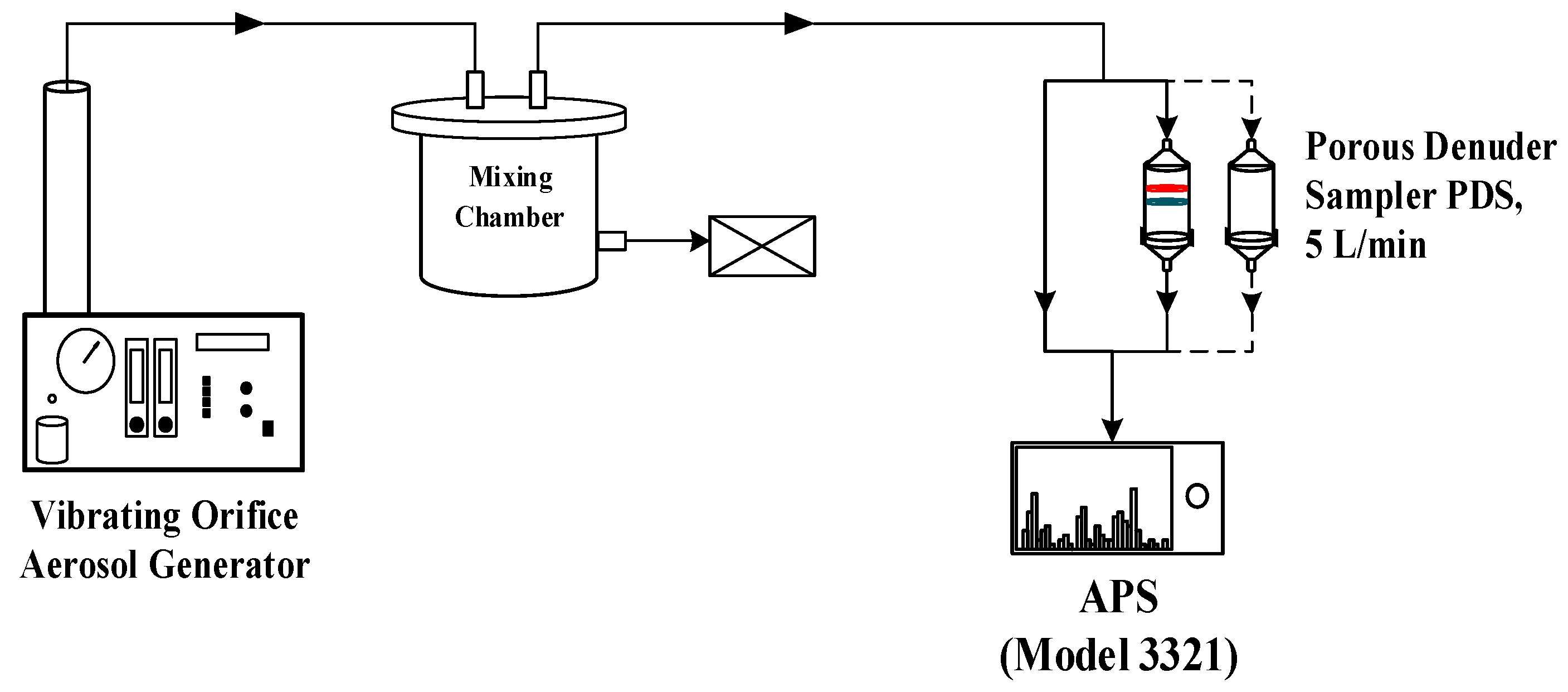
2.1. Five Liters Per Minute PDS Design and Laboratory Test
2.2. Field Comparison Test
2.3. ISORROPIA-II Thermodynamic Equilibrium Model
3. Results and Discussion
3.1. Laboratory Test Results of the 5 L/min PDS
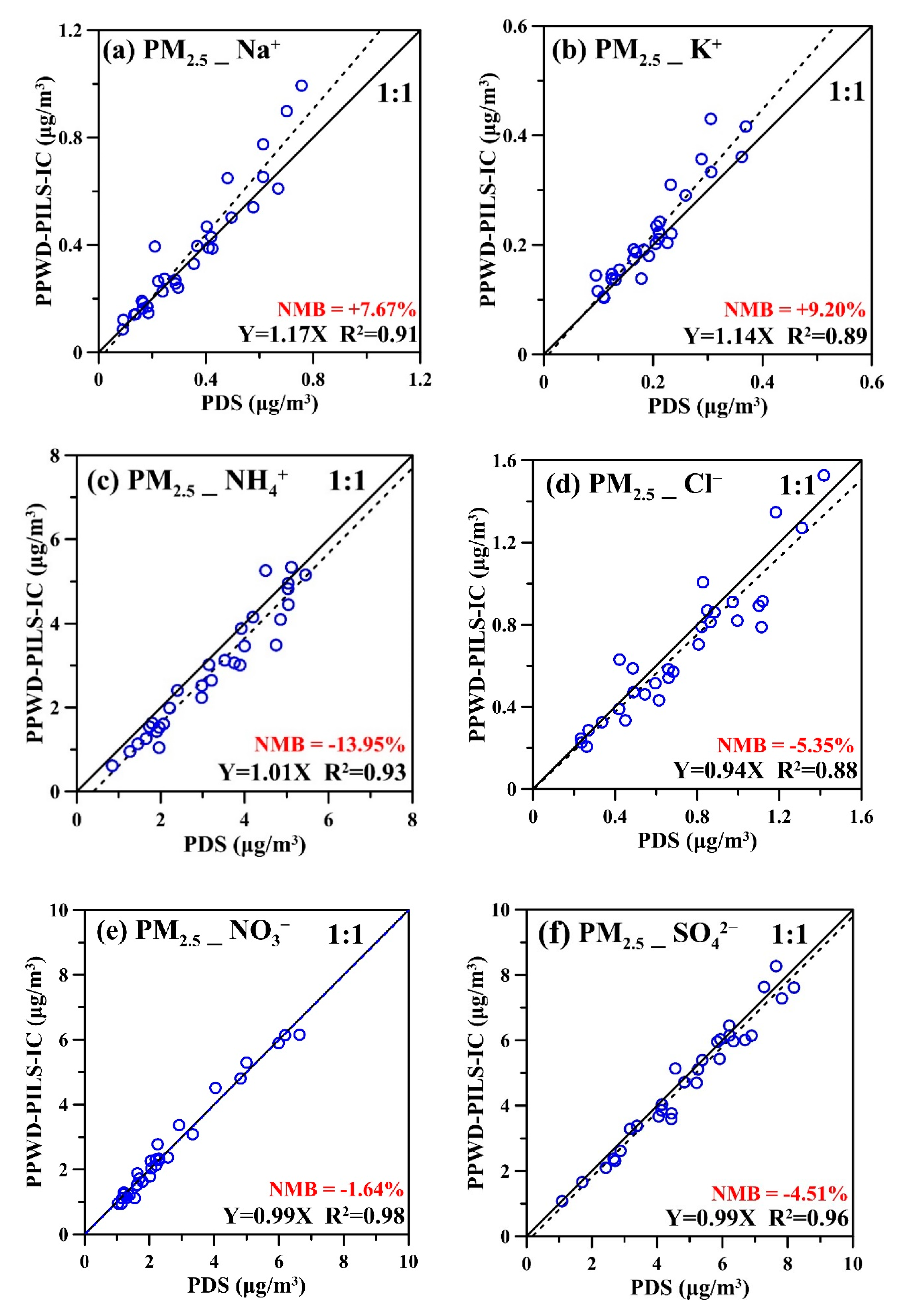
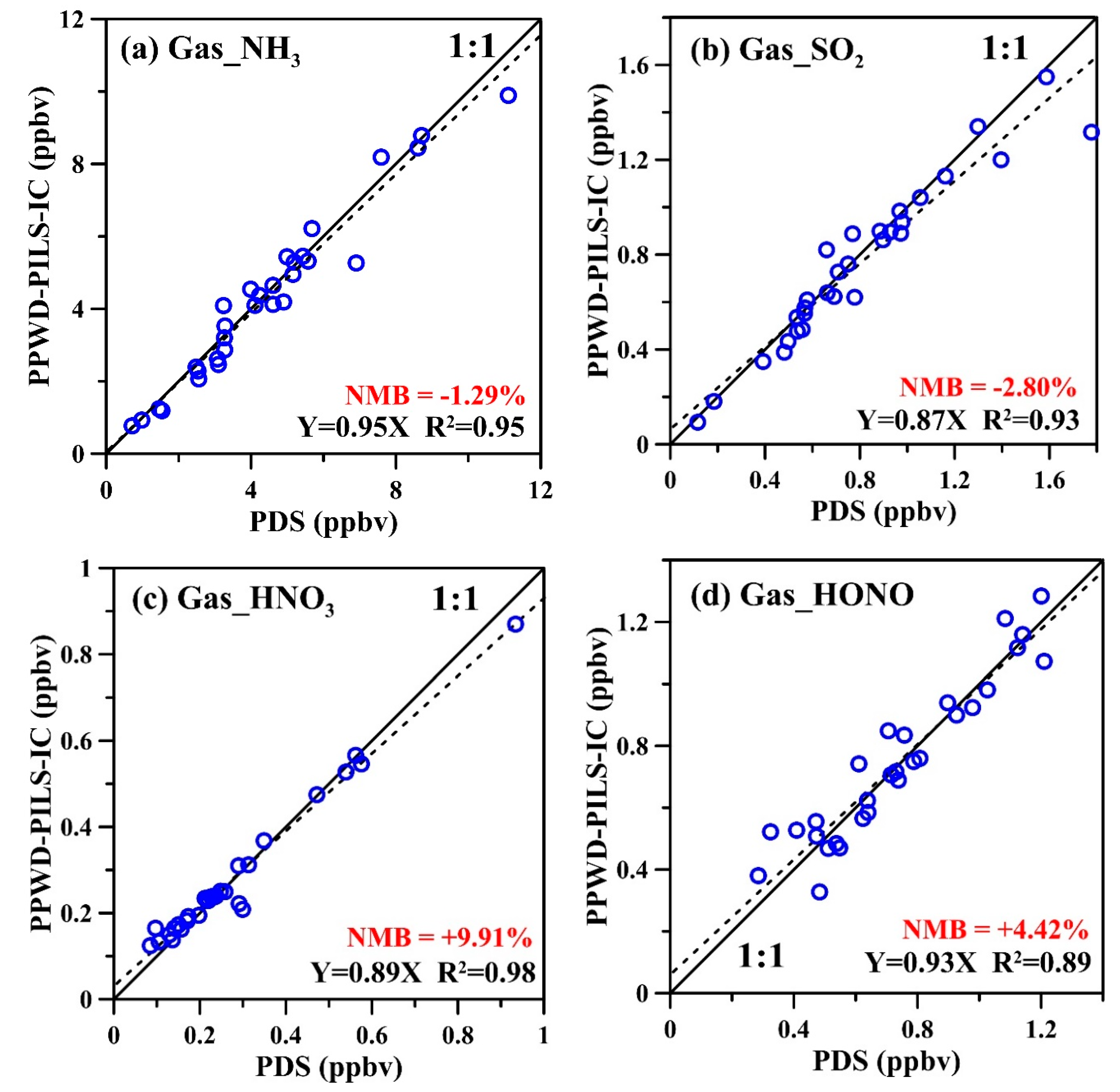
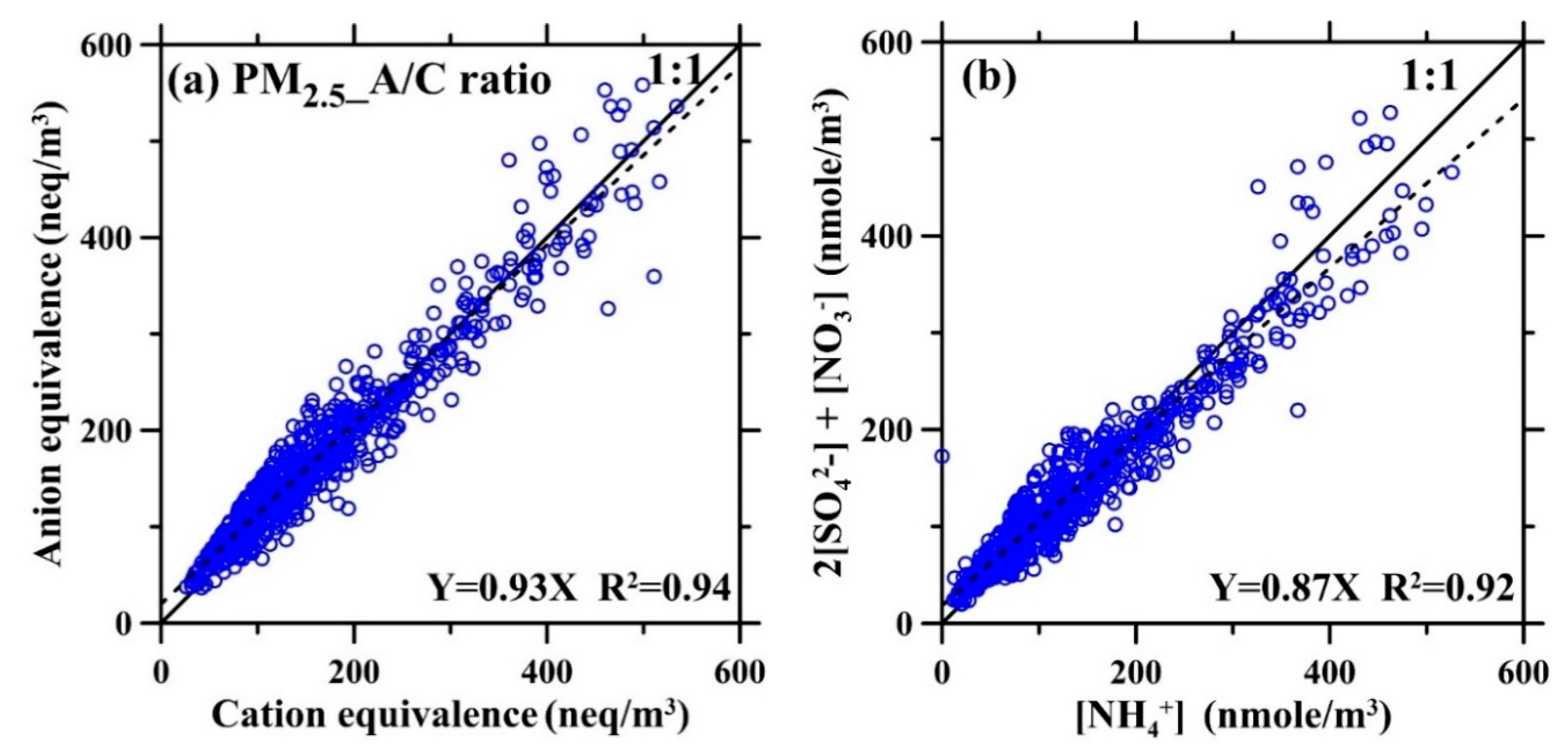
3.2. Field Comparison Test Results of PPWD-PILS-IC and PDS
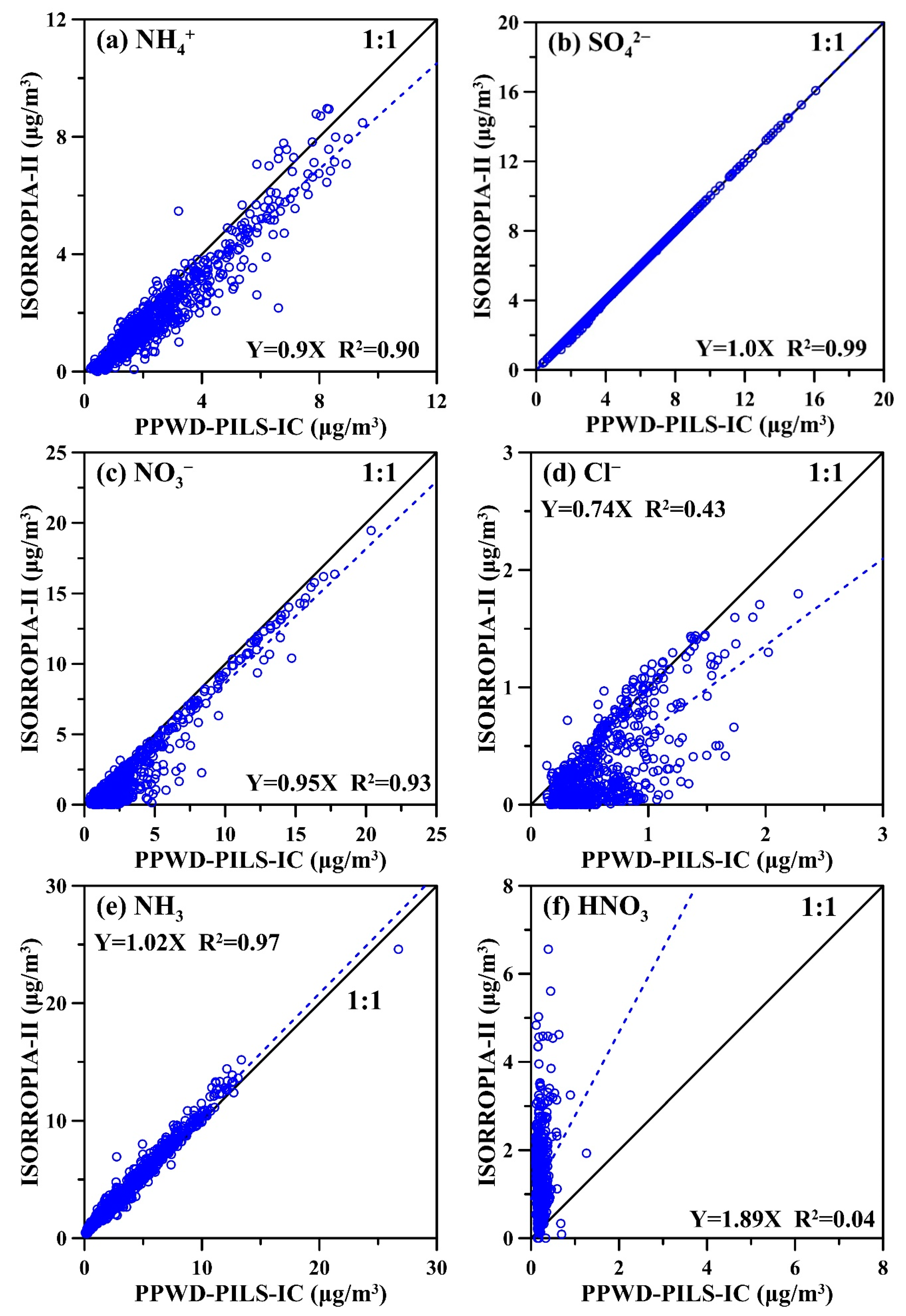
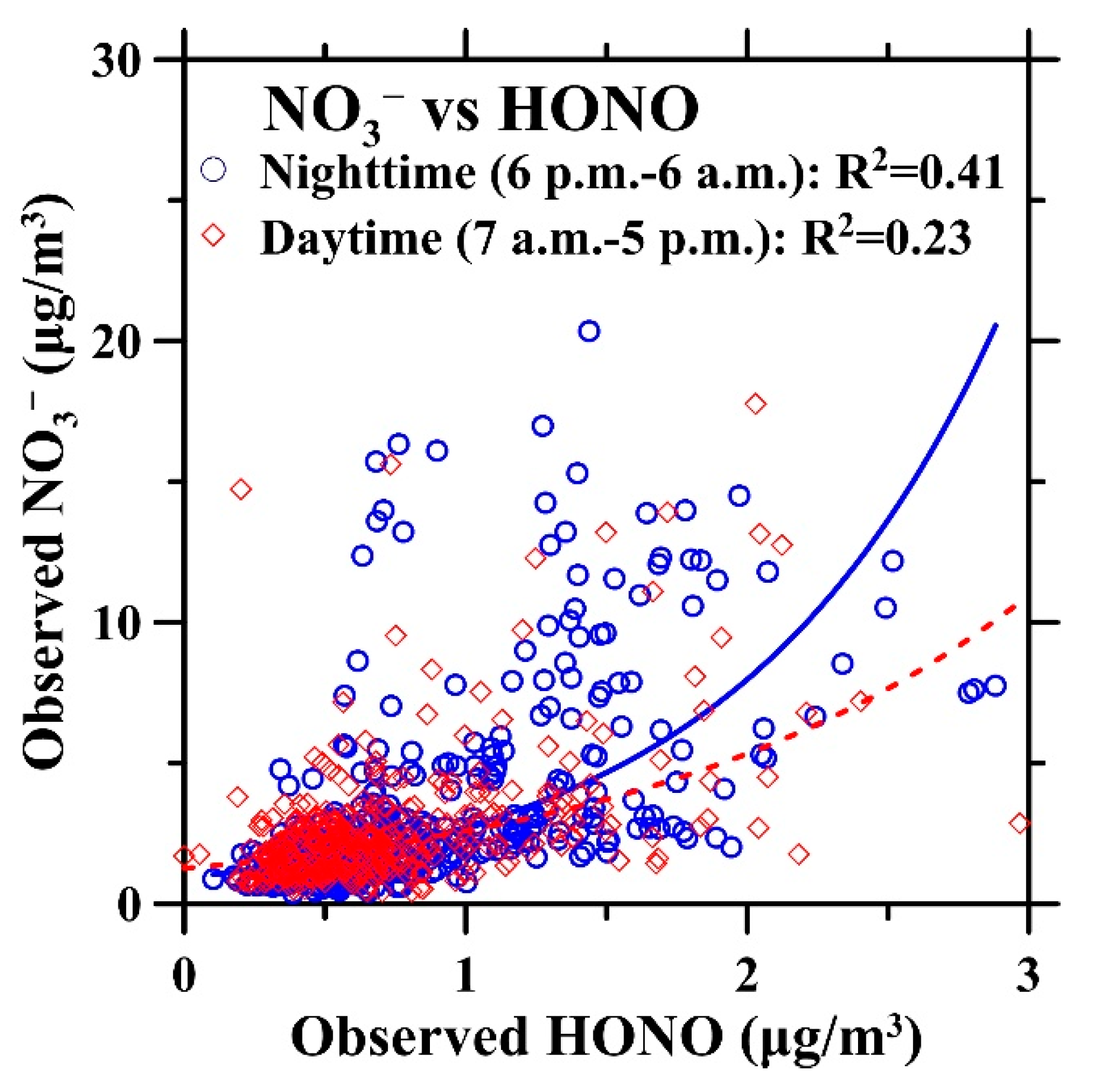
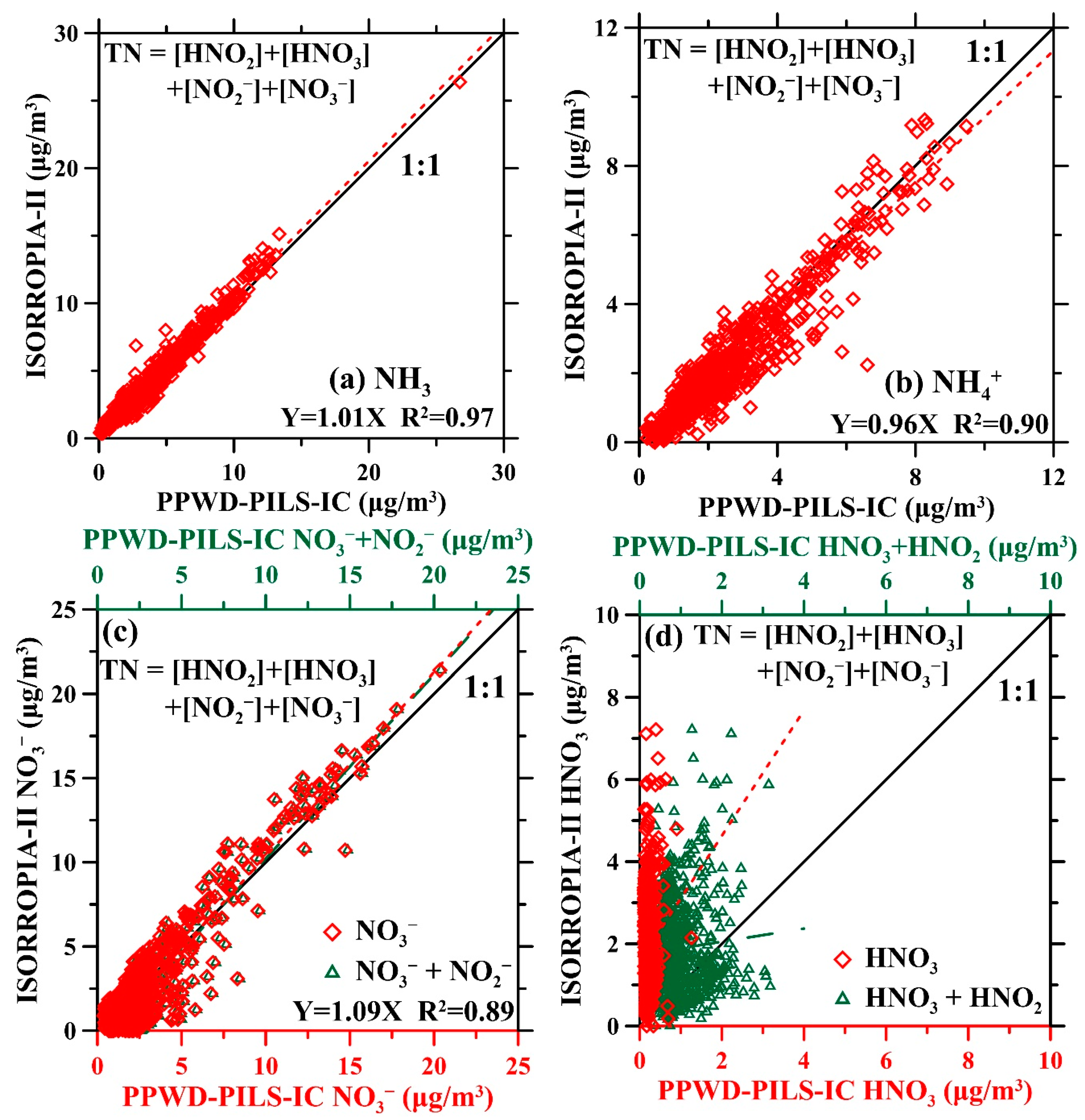
3.3. ISORROPIA-II Thermodynamic Equilibrium Model Predictions Versus PPWD-PILS-IC Observations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miller, L.; Xu, X. Ambient PM2.5 human health effects—Findings in china and research directions. Atmosphere 2018, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Braggio, J.T.; Hall, E.S.; Weber, S.A.; Huff, A.K. Contribution of satellite-derived aerosol optical depth PM2.5 bayesian concentration surfaces to respiratory-cardiovascular chronic disease hospitalizations in Baltimore, Maryland. Atmosphere 2020, 11, 209. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Tao, J.; Du, Y.; Liu, T.; Qian, Z.; Tian, L.; Di, Q.; Rutherford, S.; Guo, L.; Zeng, W.; et al. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ. Pollut. 2016, 208, 758–766. [Google Scholar] [CrossRef]
- Hwang, S.L.; Lin, Y.C.; Lin, C.M.; Hsiao, K.Y. Effects of fine particulate matter and its constituents on emergency room visits for asthma in southern Taiwan during 2008–2010: A population-based study. Environ. Sci. Pollut. Res. Int. 2017, 24, 15012–15021. [Google Scholar] [CrossRef]
- Guo, W.; Long, C.; Zhang, Z.; Zheng, N.; Xiao, H.; Xiao, H. Seasonal control of water-soluble inorganic ions in PM2.5 from nanning, a subtropical monsoon climate city in southwestern China. Atmosphere 2019, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.H.; Lin, J.H.; Yao, Y.C.; Chiang, H.L. Size distribution and water soluble ions of