The Impact of Air or Nitrogen Non-Thermal Plasma on Variations of Natural Bioactive Compounds in Djulis (Chenopodium formosanum Koidz.) Seed and the Potential Effects for Human Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Djulis Seeds Treated Using NTP
2.3. Analysis of Natural Bioactive Compounds
2.4. Analysis of Phenolic Acids and Flavonoids by HPLC/DAD
2.5. The Tests of the Thermal Effects
2.6. Cell Viability Assays of the THP-1 Cells
2.7. Intracellular ROS Staining of THP-1 Cells
2.8. Statistical Analysis
3. Results
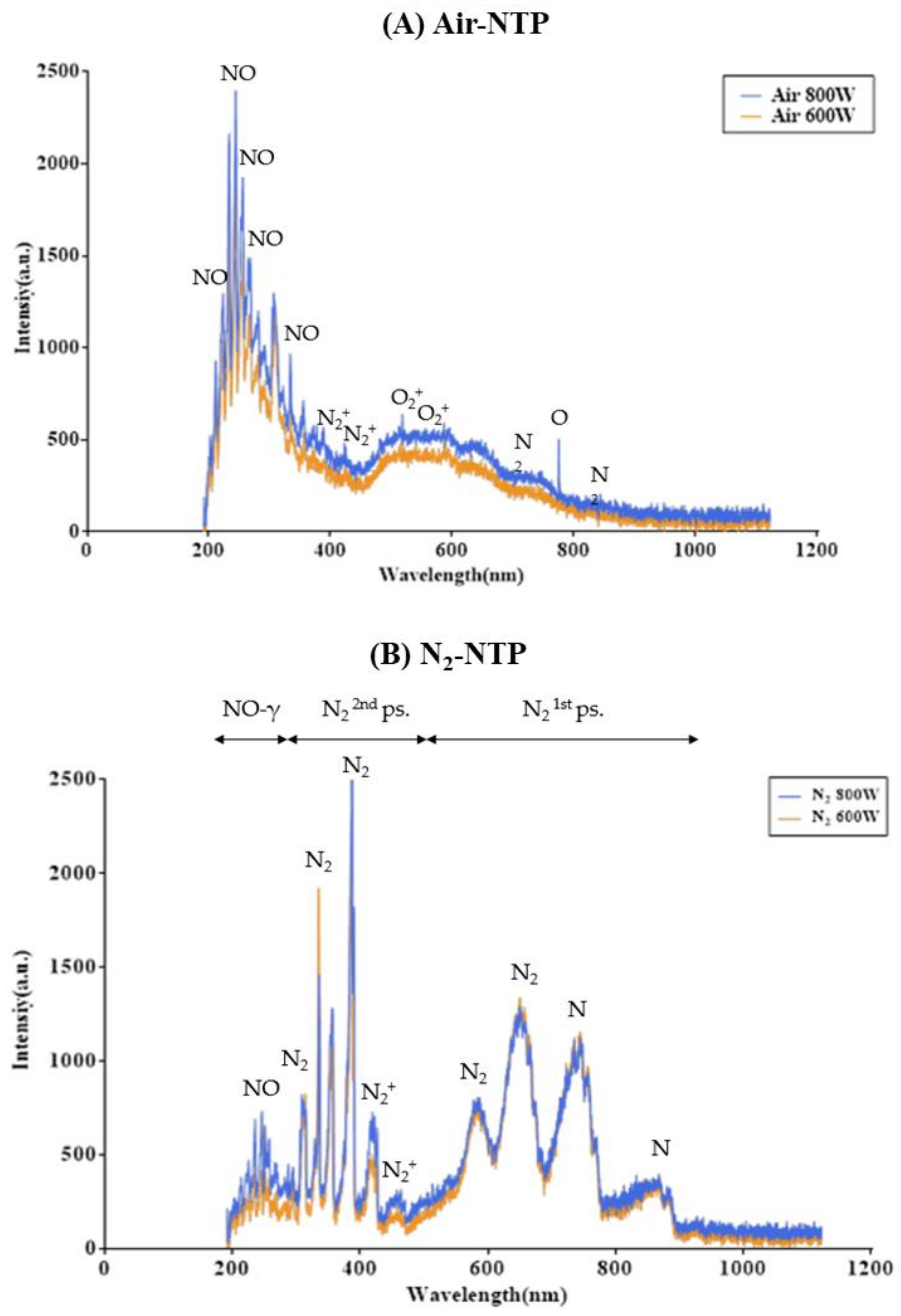
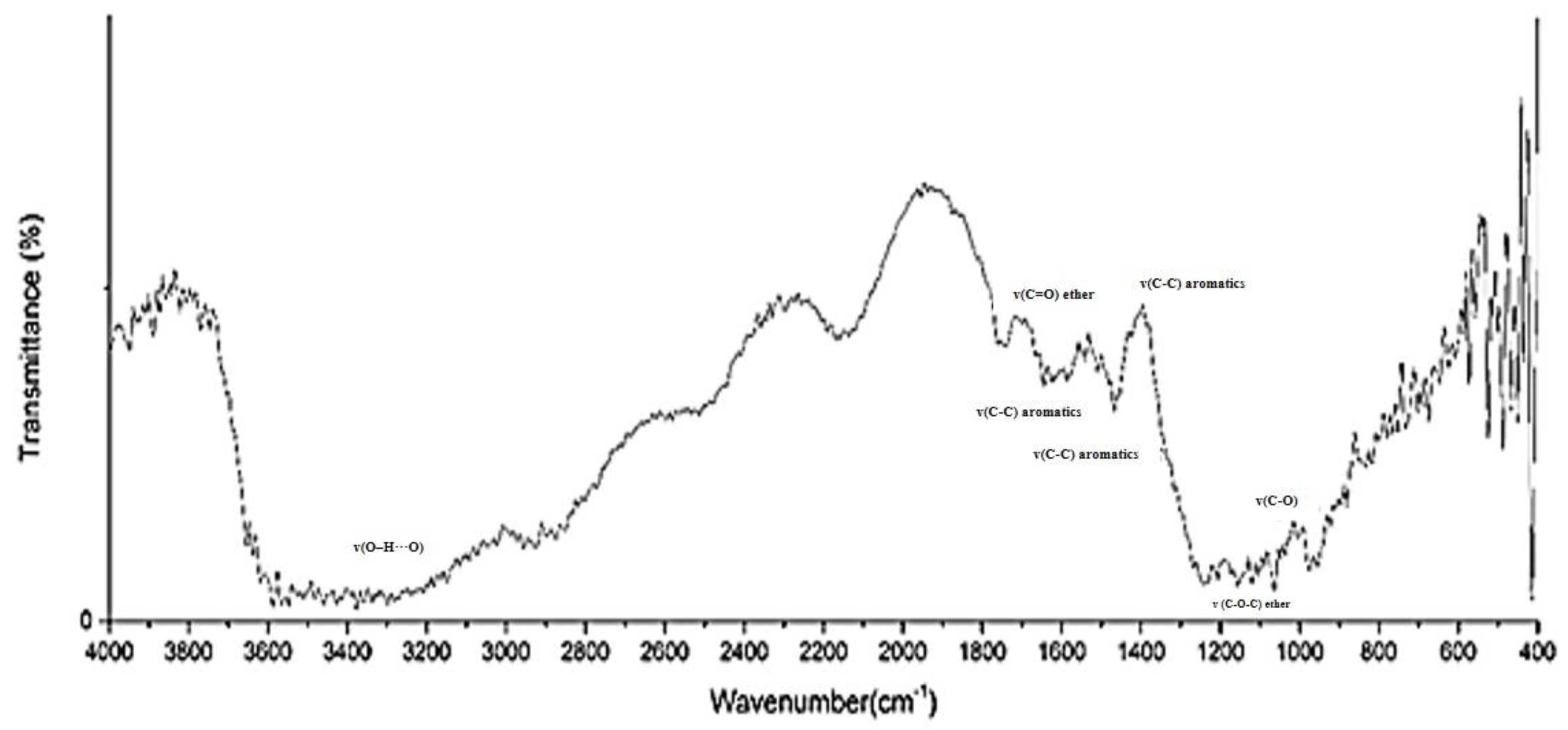
3.1. Surface Analysis of Djulis Seed after NTP Treatment
3.2. Bioactive Compounds in Djulis Seed
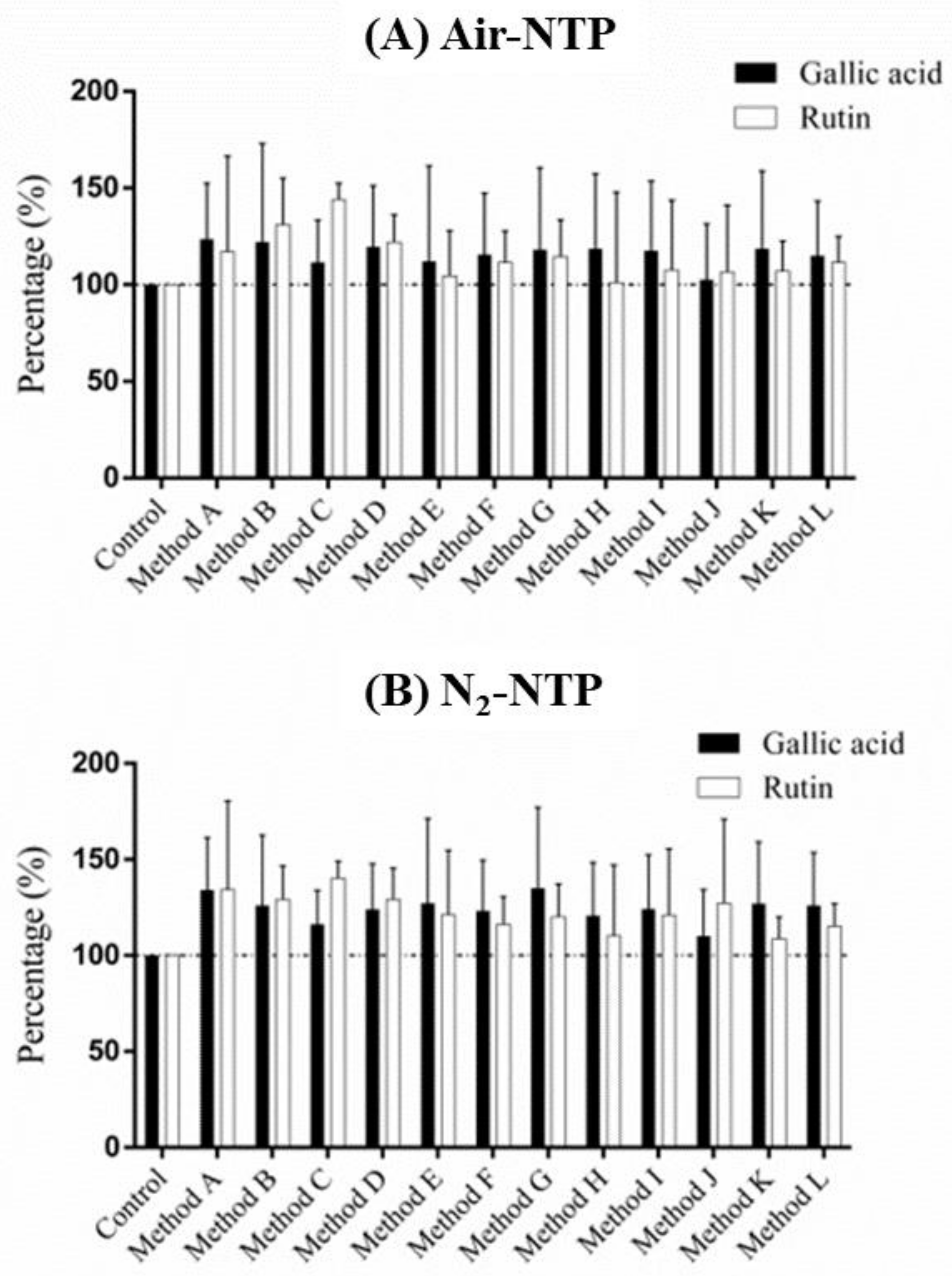
3.3. Variations in Bioactive Compounds Treated Using NTP
3.4. Changes of Bioactive Compounds on the Djulis Seed after Thermal Treatment
3.5. ROS Production with Cotreatment of Djulis Seed Extract and SWCNT-COOH in THP-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef] [Green Version]
- Hsu, B.; Lin, S.; Inbaraj, B.S.; Chen, B. Simultaneous determination of phenolic acids and flavonoids in Chenopodium formosanum Koidz(djulis) by HPLC-DAD-ESI–MS/MS. Am. Ind. Hyg. Assoc. J. 2017, 132, 109–116. [Google Scholar] [CrossRef]
- Perni, S.; Shama, G.; Kong, M.G. Cold atmospheric plasma disinfection of cut fruit surfaces contaminated with migrating microorganisms. J. Food Prot. 2008, 71, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Akan, T. Arc-free atmospheric pressure cold plasma jets: A review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Lis, K.A.; Kehrenberg, C.; Boulaaba, A.; von Köckritz-Blickwede, M.; Binder, S.; Li, Y.; Zimmermann, J.L.; Pfeifer, Y.; Ahlfeld, B. Inactivation