Concentration Characteristics and Photochemical Reactivities of VOCs in Shenyang, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Data Analysis Methods
2.2.1. Aerosol Formation
2.2.2. Ozone Formation
2.2.3. Hydroxyl Radical Consumption Rate
3. Concentration Characteristics of VOCs
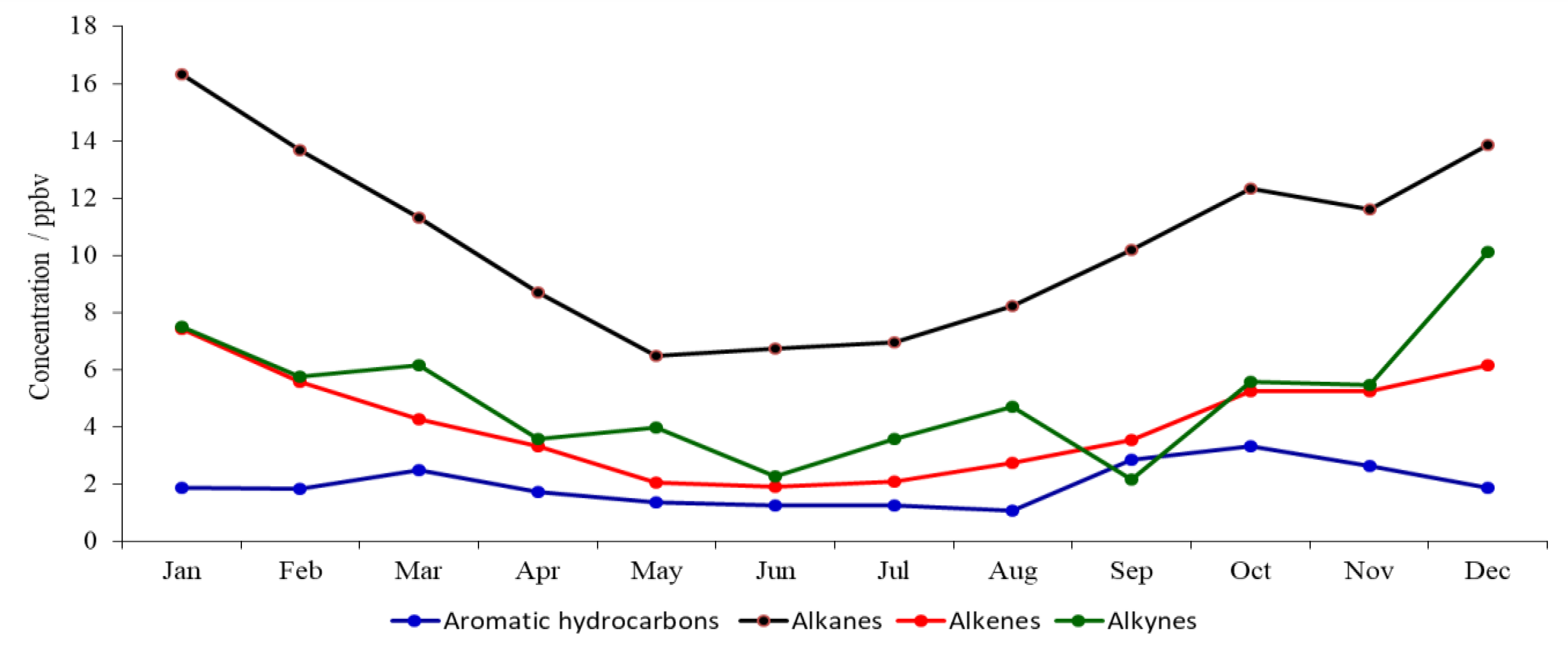
3.1. Seasonal Variations
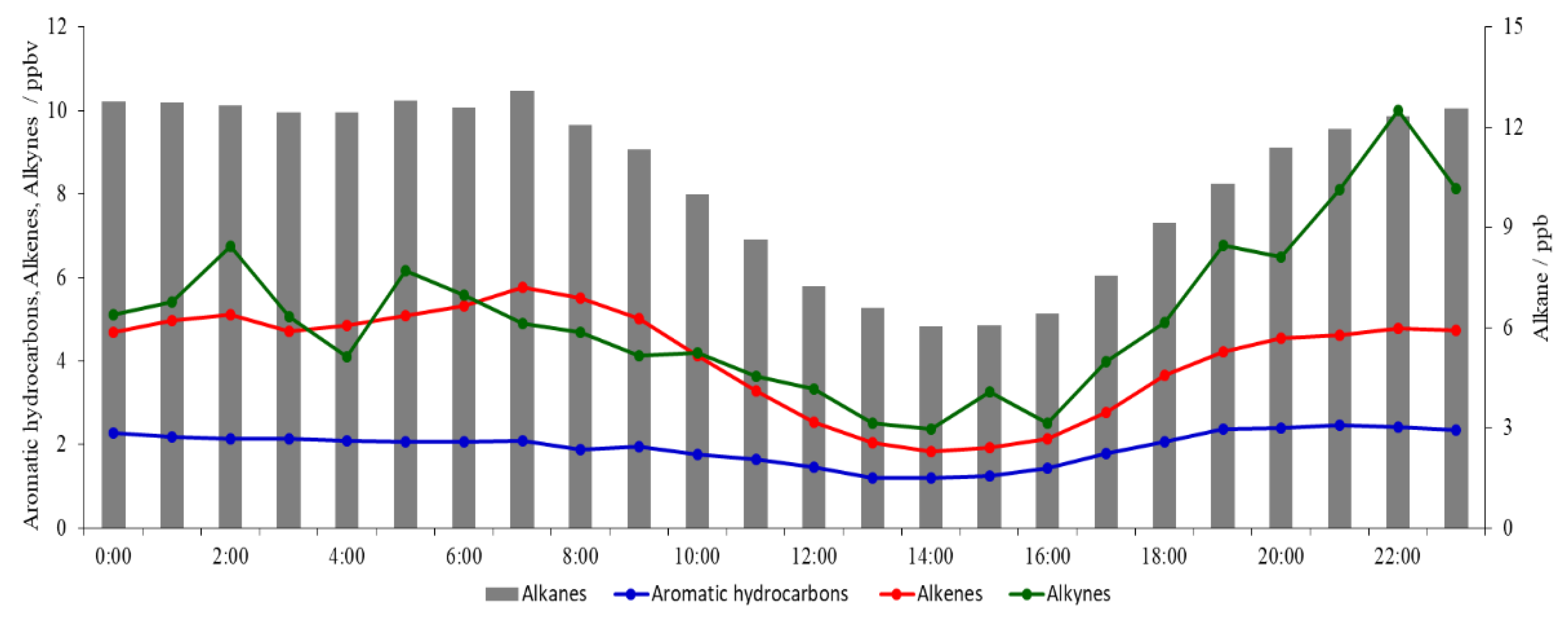
3.2. Diurnal Variation
4. Photochemical Reactivities of VOCs
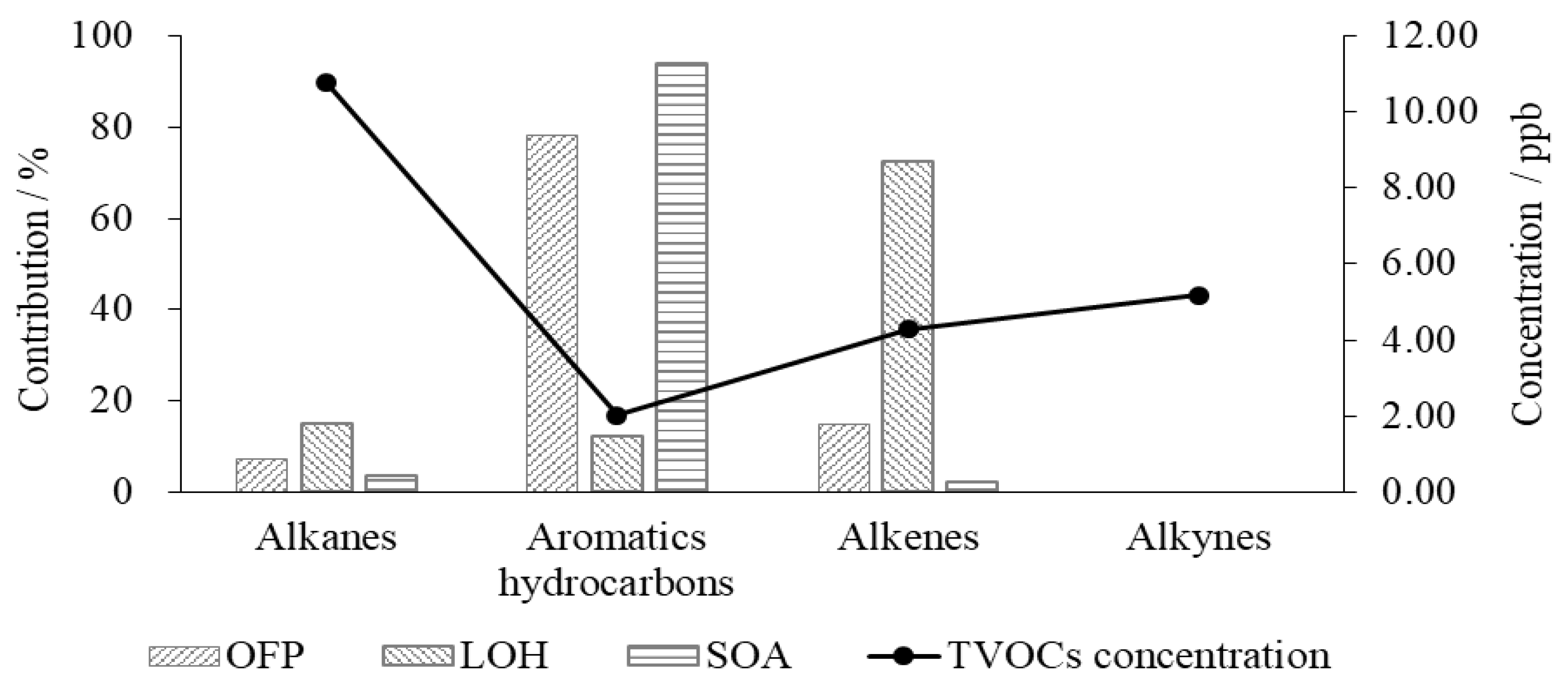
4.1. SOA Formation Potential in VOCs
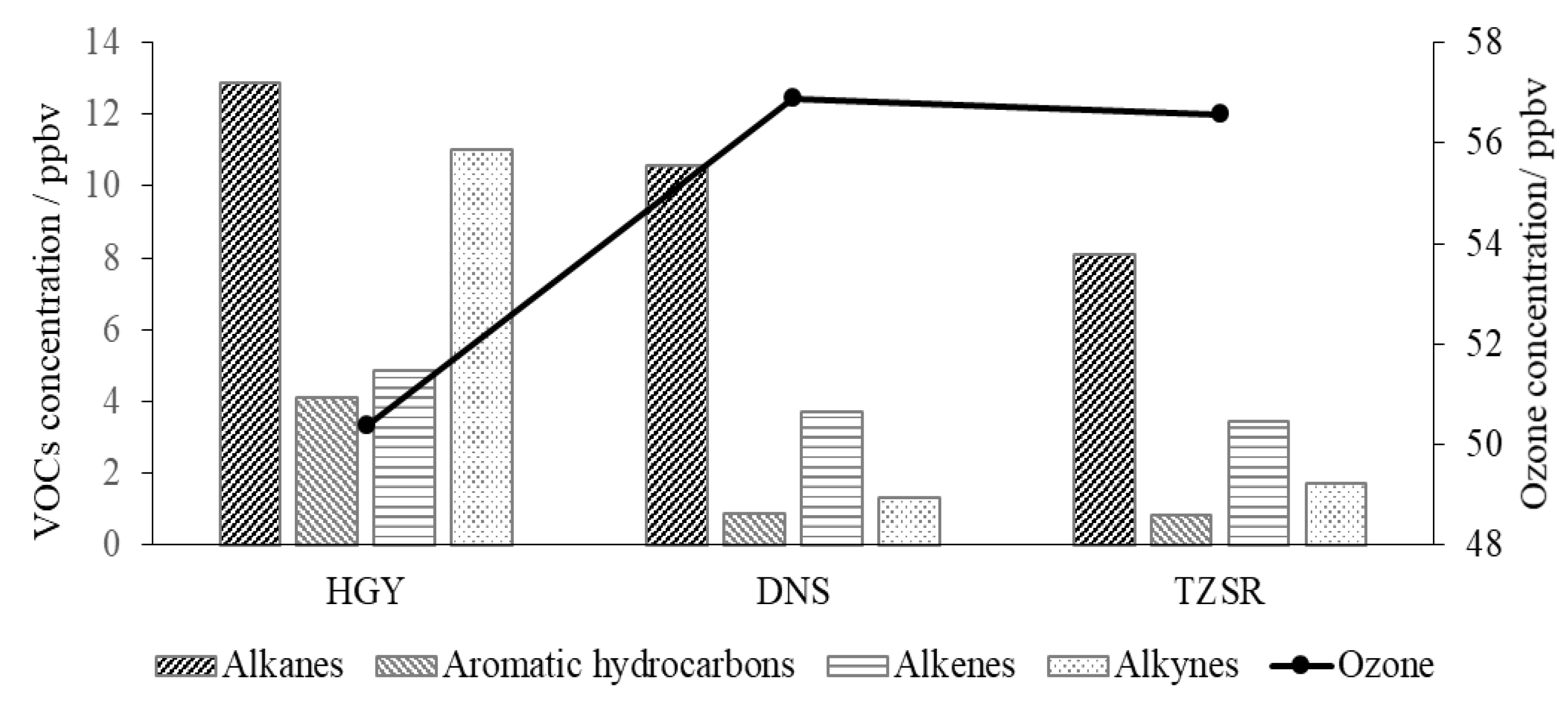
4.2. Ozone Formation Potential in VOCs
4.3. Influence of VOCs on the Consumption Rate of Hydroxyl Radicals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haagen-Smit, A.J. Chemistry and physiology of Los Angeles Smog. Ind. Eng. Chem. 1952, 44, 1342–1346. [Google Scholar] [CrossRef]
- Seinfeld, J.H. Urban air pollution: State of the science. Science 1989, 243, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; Wiley Interscience: Hoboken, NJ, USA, 1998. [Google Scholar]
- Sillman, S. The relation between ozone, NOx and hydrocarbons in urban and polluted rural environment. Atmos. Environ. 1999, 33, 1821–1845. [Google Scholar] [CrossRef]
- Cooper, O.R.; Gao, R.S.; Tarasick, D.; Leblanc, T.; Sweeney, C. Long-term ozone trends at rural ozone monitoring sites across the United States, 1990–2010. J. Geophys. Res. Atmos. 2012, 117, D22307. [Google Scholar] [CrossRef]