ambient particulate matter on episode and non-episode days in southern Taiwan. Aerosol. Air Qual. Res. 2012, 12, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Zhou, X.; Ma, Y.; Wang, L.; Wu, R.; Chen, B.; Wang, W. The concentrations, formations, relationships and modeling of sulfate, nitrate and ammonium (SNA) aerosols over China. Aerosol. Air Qual. Res. 2017, 17, 84–97. [Google Scholar] [CrossRef]
- Ramli, N.A.; Md Yusof, N.F.F.; Shith, S.; Suroto, A. Chemical and biological compositions associated with ambient respirable particulate matter: A review. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.D.; Luo, B.; Zhai, C. Characteristics and sources of water-soluble ions in PM2.5 in the sichuan basin, China. Atmosphere 2019, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Ying, Q.; Kleeman, M.J. Source contributions to the regional distribution of secondary particulate matter in California. Atmos. Environ. 2006, 40, 736–752. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Tsai, J.H.; Tsai, S.M.; Wang, W.C.; Chiang, H.L. Water-soluble ionic species of coarse and fine particulate matter and gas precursor characteristics at urban and rural sites of central Taiwan. Environ. Sci. Pollut. Res. Int. 2016, 23, 16722–16737. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Seo, J.; Kim, J.Y.; Kim, Y.P.; Jin, H.C. Local formation of sulfates contributes to the urban haze with regional transport origin. Environ. Res. Lett. 2020. [Google Scholar] [CrossRef]
- Voutsa, D.; Samara, C.; Manoli, E.; Lazarou, D.; Tzoumaka, P. Ionic composition of PM2.5 at urban sites of northern Greece: Secondary inorganic aerosol formation. Environ. Sci. Pollut. Res. Int. 2014, 21, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, R.; Zhang, Y.; Meng, Y.; Fu, H.; Chen, J. An observational study of nitrous acid (HONO) in Shanghai, China: The aerosol impact on HONO formation during the haze episodes. Sci. Total Environ. 2018, 630, 1057–1070. [Google Scholar] [CrossRef]