of multidrug-resistant pathogens and Yersinia enterocolitica with cold atmospheric-pressure plasma on stainless-steel surfaces. Int. J. Antimicrob. Agents 2018, 52, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Limsopatham, K.; Boonyawan, D.; Umongno, C.; Sukontason, K.L.; Chaiwong, T.; Leksomboon, R.; Sukontason, K. Effect of cold argon plasma on eggs of the blow fly, Lucilia cuprina (Diptera: Calliphoridae). Acta Trop. 2017, 176, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Hairston, J.S.; Patel, D.; Gott, R.P.; Xu, K.G. Cold plasma poration and corrugation of pumpkin seed coats. Bioelectrochemistry 2019, 128, 175–185. [Google Scholar] [CrossRef] [PubMed]
- de Groot, G.J.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tamošiūnė, I.; Gelvonauskienė, D.; Haimi, P.; Mildažienė, V.; Koga, K.; Shiratani, M.; Baniulis, D. Cold plasma treatment of sunflower seeds modulates plant-associated microbiome and stimulates root and lateral organ growth. Front. Plant Sci. 2020, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K.K. Effects of atmospheric-pressure N 2, He, air, and O 2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Švubová, R.; Slováková, Ľ.; Holubová, Ľ.; Rovňanová, D.; Gálová, E.; Tomeková, J. Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants 2021, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chu, Y.-L.; Sridhar, K.; Tsai, P.-J. Analysis and determination of phytosterols and triterpenes in different inbred lines of Djulis (Chenopodium formosanum Koidz.) hull: A potential source of novel bioactive ingredients. Food Chem. 2019, 297, 124948. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Hsiao, S.-M.; Chaung, H.-C.; Hong, C.-Z.; Wang, C. The LDL cholesterol-lowering effects of nanoparticled Djulis Grains. ICBBB 2011, 26, 218–221. [Google Scholar]