- Simon, H.; Reff, A.; Wells, B.; Xing, J.; Frank, N. Ozone trends across the United States over a period of decreasing NOx and VOC emissions. Environ. Sci. Technol. 2015, 49, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- China’s Ministry of Environmental Protection. China’s Ambient Air Quality Standard; Ministry of Environmental Protection: Beijing, China, 2012.
- Lin, X.; Zhu, B.; An, J.L.; Yang, H. Potential contribution of secondary organic aerosols and ozone of VOCs in the Northern Suburb of Nanjing. China Environ. Sci. 2015, 35, 976–986. [Google Scholar]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total. Environ. 2016, 541, 200–209. [Google Scholar] [CrossRef]
- Ran, L.; Zhao, C.S.; Xu, W.Y.; Lu, X.Q.; Han, M.; Lin, W.L.; Yan, P.; Xu, X.B.; Deng, Z.Z.; Ma, N.; et al. VOC reactivity and its effect on ozone production during the HaChi summer campaign. Atmos. Chem. Phys. 2011, 11, 4657–4667. [Google Scholar] [CrossRef] [Green Version]
- Shao, M.; Zhang, Y.; Zeng, L.; Tang, X.; Zhang, J.; Zhong, L.; Wang, B. Ground-level ozone in the Pearl River Delta and the roles of VOC and NOx in its production. J. Environ. Manag. 2009, 90, 512–518. