Microphysical Characteristics and Environmental Isotope Effects of the Micro-Droplet Groups under the Action of Acoustic Waves
Abstract
:1. Introduction
2. Materials and Methods
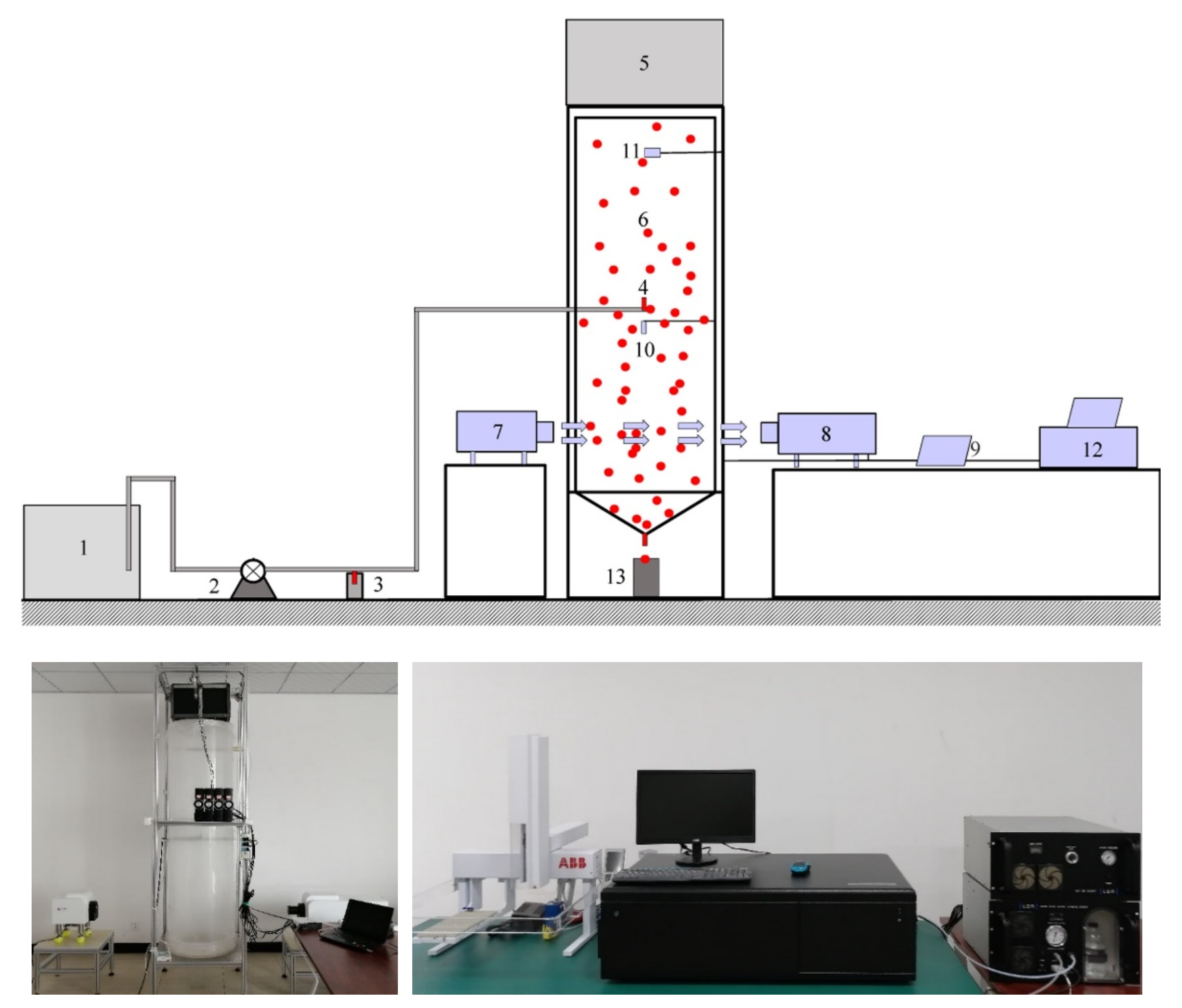
2.1. Experiment Setup
2.1.1. Micro-Droplet Generation Device
2.1.2. Acoustic Generator
2.1.3. Monitoring Devices
2.2. Experimental Process and Sample Collection
2.3. Data and Analysis Method
2.3.1. Isotopes Data
2.3.2. Significance Test Method
3. Experimental Results and Analysis
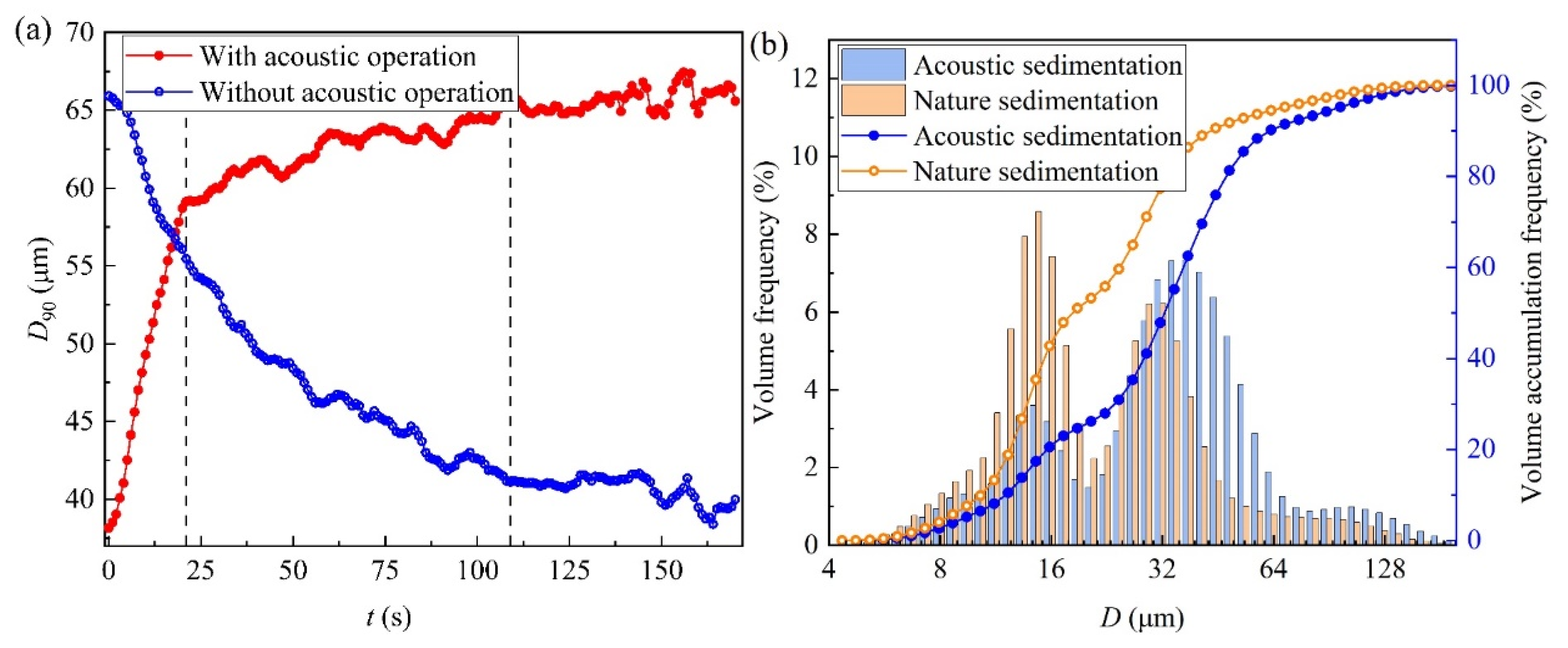
3.1. Effects of Acoustic Waves on D90
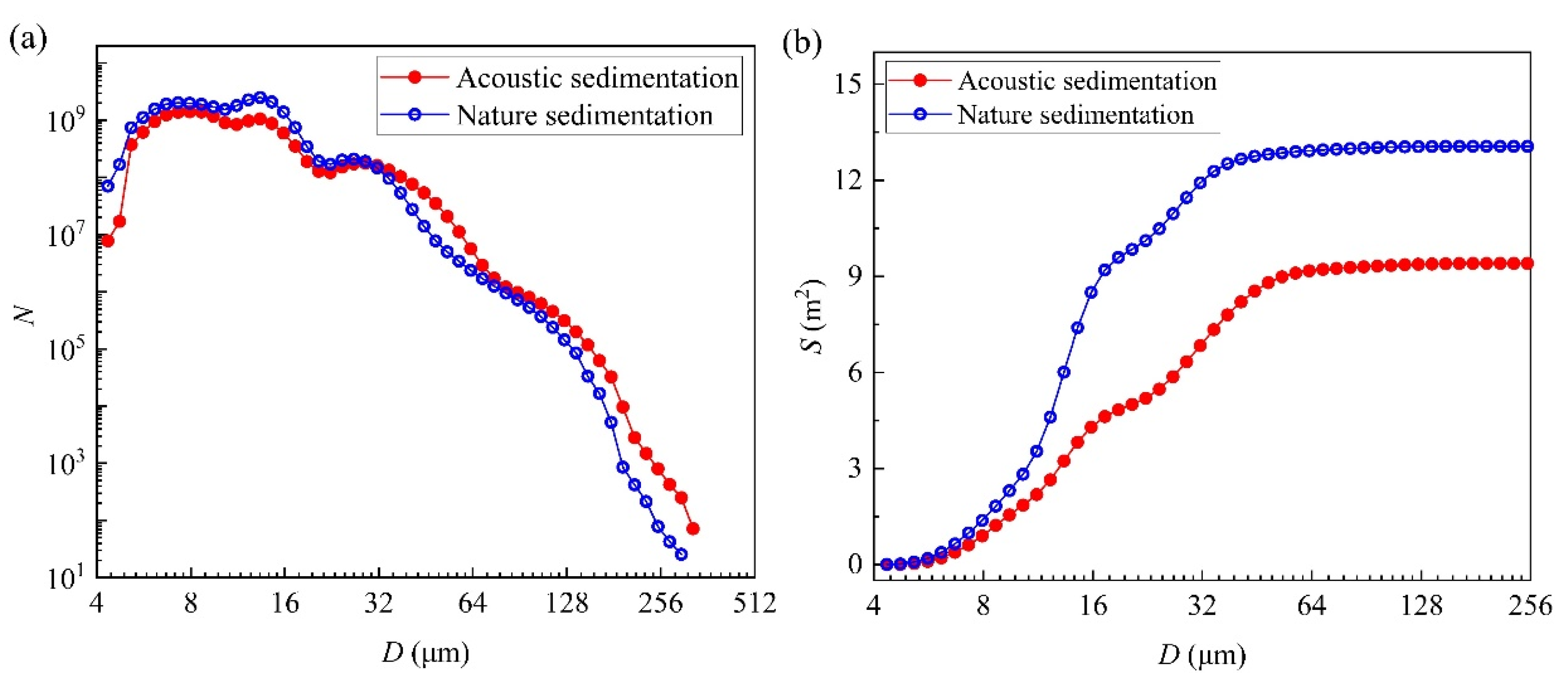
3.2. Effects of Acoustic Waves on Size Spectrum
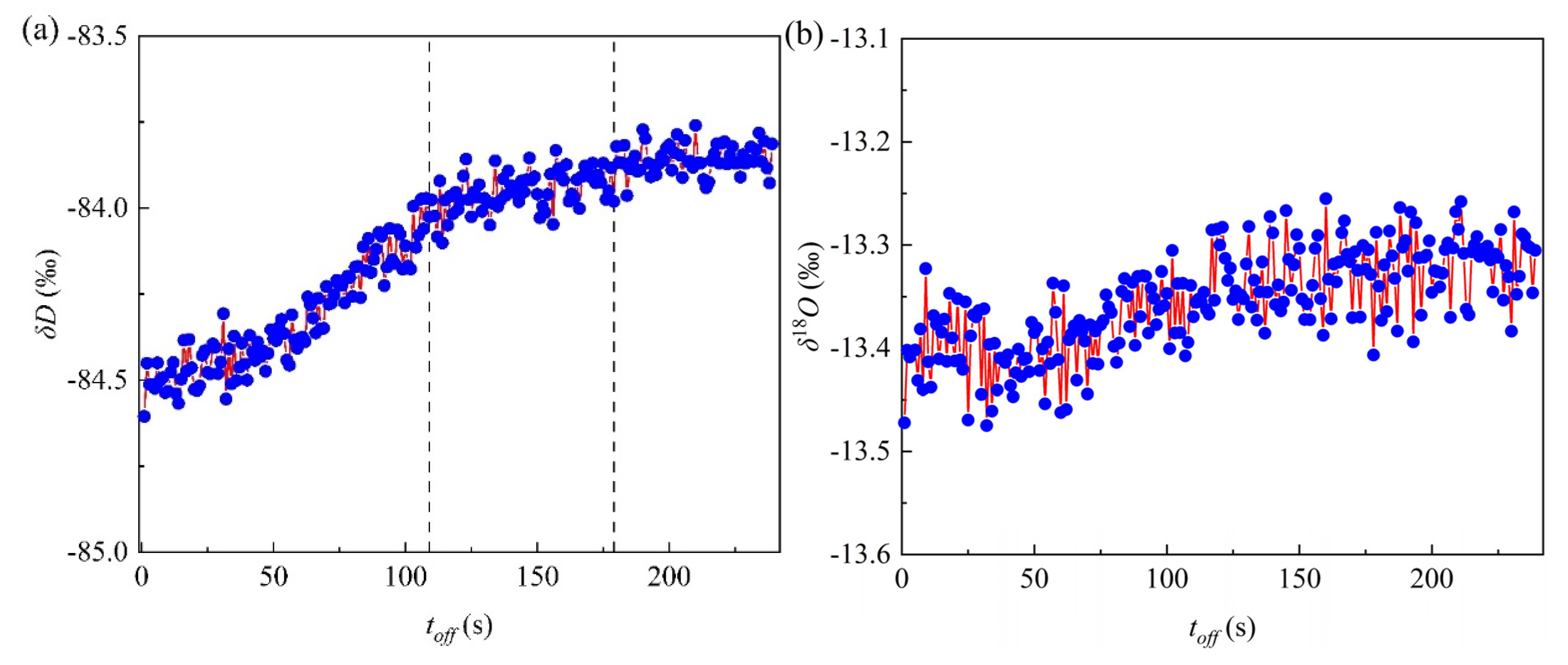
3.3. Effects of Acoustic Waves on Isotopes Exchange
4. Discussion
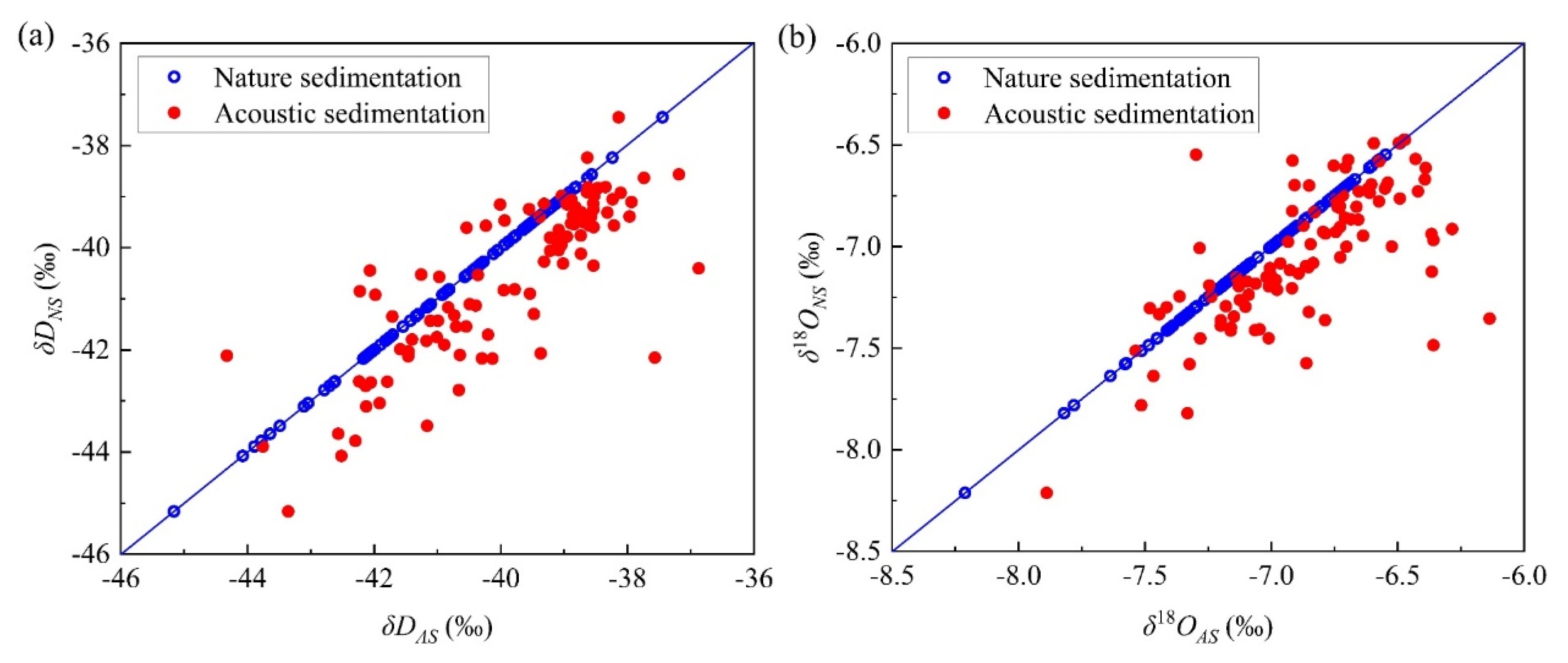
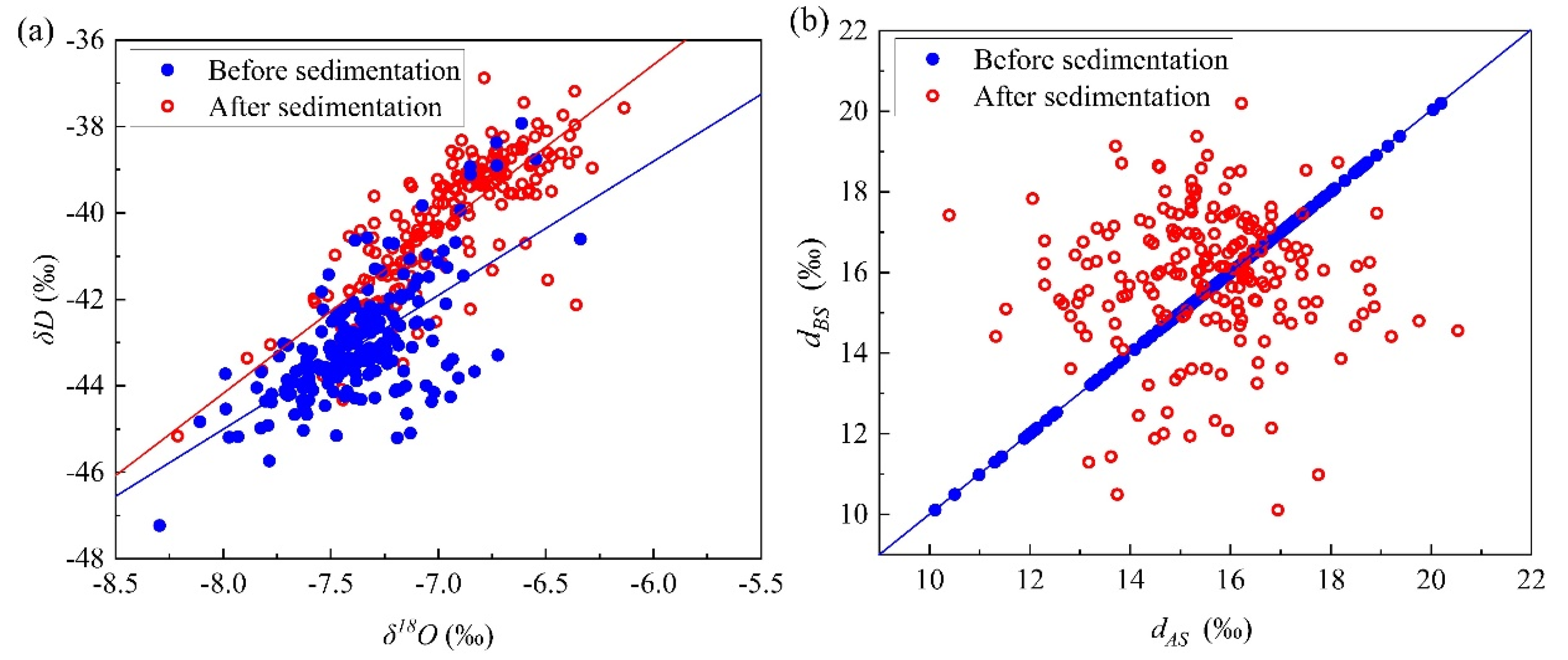
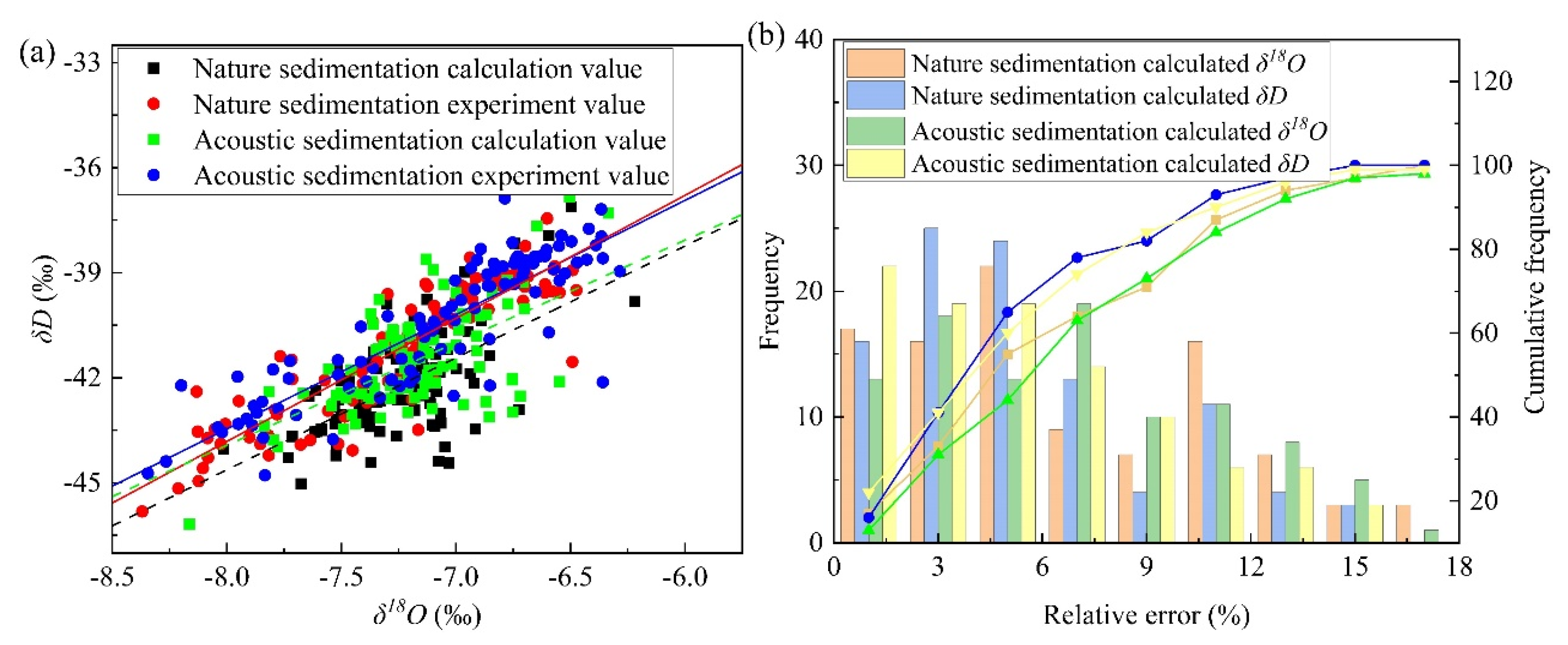
4.1. Linear Fitting Relationship between δD and δ18O Values before and after Sedimentation and Its d Change
4.2. Influence of Acoustic Waves on Particle Number and Surface Area of the Micro-Droplet Groups
4.3. Theoretical Calculation and Analysis of Isotope Exchange
4.3.1. Isotope Exchange Equation
- (1)
- The composition types of water molecules in the micro-droplets and environmental water vapor are H2O, HDO, and H218O, and there are no other types of water molecules.
- (2)
- The isotope exchange between the micro-droplets and the environmental water vapor is equal to the molecular exchange, and the influence of the increase in heavy molecule content on the mass of micro-droplets is negligible.