- Hong, Y.-H.; Huang, Y.-L.; Liu, Y.-C.; Tsai, P.-J. Djulis (Chenopodium formosanum Koidz.) water extract and its bioactive components ameliorate dermal damage in UVB-irradiated skin models. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.-J.; Sheu, C.-H.; Wu, P.-H.; Sun, Y.-F. Thermal and pH stability of betacyanin pigment of djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. J. Agric. Food Chem. 2010, 58, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Chen, Y.-S.; Sheu, C.-H.; Chen, C.-Y. Effect of nanogrinding on the pigment and bioactivity of djulis (Chenopodium formosanum Koidz.). J. Agric. Food Chem. 2011, 59, 1814–1820. [Google Scholar] [CrossRef]
- Lin, T.-A.; Ke, B.-J.; Cheng, C.-S.; Wang, J.-J.; Wei, B.-L.; Lee, C.-L. Red quinoa bran extracts protects against carbon tetrachloride-induced liver injury and fibrosis in mice via activation of antioxidative enzyme systems and blocking TGF-β1 pathway. Nutrients 2019, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.-C.; Chen, S.-Y.; Chyau, C.-C.; Fu, Z.-H.; Liu, C.-C.; Duh, P.-D. Protective effect of Djulis (Chenopodium formosanum) and its bioactive compounds against carbon tetrachloride-induced liver injury, in vivo. J. Funct. Foods 2016, 26, 585–597. [Google Scholar] [CrossRef]
- Lee, C.-W.; Chen, H.-J.; Chien, Y.-H.; Hsia, S.-M.; Chen, J.-H.; Shih, C.-K. Synbiotic combination of Djulis (Chenopodium formosanum) and Lactobacillus acidophilus inhibits colon carcinogenesis in rats. Nutrients 2020, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-W.; Chen, H.-J.; Xie, G.-R.; Shih, C.-K. Djulis (Chenopodium formosanum) prevents colon carcinogenesis via regulating antioxidative and apoptotic pathways in rats. Nutrients 2019, 11, 2168. [Google Scholar] [CrossRef] [Green Version]