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zhang, Y.H.; Shao, M. Atmospheric Environmental Chemistry, 2nd ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Xue, L.K.; Wang, T.; Gao, J.; Ding, A.J.; Zhou, X.H.; Blake, D.R.; Wang, X.F.; Saunders, S.M.; Fan, S.J.; Zuo, H.C.; et al. Ground-level ozone in four Chinese cities: Precursors, regional transport and heterogeneous processes. Atmos. Chem. Phys. 2014, 14, 13175–13188. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.X.; Tang, L.L.; Zhang, Y.J.; Mu, Y.F.; Wang, M.; Chen, W.T.; Zhou, H.C.; Hua, Y.; Jiang, R.X. Correlation analysis between characteristics of VOCs and ozone formation potential in summer in Nanjing urban district. Environ. Sci. 2016, 37, 443–451. [Google Scholar]
- Yu, X.F.; Cheng, P.; Gu, Y.G.; Li, M.; Tian, Z. Formation potential of ozone and secondary organic aerosol from VOCs oxidation in summer in Guangzhou, China. China Environ. Sci. 2018, 38, 830–837. [Google Scholar]
- Zhang, J.; Wang, T.; Chameides, W.L.; Cardelino, C.; Kwok, J.; Blake, D.R.; Ding, A.; So, K.L. Ozone production and hydrocarbon reactivity in Hongkong, Southern China. Atmos. Chem. Phys. 2007, 7, 557–573. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.J.; Zhang, Y.L.; Carlo, B.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.L.; Wang, Z.F.; Fu, P.Q.; Yang, T.; Jiang, Q.; Dong, H.B.; Li, J.; Jia, J.J. Aerosol composition, sources and processes during wintertime in Beijing, China. Atmos. Chem. Phys. 2013, 13, 4577–4592. [Google Scholar] [CrossRef] [Green Version]