- Xu, W.; Kuang, Y.; Zhao, C.; Tao, J.; Zhao, G.; Bian, Y.; Yang, W.; Yu, Y.; Shen, C.; Liang, L.; et al. NH3-promoted hydrolysis of NO2 induces explosive growth in HONO. Atmos. Chem. Phys. 2019, 19, 10557–10570. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Chen, Y.; Liu, X.; Zhang, J.; Guo, Y.; An, J. Seasonal effects of additional HONO sources and the heterogeneous reactions of N2O5 on nitrate in the North China Plain. Sci. Total Environ. 2019, 690, 97–107. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, N.; Gao, H.; Zhou, X. Photolysis of particulate nitrate as a source of HONO and NOx. Environ. Sci. Technol. 2017, 51, 6849–6856. [Google Scholar] [CrossRef]
- Tsai, C.; Spolaor, M.; Colosimo, S.F.; Pikelnaya, O.; Cheung, R.; Williams, E.; Gilman, J.B.; Lerner, B.M.; Zamora, R.J.; Warneke, C.; et al. Nitrous acid formation in a snow-free wintertime polluted rural area. Atmos. Chem. Phys. 2018, 18, 1977–1996. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Duan, J.; Tan, J.; Ma, Y.; He, K.; Wang, S.; Huang, X.; Zhang, Y. Gas-to-particle conversion of atmospheric ammonia and sampling artifacts of ammonium in spring of Beijing. Sci. China Earth Sci. 2014, 58, 345–355. [Google Scholar] [CrossRef]
- Le, T.C.; Shukla, K.K.; Chen, Y.T.; Chang, S.C.; Lin, T.Y.; Li, Z.; Pui, D.Y.H.; Tsai, C.J. On the concentration differences between PM2.5 FEM monitors and FRM samplers. Atmos. Environ. 2020, 222, 117138. [Google Scholar] [CrossRef]
- Liu, C.N.; Lin, S.F.; Tsai, C.J.; Wu, Y.C.; Chen, C.F. Theoretical model for the evaporation loss of PM2.5 during filter sampling. Atmos. Environ. 2015, 109, 79–86. [Google Scholar] [CrossRef]
- Liu, C.N.; Awasthi, A.; Hung, Y.H.; Gugamsetty, B.; Tsai, C.J.; Wu, Y.C.; Chen, C.F. Differences in 24-h average PM2.5 concentrations between the beta attenuation monitor (BAM) and the dichotomous sampler (Dichot). Atmos. Environ. 2013, 75, 341–347. [Google Scholar] [CrossRef]
- Liu, C.N.; Lin, S.F.; Awasthi, A.; Tsai, C.J.; Wu, Y.C.; Chen, C.F. Sampling and conditioning artifacts of PM2.5 in filter-based samplers. Atmos. Environ. 2014, 85, 48–53. [Google Scholar] [CrossRef]