- (3)
- The initial isotopic values of the micro-droplets with different particle sizes are the same.
- (4)
- The air temperature and saturated water content in the air chamber remain unchanged.
4.3.2. Calculation Parameters
4.3.3. Theoretical Calculation and Analysis
5. Conclusions
- (1)
- The micro-droplets could agglomerate under the influence of acoustic waves and could accelerate the isotope exchange rate. Isotope exchange continuously occurred during the sedimentation process. The acoustic waves had a “trigger effect” on D90. The acoustic waves only reduced and transferred the peak volume frequency, but did not change the peak shape.
- (2)
- The relative variations in the theoretical values for different sedimentation conditions were consistent with the experimental results. The cumulative frequencies of the relative error less than 11% of δD and δ18O were more than 84, indicating high precision. There were some shortcomings, such as the narrow frequency range of acoustic waves and the lack of experimental support for accurate parameters, such as velocity and ventilation coefficient, which will be continuously improved in future studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, A.; Zhang, H.; Wang, L.; Wang, Q.; Wu, X. Source Apportionment and Health Risk Assessment of Heavy Metals in PM2.5 in Handan: A Typical Heavily Polluted City in North China. Atmosphere 2021, 12, 1232. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, H.; Li, Q.; Li, L.; Ge, C.; Li, L.; Feng, J. Secondary Organic Aerosols in PM2.5 in Bengbu, a Typical City in Central China: Concentration, Seasonal Variation and Sources. Atmosphere 2021, 12, 854. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Li, J.; Sun, H.; Qian, Y.; Ding, L.; Ma, H.; Li, J. Seasonal Variation Characteristics of Bacteria and Fungi in PM2.5 in Typical Basin Cities of Xi’an and Linfen, China. Atmosphere 2021, 12, 809. [Google Scholar] [CrossRef]
- Maciejczyk, P.; Chen, L.C.; Thurston, G. The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations. Atmosphere 2021, 12, 1086. [Google Scholar] [CrossRef]
- Yang, S.Q.; Xing, J.; Chen, W.Y.; Li, F.; Zhu, Y. Rapid Evaluation of the Effects of Policies Corresponding to Air Quality, Carbon Emissions and Energy Consumption: An Example from Shenzhen, China. Atmosphere 2021, 12, 1221. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, Y.; Zhang, B. Prediction of PM2.5 Concentration Based on the LSTM-TSLightGBM Variable Weight Combination Model. Atmosphere 2021, 12, 1211. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, S.Y.; Byun, J.Y.; Kim, H.; Kim, W.J.; Kim, P.R.; Han, Y.J. Different Characteristics of PM2.5 Measured in Downtown and Suburban Areas of a Medium-Sized City in South Korea. Atmosphere 2021, 12, 832. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, L.; Ding, W.; Liu, R.; Wang, H.; Wang, S. Analysis of Chemical Composition Characteristics and Source of PM2.5 under Different Pollution Degrees in Autumn and Winter of Liaocheng, China. Atmosphere 2021, 12, 1180. [Google Scholar] [CrossRef]