- Grosjean, D.; Seinfeld, J.H. Parameterization of the formation potential of secondary organic aerosols. Atmos. Environ. 1989, 23, 1733–1747. [Google Scholar] [CrossRef]
- Barthelmie, R.J.; Pryor, S.C. Secondary organic aerosols: Formation potential and ambient data. Sci. Total. Environ. 1997, 205, 167–178. [Google Scholar] [CrossRef]
- Lv, Z.F.; Hao, J.M.; Duan, J.C.; Li, J.H. Estimate of the Formation Potential of Secondary Organic Aerosol in Beijing Summertime. Environ. Sci. 2009, 34, 424–433. [Google Scholar]
- Wang, Q.; Chen, C.H.; Wang, H.L.; Zhou, M.; Lou, S.R.; Qiao, L.P.; Huang, C.; Li, L.; Su, L.Y.; Mu, Y.Y.; et al. Forming potential of secondary organic aerosols and sources apportionment of VOCs in autumn of Shanghai, China. Environ. Sci. 2013, 30, 969–975. [Google Scholar]
- Wang, F.P.; Zhu, Q.; Feng, N.; Liu, R.L.; Huang, X.F.; He, L.Y. The generation potential of secondary organic aerosol of atmospheric VOCs in Shenzhen. China Environ. Sci. 2014, 34, 2449–2457. [Google Scholar]
- Qi, A.A.; Zhou, X.P.; Lei, C.N.; Ma, X.; Xie, Y.S.; Pang, W.Q.; Hao, L.L. Key active components and sources of volatile organic compounds in ambient air of Lanzhou city. Environ. Chem. 2020, 39, 3083–3093. [Google Scholar]
- Liu, N.W.; Ren, W.H.; Li, X.L.; Ma, X.; Zhang, Y.; Li, B. Distribution and urban–suburban differences in ground-level ozone and its precursors over Shenyang, China. Meteorol. Atmos. Phys. 2019, 131, 669–679. [Google Scholar] [CrossRef]