- Tsai, C.J.; Huang, C.H.; Wang, S.H.; Shih, T.S. Design and testing of a personal porous-metal denuder. Aerosol. Sci. Technol. 2001, 35, 611–616. [Google Scholar] [CrossRef]
- Tsai, C.J.; Huang, C.H.; Lin, Y.C.; Shih, T.S.; Shih, B.H. Field test of a porous-metal denuder sampler. Aerosol. Sci. Technol. 2003, 37, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Trebs, I.; Meixner, F.; Slanina, J.; Otjes, R.; Jongejan, P.; Andreae, M. Real-time measurements of ammonia, acidic trace gases and water-soluble inorganic aerosol species at a rural site in the Amazon Basin. Atmos. Chem. Phys. 2004, 4, 967–987. [Google Scholar] [CrossRef] [Green Version]
- ten Brink, H.; Otjes, R.; Jongejan, P.; Slanina, S. An instrument for semi-continuous monitoring of the size-distribution of nitrate, ammonium, sulphate and chloride in aerosol. Atmos. Environ. 2007, 41, 2768–2779. [Google Scholar] [CrossRef] [Green Version]
- Godri, K.; Evans, G.; Slowik, J.; Knox, A.; Abbatt, J.; Brook, J.; Dann, T.; Dabek-Zlotorzynska, E. Evaluation and application of a semi-continuous chemical characterization system for water soluble inorganic PM2.5 and associated precursor gases. Atmos. Meas. Tech. 2009, 2, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Nie, W.; Wang, T.; Gao, X.; Pathak, R.K.; Wang, X.; Gao, R.; Zhang, Q.; Yang, L.; Wang, W. Comparison among filter-based, impactor-based and continuous techniques for measuring atmospheric fine sulfate and nitrate. Atmos. Environ. 2010, 44, 4396–4403. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Lin, Y.; Gautam, S.; Kuo, H.C.; Tsai, C.J.; Yeh, H.; Huang, W.; Li, S.W.; Wu, G.J. Development of an automated system (PPWD/PILS) for studying PM2.5 water-soluble ions and precursor gases: Field measurements in two cities, Taiwan. Aerosol. Air Qual. Res. 2017, 17, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Rumsey, I.; Cowen, K.; Walker, J.; Kelly, T.; Hanft, E.; Mishoe, K.; Rogers, C.; Proost, R.; Beachley, G.; Lear, G. An assessment of the performance of the monitor for AeRosols and GAses in ambient air (MARGA): A semi-continuous method for soluble compounds. Atmos. Chem. Phys. 2014, 14, 5639–5658. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Shairsingh, K.; Lam, P.H.; Evans, G.J. Underestimation of sulfate concentration in PM 2.5 using a semi-continuous particle instrument based on ion chromatography. J. Environ. Monit. 2009, 11, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S.; Wang, T. On the performance of a semi-continuous PM2.5 sulphate and nitrate instrument under high loadings of particulate and sulphur dioxide. Atmos. Environ. 2007, 41, 5442–5451. [Google Scholar] [CrossRef]
- Markovic, M.Z.; VandenBoer, T.C.; Murphy, J.G. Characterization and optimization of an online system for the simultaneous measurement of atmospheric water-soluble constituents in the gas and particle phases. J. Environ. Monit. 2012, 14, 1872–1884. [Google Scholar] [CrossRef]
- Ku, Y.P.; Yang, C.; Lin, G.Y.; Tsai, C.J. An online parallel-plate wet denuder system for monitoring acetic acid gas. Aerosol. Air Qual. Res. 2010, 10, 479–488. [Google Scholar] [CrossRef]
- Tsai, C.J.; Lin, G.Y.; Chen, S.C. A parallel plate wet denuder for acidic gas measurement. AIChE 2008, 54, 2198–2205. [Google Scholar] [CrossRef]
- Weber, R.J.; Orsini, D.; Daun, Y.; Lee, Y.N.; Klotz, P.J.; Brechtel, F. A particle-into-liquid collector for rapid measurement of aerosol bulk chemical composition. Aerosol. Sci. Technol. 2001, 35, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Weber, R.; Orsini, D.; Duan, Y.; Baumann, K.; Kiang, C.; Chameides, W.; Lee, Y.; Brechtel, F.; Klotz, P.; Jongejan, P. Intercomparison of near real time monitors of PM2.5 nitrate and sulfate at the US environmental protection agency atlanta supersite. J. Geophys. Res. 2003, 108, 1984–2012. [Google Scholar] [CrossRef]