- Lee, K.; Lee, K.I.; Jeon, S.Y.; Kim, S. Preparation of monodisperse charged droplets via electrohydrodynamic device for the removal of fine dust particles smaller than 10 μm. Adv. Powder Technol. 2019, 30, 190–198. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, W.; Peng, H.; Xie, Y. An investigation of the nozzle’s atomization dust suppression rules in a fully-mechanized excavation face based on the airflow-droplet-dust three-phase coupling model. Adv. Powder Technol. 2018, 29, 941–956. [Google Scholar] [CrossRef]
- Kan, H.; Nakamura, H.; Watano, S. Effect of collision angle on particle-particle adhesion of colliding particles through liquid droplet. Adv. Powder Technol. 2018, 29, 1317–1322. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, J.; Qiu, J.; Chu, H.; Bai, W.; Wang, G. Numerical study of acoustic agglomeration process of droplet aerosol using a three-dimensional CFD-DEM coupled model. Powder Technol. 2020, 362, 37–53. [Google Scholar] [CrossRef]
- Zu, K.; Yao, Y.; Cai, M.; Zhao, F.; Cheng, D. Modeling and experimental study on acoustic agglomeration for dust particle removal. J. Aerosol Sci. 2017, 114, 62–76. [Google Scholar] [CrossRef]
- Cheng, M.T.; Lee, P.S.; Berner, A.; Shaw, D.T. Orthokinetic agglomeration in an intense acoustic field. J. Colloid Interface Sci. 1983, 91, 176–187. [Google Scholar] [CrossRef]