- Mancinelli, A.; Yang, L.P.; Lidquist, P.; Anderson, R.; Rabino, I. Photocontrol of anthocyanin synthesis III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant. Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta v ulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Jin, Z.-Q.; Chen, X. A simple reproducible model of free radical-injured isolated heart induced by 1, 1-diphenyl-2-picryl-hydrazyl (DPPH). J. Pharm. Toxicol Methods 1998, 39, 63–70. [Google Scholar] [CrossRef]
- Klas, M.; Ptasinska, S. Characteristics of N2 and N2/O2 atmospheric pressure glow discharges. Plasma Sources Sci. Technol. 2013, 22, 025013. [Google Scholar] [CrossRef]
- Han, X.; Klas, M.; Liu, Y.; Sharon Stack, M.; Ptasinska, S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl. Phys. Lett. 2013, 102, 233703. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [Green Version]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Mohammadi, E.; Zeinali, M.; Mohammadi-Sardoo, M.; Iranpour, M.; Behnam, B.; Mandegary, A. The effects of functionalization of carbon nanotubes on toxicological parameters in mice. Hum. Exp. Toxicol. 2020, 39, 1147–1167. [Google Scholar] [CrossRef]
- Öner, D.; Moisse, M.; Ghosh, M.; Duca, R.C.; Poels, K.; Luyts, K.; Putzeys, E.; Cokic, S.M.; Van Landuyt, K.; Vanoirbeek, J. Epigenetic effects of carbon nanotubes in human monocytic cells. Mutagenesis 2017, 32, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Kiliç, G.; Costa, P.M.; Fadeel, B. Cytotoxicity screening and cytokine profiling of nineteen nanomaterials enables hazard ranking and grouping based on inflammogenic potential. Nanotoxicology 2017, 11, 809–826. [Google Scholar]