- Shao, M.; Wang, W.J.; Yuan, B.; Parrish, D.D.; Li, X.; Lu, K.; Wu, L.; Wang, X.; Mo, Z.; Yang, S.; et al. Quantifying the role of PM2.5 dropping in variations of ground-level ozone: Inter-comparison between Beijing and Los Angeles. Sci. Total. Environ. 2021, 788, 147712. [Google Scholar] [CrossRef]
- Grosjean, D. In situ organic aerosol formation during a smog episode: Estimated production and chemical functionality. Atmos. Environ. 1992, 24A, 953–963. [Google Scholar] [CrossRef]
- Izumi, K.; Fukuyama, T. Photochemical aerosol formation from aromatic hydrocarbons in the presence of NOx. Atmos. Environ. 1990, 24A, 1433–1441. [Google Scholar] [CrossRef]
- Carter, W.P.L. Development of ozone reactivity scales for volatile Organic compounds. Air Waste 1994, 44, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Liu, N.; Ma, J.Z. Seasonal relationships between tropospheric ozone and its precursors over East Asia. J. Appl. Meteorol. Sci. 2017, 28, 427–435. [Google Scholar]
- Zhang, R.X.; Liu, H.W.; Deng, S.X. Characteristics of VOCs and formation potential of O3 and SOA in autumn and winter in Baoji, China. China Environ. Sci. 2020, 40, 983–996. [Google Scholar]
- Shenyang Ecological Environment Bureau. Shenyang Ecological Environment Quality Report in 2016 to 2020; Shenyang Ecological Environment Bureau: Shenyang, China, 2021.