- Dong, S.; Lipkens, B.; Cameron, T.M. The effects of orthokinetic collision, acoustic wake, and gravity on acoustic agglomeration of polydisperse aerosols. J. Aerosol Sci. 2006, 37, 540–553. [Google Scholar] [CrossRef]
- González, I.; Hoffmann, T.L.; Gallego, J.A. Precise measurements of particle entrainment in a standing-wave acoustic field between 20 and 3500 Hz. J. Aerosol Sci. 2000, 31, 1461–1468. [Google Scholar] [CrossRef]
- Song, L.; Koopmann, G.; Hoffmann, T. An improved theoretical model of acoustic agglomeration. J. Vib. Acoust. 1994, 116, 208–214. [Google Scholar] [CrossRef]
- Hoffmann, T.L.; Koopmann, G.H. Visualization of acoustic particle interaction and agglomeration: Theory and experiments. J. Acoust. Soc. Am. 1996, 99, 2130–2141. [Google Scholar] [CrossRef]
- Shaw, D.; Tu, K. Acoustic particle agglomeration due to hydrodynamic interaction between monodisperse aerosols. J. Aerosol. Sci. 1979, 10, 317–328. [Google Scholar] [CrossRef]
- Tiwary, R.; Reethof, G. Hydrodynamic interaction of spherical aerosol particles in a high intensity acoustic field. J. Sound Vib. 1986, 108, 33–49. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, B.; Xu, J.; Shen, X. Experimental study on acoustic agglomeration of ultrafine fly ash particles. Proc. CSEE 2007, 27, 28–32. (In Chinese) [Google Scholar]
- Chang, H.; Kan, C.; Wei, Y.T. Sonic dissipation of water fog-A preliminary experimental study. J. Nanjing Univ. Nat. Sci. 1963, 2, 21–28. (In Chinese) [Google Scholar]