- Clegg, S.L.; Pitzer, K.S. Thermodynamics of multicomponent, miscible, ionic solutions: Generalized equations for symmetrical electrolytes. J. Phys. Chem. 1992, 96, 3513–3520. [Google Scholar] [CrossRef]
- Kim, Y.P.; Seinfeld, J.H.; Saxena, P. Atmospheric gas-aerosol equilibrium I. Thermodynamic model. Aerosol. Sci. Technol. 1993, 19, 157–181. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Tabazadeh, A.; Turco, R.P. Simulating equilibrium within aerosols and nonequilibrium between gases and aerosols. J. Geophys. Res. 1996, 101, 9079–9091. [Google Scholar] [CrossRef]
- Nenes, A.; Pandis, S.N.; Pilinis, C. ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aquat. Geochem. 1998, 4, 123–152. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-NH4+-Na+-SO42−-NO3−-Cl−-H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.S.; Pandis, S.N. An analysis of four models predicting the partitioning of semivolatile inorganic aerosol components. Aerosol. Sci. Technol. 1999, 31, 129–153. [Google Scholar] [CrossRef]
- Metzger, S.; Dentener, F.; Pandis, S.; Lelieveld, J. Gas/aerosol partitioning: 1. A computationally efficient model. J. Geophys. Res. 2002, 107, 1611–1624. [Google Scholar] [CrossRef] [Green Version]
- Makar, P.; Bouchet, V.; Nenes, A. Inorganic chemistry calculations using HETV—A vectorized solver for the SO42−–NO3−–NH4+ system based on the ISORROPIA algorithms. Atmos. Environ. 2003, 37, 2279–2294. [Google Scholar] [CrossRef]
- Zaveri, R.A.; Easter, R.C.; Peters, L.K. A computationally efficient multicomponent equilibrium solver for aerosols (MESA). J. Geophys. Res. 2005, 110, D24203. [Google Scholar] [CrossRef]
- Amundson, N.; Caboussat, A.; He, J.; Martynenko, A.; Savarin, V.; Seinfeld, J.; Yoo, K. A new inorganic atmospheric aerosol phase equilibrium model (UHAERO). Atmos. Chem. Phys. 2006, 6, 975–992. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, A.; Rengarajan, R. Time-resolved inorganic chemical composition of fine aerosol and associated precursor gases over an urban environment in western India: Gas-aerosol equilibrium characteristics. Atmos. Environ. 