- Guo, H.; Wang, T.; Louie, P.K.K. Source apportionment of ambient non-methane hydrocarbons in Hong Kong: Application of a principal component analysis (PCA)/absolute principal component scores (APCS) receptor model. Environ. Pollut. 2004, 129, 489–498. [Google Scholar] [CrossRef]
- Ye, W.H.; Sun, X.H.; Liu, J.S.; Pan, H.F.; Pang, X.L. Study on air pollution of VOC in arterial traffic. Environ. Monit. China 2009, 25, 85–89. [Google Scholar]
- Zou, Y.; Deng, X.J.; Zhu, D.; Gong, D.C.; Wang, H.; Li, F.; Tan, H.B.; Deng, T.; Mai, B.R.; Liu, X.T.; et al. Characteristics of 1year of observational data of VOCs, NOx and O3 at a suburban site in Guangzhou, China. Atmos. Chem. Phys. 2015, 15, 6625–6636. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.N. Observation Based Study of VOCs and Their Influence on Ozone and Secondary Organic Formation in the Western YRD Region, China; Nanjing University: Nanjing, China, 2019. [Google Scholar]
- Liu, Y.; Shao, M.; Lu, S. Volatile organic compound (VOC) measurements in the Pearl River Delta (PRD) region, China. Atmos. Chem. Phys. 2008, 8, 1531–1545. [Google Scholar] [CrossRef] [Green Version]
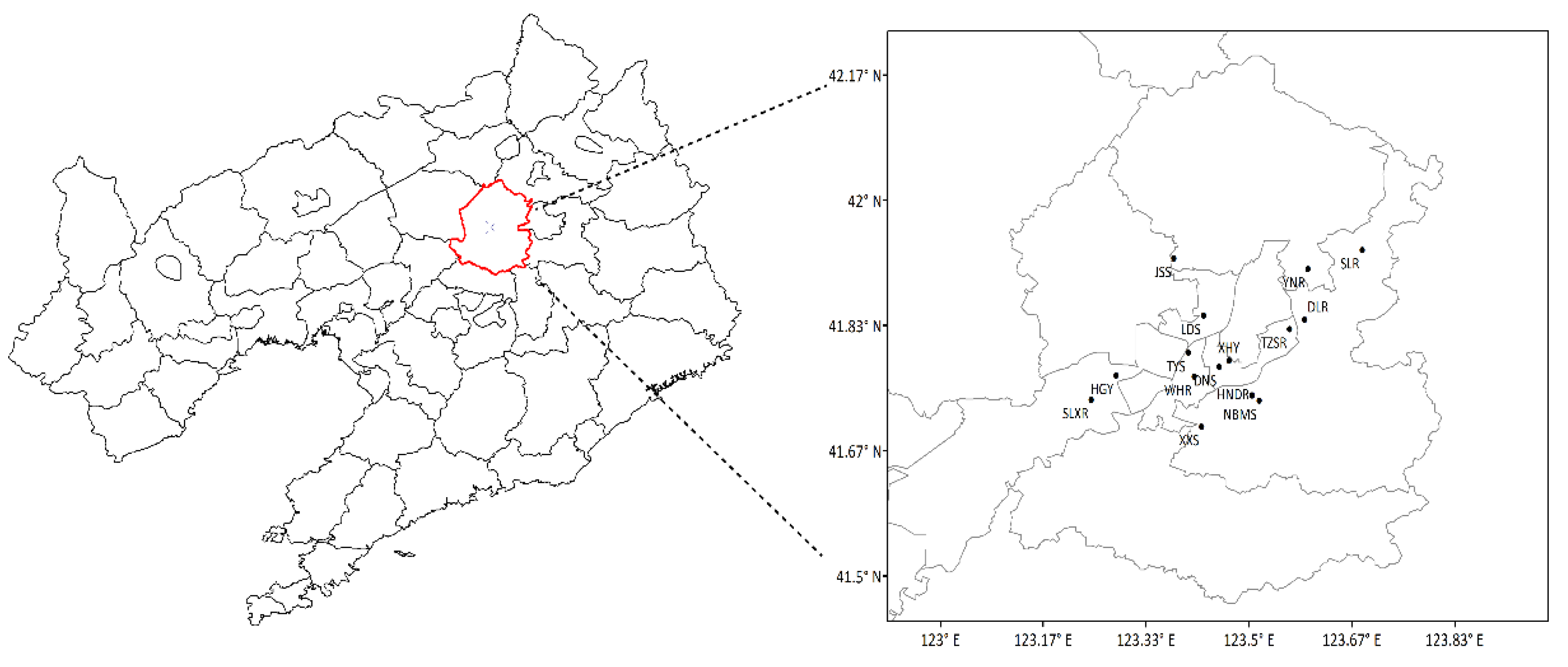


| Information of Each Site | VOCs’ Environmental Monitoring Site | State-Controlled Ambient Air Quality Monitoring Site | NBMS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | HGY | DNS | TZSR | LDS | TYS | WHR | XHY | DLR | HNR | XXS | YNR | SLXR | JSS | SLR | NBMS |
| Latitude | 41.77° N | 41.78° N | 41.83° N | 41.85° N | 41.80° N | 41.77° N | 41.79° N | 41.84° N | 41.74° N | 41.70° N | 41.91° N | 41.73° N | 41.92° N | 41.93° N | 41.73 |
| Longitude | 123.29° E | 123.45° E | 123.57° E | 123.43° E | 123.40° E | 123.41° E | 123.47° E | 123.59° E | 123.51° E | 123.42° E | 123.60° E | 123.24° E | 123.38° E | 123.68° E | 123.52 |
| Location | Urban | Urban | Suburbs | Urban | Urban | Urban | Urban | Suburbs | Suburbs | Suburbs | Suburbs | Suburbs | Suburbs | Rural | Urban |