- Hou, S.; Wu, J.; Xi, B. Experiments on acoustic dissipation of water fog at low frequency. Exp. Meas. Fluid Mech. 2002, 16, 52–56. (In Chinese) [Google Scholar]
- Bai, W.; Wei, J.; Ni, S.; Shi, Y. Experimental study on micro-droplet sedimentation under the action of low-frequency acoustic wave. J. Basic Sci. Eng. 2020, 28, 247–258. (In Chinese) [Google Scholar]
- Shi, Y.; Wei, J.; Bai, W.; Wang, G. Numerical investigations of acoustic agglomeration of liquid droplet using a coupled CFD-DEM model. Adv. Powder Technol. 2020, 31, 2394–2411. [Google Scholar] [CrossRef]
- Gu, W.; Pang, Z.; Wang, J. Isotope Hydrology; Science Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Friedman, I.; Machta, L.; Soller, R. Water-vapor exchange between a water droplet and its environment. J. Geophys. Res. 1962, 67, 2761–2766. [Google Scholar] [CrossRef]
- Stewart, M.K. Stable isotope fractionation due to evaporation and isotopic exchange of falling waterdrops: Applications to atmospheric processes and evaporation of lakes. J. Geophys. Res. 1975, 80, 1133–1146. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, J.; Li, T.; Huang, Y.; Qiao, Z.; Cao, J.; Zhong, D.; Wang, G. Cloud and precipitation interference by strong low-frequency sound wave. Sci. China Tech. Sci. 2021, 64, 261–272. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, J.; Ren, Y.; Qiao, Z.; Li, Q.; Zhu, X.; Kang, B.; Pan, P.; Cao, J.; Qiu, J.; et al. Investigation of Precipitation Characteristics under the Action of Acoustic Waves in the Source Region of the Yellow River. J. Appl. Meteorol. Climatol. 2021, 60, 951–966. [Google Scholar]