2015, 109, 217–227. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A.; Sullivan, A.; Weber, R.; Reken, T.V.; Fischer, M.; Matias, E.; Moya, M.; Farmer, D.; Cohen, R.C. Thermodynamic characterization of Mexico city aerosol during MILAGRO 2006. Atmos. Chem. Phys. 2009, 9, 2141–2156. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Liu, J.; Froyd, K.D.; Roberts, J.M.; Veres, P.R.; Hayes, P.L.; Jimenez, J.L.; Nenes, A.; Weber, R.J. Fine particle pH and gas–particle phase partitioning of inorganic species in Pasadena, California, during the 2010 CalNex campaign. Atmos. Chem. Phys. 2017, 17, 5703–5719. [Google Scholar] [CrossRef] [Green Version]
- Le, T.C.; Tsai, C.J. Novel non-bouncing PM2.5 impactor modified from well impactor ninety-six. Aerosol. Sci. Technol. 2017, 51, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Le, T.C.; Shukla, K.K.; Sung, J.C.; Li, Z.; Yeh, H.; Huang, W.; Tsai, C.J. Sampling efficiency of low-volume PM10 inlets with different impaction substrates. Aerosol. Sci. Technol. 2019, 53, 295–308. [Google Scholar] [CrossRef]
- Chen, S.C.; Tsai, C.J.; Huang, C.Y.; Chen, H.D.; Chen, S.J.; Lin, C.C.; Tsai, J.H.; Chou, C.C.K.; Lung, S.C.C.; Huang, W.R. Chemical mass closure and chemical characteristics of ambient ultrafine particles and other PM fractions. Aerosol. Sci. Technol. 2010, 44, 713–723. [Google Scholar] [CrossRef]
- Le, T.C.; Fu, C.X.; Sung, C.J.; Li, Z.Y.; Pui, D.Y.H.; Tsai, C.J. The performance of the PM2.5 VSCC and oil-Wetted M-WINS in long-Term field sampling studies. Atmos. Environ. 2020. [Google Scholar] [CrossRef]
- Le, T.C.; Tsai, C.J. Inertial impaction technique for the classification of particulate matters and nanoparticles: A review. KONA Powder Part J. 2020. [Google Scholar] [CrossRef] [Green Version]
- U.S. EPA. Quality Assurance Guidance Document 2.12, Monitoring PM2.5 in Ambient Air Using Designated Reference or Class. I. Equivalent Methods; Environmental Protection Agency: Research Triangle Park (RTP), NC, USA, 1998.