| Method | Plasma Nozzle A50/A30/A10 | Distance a 5//10/15 mm | Plasma Gas Air/N2 | Power Input 0.6/0.8 kW | Sweep Speed 100/150 mm/s | Temperature b (°C) | Torch Diameter c (mm) | Treatment Time (Sec) |
|---|---|---|---|---|---|---|---|---|
| Control | None | None | None | None | None | None | None | None |
| Method A | A50 | 5 | Air/N2 | 0.6 | 100 | 49 | 42 | 1.02 |
| Method B | A50 | 5 | Air/N2 | 0.8 | 100 | 59 | 42 | 1.02 |
| Method C | A50 | 5 | Air/N2 | 0.8 | 150 | 46 | 42 | 0.68 |
| Method D | A50 | 10 | Air/N2 | 0.8 | 100 | 54 | 42 | 1.02 |
| Method E | A30 | 5 | Air/N2 | 0.8 | 100 | 55 | 30 | 0.90 |
| Method F | A30 | 10 | Air/N2 | 0.8 | 100 | 52 | 30 | 0.90 |
| Method G | A30 | 5 | Air/N2 | 0.8 | 150 | 47 | 30 | 0.60 |
| Method H | A30 | 5 | Air/N2 | 0.6 | 100 | 49 | 30 | 0.90 |
| Method I | A10 | 15 | Air/N2 | 0.8 | 100 | 75 | 17 | 0.77 |
| Method J | A10 | 15 | Air/N2 | 0.8 | 150 | 57 | 17 | 0.51 |
| Method K | A10 | 15 | Air/N2 | 0.6 | 100 | 67 | 17 | 0.77 |
| Method L | A10 | 10 | Air/N2 | 0.8 | 100 | 77 | 17 | 0.77 |
| Air-NTP (n = 3) a (Retail Store) | N2-NTP (n = 6) b (SAF/NPUST) | |||||
|---|---|---|---|---|---|---|
| Compounds | Range | Median | Mean | Range | Median | Mean |
| Betacyanin (mg/100 g) | 9.50–9.06 | 9.50 | 9.50 ± 0.005 | 3.99–4.03 | 4.00 | 4.01 ± 0.019 |
| Anthocyanin (mg/100 g) | 24.5–25.6 | 24.5 | 24.8 ± 0.633 | 25.3–25.8 | 25.7 | 25.6 ± 0.633 |
| Total phenolic content (mg GAE/100 g) | 34.6–36.1 | 35.9 | 35.55 ± 0.830 | 86.8–89.0 | 88.2 | 88.0 ± 1.12 |
| Total flavonoids (mg QU/100 g) | 1.39–1.89 | 1.79 | 1.69 ± 0.265 | 4.85–5.66 | 5.25 | 5.25 ± 0.401 |