| Land-use | City | City | City/ cropland | City | City | City | City | City/ cropland | City/ cropland | City/ cropland | City/ cropland | City/ cropland | City/ cropland | Forest / reservoir | City |
| Ozone—mean | 26 | 29 | 29 | 29 | 28 | 30 | 27 | 29 | 27 | 30 | 32 | 31 | 31 | 31 | |
| Ozone—max | 479 | 54 | 52 | 52 | 52 | 53 | 48 | 50 | 47 | 52 | 51 | 51 | 52 | 47 | |
| Ozone—min | 9 | 10 | 11 | 12 | 10 | 12 | 10 | 12 | 10 | 13 | 14 | 13 | 14 | 16 | |
| NOx—mean | 51 | 34 | 25 | 37 | 33 | 30 | 30 | 22 | 31 | 36 | 20 | 30 | 28 | 15 | |
| NOx—max | 78 | 49 | 28 | 60 | 53 | 52 | 49 | 35 | 53 | 55 | 33 | 46 | 44 | 21 | |
| NOx—min | 26 | 19 | 14 | 16 | 15 | 11 | 14 | 12 | 17 | 19 | 13 | 12 | 15 | 10 | |
| PM2.5—mean | 48 | 50 | 44 | 40 | 44 | 44 | 42 | 41 | 49 | 45 | 37 | 47 | 39 | 41 | |
| PM2.5—max | 76 | 82 | 70 | 63 | 70 | 73 | 71 | 71 | 83 | 78 | 59 | 81 | 68 | 66 | |
| PM2.5—min | 22 | 22 | 22 | 18 | 21 | 21 | 17 | 16 | 20 | 18 | 17 | 20 | 19 | 20 | |
| Season | Photochemical Reactivities | VOC (ppbv) | Alkanes (%) | Aromatic Hydrocarbons (%) | Alkenes (%) |
|---|---|---|---|---|---|
| Spring | OFP | 5.09 | 7 | 79 | 14 |
| LOH | 82.68 | 15 | 12 | 73 | |
| SOAp | 7.46 | 3 | 95 | 2 | |
| Summer | OFP | 4.99 | 6 | 61 | 33 |
| LOH | 64.42 | 18 | 13 | 69 | |
| SOAp | 5.40 | 4 | 89 | 7 | |
| Autumn | OFP | 6.46 | 7 | 86 | 7 |
| LOH | 114.67 | 15 | 14 | 71 | |
| SOAp | 10.40 | 3 | 96 | 1 | |
| Winter | OFP | 6.84 | 8 | 82 | 10 |
| LOH | 136.13 | 13 | 11 | 76 | |
| SOAp | 8.79 | 4 | 94 | 2 |
| Units/ppbv. | Mean Level (ppbv) | FAC (%) | FVOCr (%) | SOAp (ppbv) | SOAp (%) |
|---|---|---|---|---|---|
| toluene | 0.55 | 5.40 | 12.00 | 3.15 | 39.21 |
| benzene | 1.12 | 2.00 | 10.00 | 2.50 | 31.07 |
| o-xylene | 0.07 | 5.00 | 26.00 | 0.48 | 5.93 |
| ethylbenzene | 0.07 | 5.40 | 15.00 | 0.44 | 5.52 |
| isoprene | 0.09 | 2.00 | 0.00 | 0.19 | 2.33 |
| 1,3,5-trimethylbenzene | 0.02 | 2.90 | 74.00 | 0.17 | 2.12 |
| m-ethyltoluene | 0.02 | 6.30 | 31.00 | 0.17 | 2.09 |
| methylcyclohexane | 0.04 | 2.70 | 20.00 | 0.14 | 1.80 |