- Wei, J.; Shi, Y.; Ren, Y.; Li, Q.; Qiao, Z.; Cao, J.; Ayantobo, O.O.; Yin, J.; Wang, G. Application of Ground-Based Microwave Radiometer in Retrieving Meteorological Characteristics of Tibet Plateau. Remote Sens. 2021, 13, 2527. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, J.; Li, Q.; Yang, H.; Qiao, Z.; Ren, Y.; Ni, S.; He, J.; Shen, W.; Cao, S.; et al. Investigation of vertical microphysical characteristics of precipitation under the action of low-frequency acoustic waves. Atmos. Res. 2021, 249, 105283. [Google Scholar] [CrossRef]
- Bai, W.; Wei, J.; Li, Q.; Shi, Y.; Ni, S.; Lei, F. The hydrogen and oxygen isotope characteristic of droplet collision and deposition under the action of acoustic wave. J. Appl. Acoust. 2021, 40, 1–11. (In Chinese) [Google Scholar]
- Yang, J.; Chen, B.; Yin, Y. Cloud Precipitation Physics; China Meteorological Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Han, X.; Xie, Y. Probability Theory and Mathematical Statistics; Peking University Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Beard, K.V.; Pruppacher, H.R. A wind tunnel investigation of the rate of evaporation of small water drops falling at terminal velocity in air. J. Atmos. Sci. 1971, 28, 1455–1464. [Google Scholar] [CrossRef] [Green Version]

| Isotope | Source of Difference | SS | f | MS | F | p-Value |
|---|---|---|---|---|---|---|
| δD | Between groups | 23.42 | 1.00 | 23.42 | 9.85 | 1.95 × 10−3 |