| DPPH c (Percentage, %) | 72.5–81.2 | 79.1 | 77.6 ± 4.52 | 85.5–86.2 | 86.0 | 85.9 ± 0.355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.-J.; Lin, T.-C.; Chao, H.-R.; Tsai, C.-H.; Lu, J.-H.; Tsai, M.-H.; Chang, C.-T.; Hsieh, H.; Lu, I.-C.; Arcega, R.D.; et al. The Impact of Air or Nitrogen Non-Thermal Plasma on Variations of Natural Bioactive Compounds in Djulis (Chenopodium formosanum Koidz.) Seed and the Potential Effects for Human Health. Atmosphere 2021, 12, 1375. https://doi.org/10.3390/atmos12111375
Lu B-J, Lin T-C, Chao H-R, Tsai C-H, Lu J-H, Tsai M-H, Chang C-T, Hsieh H, Lu I-C, Arcega RD, et al. The Impact of Air or Nitrogen Non-Thermal Plasma on Variations of Natural Bioactive Compounds in Djulis (Chenopodium formosanum Koidz.) Seed and the Potential Effects for Human Health. Atmosphere. 2021; 12(11):1375. https://doi.org/10.3390/atmos12111375
Chicago/Turabian StyleLu, Bing-Jyh, Tzu-Che Lin, How-Ran Chao, Cheng-Hsian Tsai, Jian-He Lu, Ming-Hsien Tsai, Ching-Tzu Chang, Hao Hsieh, I-Cheng Lu, Rachelle D. Arcega, and et al. 2021. "The Impact of Air or Nitrogen Non-Thermal Plasma on Variations of Natural Bioactive Compounds in Djulis (Chenopodium formosanum Koidz.) Seed and the Potential Effects for Human Health" Atmosphere 12, no. 11: 1375. https://doi.org/10.3390/atmos12111375
APA StyleLu, B.-J., Lin, T.-C., Chao, H.-R., Tsai, C.-H., Lu, J.-H., Tsai, M.-H., Chang, C.-T., Hsieh, H., Lu, I.-C., Arcega, R. D., Chang, W.-H., Chen, H.-L., Mansor, W. N. W., & Lee, Y.-C. (2021). The Impact of Air or Nitrogen Non-Thermal Plasma on Variations of Natural Bioactive Compounds in Djulis (Chenopodium formosanum Koidz.) Seed and the Potential Effects for Human Health. Atmosphere, 12(11), 1375. https://doi.org/10.3390/atmos12111375