| p-diethylbenzene | 0.01 | 6.30 | 47.00 | 0.12 | 1.51 |
| 1,2,3-trimethylbenzene | 0.02 | 3.60 | 51.00 | 0.12 | 1.47 |
| Units/ppbv. | Mean Level (ppbv) | MIR | FVOCr (%) | OFP (ppbv) | OFP (%) |
|---|---|---|---|---|---|
| toluene | 0.55 | 2.70 | 12.00 | 1.70 | 29.64 |
| isoprene | 0.09 | 9.10 | 0.00 | 0.85 | 14.83 |
| o-xylene | 0.07 | 6.50 | 26.00 | 0.62 | 10.79 |
| 1,3,5-trimethylbenzene | 0.02 | 10.10 | 74.00 | 0.59 | 10.36 |
| benzene | 1.12 | 0.42 | 10.00 | 0.52 | 9.13 |
| 1,2,4-trimethylbenzene | 0.02 | 8.80 | 58.00 | 0.48 | 8.32 |
| 1,2,3-trimethylbenzene | 0.02 | 8.90 | 51.00 | 0.29 | 5.09 |
| ethylbenzene | 0.07 | 2.70 | 15.00 | 0.22 | 3.87 |
| methylcyclopentane | 0.04 | 2.80 | 10.00 | 0.12 | 2.06 |
| methylcyclohexane | 0.04 | 1.80 | 20.00 | 0.10 | 1.68 |
| Units/ppbv. | Mean Level (ppbv) | KOH | LOH (ppbv) | LOH (%) |
|---|---|---|---|---|
| ethylene | 2.88 | 8.52 | 24.51 | 24.64 |
| propylene | 0.61 | 26.30 | 16.07 | 16.16 |
| 1-hexene | 0.27 | 37.00 | 9.88 | 9.93 |
| 1-butene | 0.16 | 31.40 | 5.14 | 5.17 |
| trans-2-butene | 0.08 | 64.00 | 4.95 | 4.98 |
| cis-2-butene | 0.06 | 56.40 | 3.60 | 3.62 |
| propane | 3.17 | 1.09 | 3.46 | 3.47 |
| toluene | 0.55 | 5.96 | 3.31 | 3.32 |
| trans-2-pentene | 0.05 | 65.00 | 3.14 | 3.16 |
| styrene | 0.05 | 58.00 | 3.13 | 3.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Li, X.; Ren, W.; Li, L.; Su, C.; Wang, C. Concentration Characteristics and Photochemical Reactivities of VOCs in Shenyang, China. Atmosphere 2021, 12, 1240. https://doi.org/10.3390/atmos12101240
Liu N, Li X, Ren W, Li L, Su C, Wang C. Concentration Characteristics and Photochemical Reactivities of VOCs in Shenyang, China. Atmosphere. 2021; 12(10):1240. https://doi.org/10.3390/atmos12101240
Chicago/Turabian StyleLiu, Ningwei, Xiaolan Li, Wanhui Ren, Liguang Li, Congcong Su, and Chuang Wang. 2021. "Concentration Characteristics and Photochemical Reactivities of VOCs in Shenyang, China" Atmosphere 12, no. 10: 1240. https://doi.org/10.3390/atmos12101240