| Within group | 470.64 | 198.00 | 2.38 | |||
| δ18O | Between groups | 1.44 | 1.00 | 1.44 | 13.41 | 3.22 × 10−4 |
| Within group | 21.33 | 198.00 | 0.11 |
| Condition | m (g) | ρs (g/m3) | ε | v (m/s) | t (s) | βγ19 | βγ20 |
|---|---|---|---|---|---|---|---|
| Acoustic | 0.36 | 15 | 2.2 | 0.016 | 109 | 0.35 | 0.34 |
| Nature | 0.36 | 15 | 2.0 | 0.016 | 109 | 0.35 | 0.34 |
| Condition | Experiment | Calculation | ||||||
|---|---|---|---|---|---|---|---|---|
| k | b | R2 | N | k | b | R2 | N | |
| Acoustic | 3.26 ± 0.19 | −17.35 ± 1.33 | 0.75 | 100 | 2.93 ± 0.38 | −20.50 ± 2.75 | 0.37 | 100 |
| Nature | 3.51 ± 0.18 | −15.70 ± 1.30 | 0.80 | 100 | 3.20 ± 0.41 | −19.06 ± 3.00 | 0.38 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, W.; Wei, J.; Shi, Y.; Zhao, Z.; Li, Q. Microphysical Characteristics and Environmental Isotope Effects of the Micro-Droplet Groups under the Action of Acoustic Waves. Atmosphere 2021, 12, 1488. https://doi.org/10.3390/atmos12111488
Bai W, Wei J, Shi Y, Zhao Z, Li Q. Microphysical Characteristics and Environmental Isotope Effects of the Micro-Droplet Groups under the Action of Acoustic Waves. Atmosphere. 2021; 12(11):1488. https://doi.org/10.3390/atmos12111488
Chicago/Turabian StyleBai, Wenwen, Jiahua Wei, Yang Shi, Zhifeng Zhao, and Qiong Li. 2021. "Microphysical Characteristics and Environmental Isotope Effects of the Micro-Droplet Groups under the Action of Acoustic Waves" Atmosphere 12, no. 11: 1488. https://doi.org/10.3390/atmos12111488