- Sanusi, A.; Wortham, H.; Millet, M.; Mirabel, P. Chemical composition of rainwater in eastern France. Atmos. Environ. 1996, 30, 59–71. [Google Scholar] [CrossRef]
- Sorooshian, A.; Brechtel, F.J.; Ma, Y.; Weber, R.J.; Corless, A.; Flagan, R.C.; Seinfeld, J.H. Modeling and characterization of a particle-into-liquid sampler (PILS). Aerosol. Sci. Technol. 2006, 40, 396–409. [Google Scholar] [CrossRef] [Green Version]
- Huy, D.H.; Thanh, L.T.; Hien, T.T.; Takenaka, N. Comparative study on water-soluble inorganic ions in PM2.5 from two distinct climate regions and air quality. J. Environ. Sci. 2020, 88, 349–360. [Google Scholar] [CrossRef]
- Liu, C.N.; Awasthi, A.; Hung, Y.H.; Tsai, C.J. Collection efficiency and interstage loss of nanoparticles in micro-orifice-based cascade impactors. Atmos. Environ. 2013, 69, 325–333. [Google Scholar] [CrossRef]
- Chien, C.L.; Tien, C.Y.; Liu, C.N.; Ye, H.; Huang, W.; Tsai, C.J. Design and testing of the NCTU Micro-Orifice Cascade Impactor (NMCI) for the measurement of nanoparticle size distributions. Aerosol. Sci. Technol. 2015, 49, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Tobo, Y.; Zhang, D.; Nakata, N.; Yamada, M.; Ogata, H.; Hara, K.; Iwasaka, Y. Hygroscopic mineral dust particles as influenced by chlorine chemistry in the marine atmosphere. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; Kang, C.M.; Koutrakis, P. Composition and sources of fine and coarse particles collected during 2002–2010 in Boston, MA. J. Air Waste Manag. Assoc. 2015, 65, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, E.T.; Griffin, R.J.; Steiner, A.L.; Dibb, J.; Scheuer, E.; Gong, L.; Rutter, A.P.; Cevik, B.K.; Kim, S.; Lefer, B.; et al. Evaluation of nitrous acid sources and sinks in urban outflow. Atmos. Environ. 2016, 127, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Gil, J.; Kim, J.; Lee, M.; Lee, G.; Lee, D.; Jung, J.; An, J.; Hong, J.; Cho, S.; Lee, J.; et al. The role of HONO in O3 formation and insight into its formation mechanism during the KORUS-AQ Campaign. Atmos. Chem. Phys. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Wang, G.; Zhang, S.; Li, D.; Xie, Y.; Wu, C.; Yuan, Q.; Chen, J.; Zhang, H. Abundant NH3 in China enhances atmospheric HONO production by promoting the heterogeneous reaction of SO2 with NO2. Environ. Sci. Technol. 2019, 53, 14339–14347. [Google Scholar] [CrossRef]
- TWEPA. Taiwan Air Quality Index. Available online: https://airtw.epa.gov.tw/ENG/default.aspx (accessed on 30 June 2020).
- Kleffmann, J.; Wiesen, P. Heterogeneous conversion of NO2 and NO on HNO3 treated soot surfaces: Atmospheric implications. Atmos. Chem. Phys. 2005, 5, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Tsai, C.J.; Chou, C.C.K.; Roam, G.D.; Cheng, S.S.; Wang, Y.N. Ultrafine particles at three different sampling locations in Taiwan. Atmos. Environ. 2010, 44, 533–540. [Google Scholar] [CrossRef]



| NCTU | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F− | Cl− | NO2− | NO3− | SO42− | Total | A/C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass conc. (μg/m3) | 0.35 ± 0.19 | 3.22 ± 1.38 | 0.20 ± 0.07 | 0.07 ± 0.02 | 0.04 ± 0.02 | 0.31 ± 0.15 | 0.72 ± 0.33 | 0.22 ± 0.33 | 2.58 ± 1.60 | 4.02 ± 1.87 | 12.63 ± 1.71 | 0.92 ± 0.2 |
| Percentage (%) | 2.74 | 25.52 | 1.57 | 0.58 | 0.32 | 2.47 | 5.71 | 1.75 | 20.41 | 38.94 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.-C.; Wang, Y.-C.; Pui, D.Y.H.; Tsai, C.-J. Characterization of Atmospheric PM2.5 Inorganic Aerosols Using the Semi-Continuous PPWD-PILS-IC System and the ISORROPIA-II. Atmosphere 2020, 11, 820. https://doi.org/10.3390/atmos11080820
Le T-C, Wang Y-C, Pui DYH, Tsai C-J. Characterization of Atmospheric PM2.5 Inorganic Aerosols Using the Semi-Continuous PPWD-PILS-IC System and the ISORROPIA-II. Atmosphere. 2020; 11(8):820. https://doi.org/10.3390/atmos11080820
Chicago/Turabian StyleLe, Thi-Cuc, Yun-Chin Wang, David Y. H. Pui, and Chuen-Jinn Tsai. 2020. "Characterization of Atmospheric PM2.5 Inorganic Aerosols Using the Semi-Continuous PPWD-PILS-IC System and the ISORROPIA-II" Atmosphere 11, no. 8: 820. https://doi.org/10.3390/atmos11080820
APA StyleLe, T.-C., Wang, Y.-C., Pui, D. Y. H., & Tsai, C.-J. (2020). Characterization of Atmospheric PM2.5 Inorganic Aerosols Using the Semi-Continuous PPWD-PILS-IC System and the ISORROPIA-II. Atmosphere, 11(8), 820. https://doi.org/10.3390/atmos11080820





