Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls
Abstract
:1. Introduction
2. Indoor Air Pollution: Sources and Their Health Effects
2.1. Inorganic Pollutants
2.2. Organic Pollutants
VOCs
3. Indoor Air Pollution Control Techniques
4. The Role of Plants for Indoor Pollutant Removal
4.1. Green Walls
Botanical and Biofiltration System
4.2. Mechanisms of Air Pollutant Removal by Plants
4.3. Volatile Organic Compounds (VOCs) Removal from Indoor Air by Plants
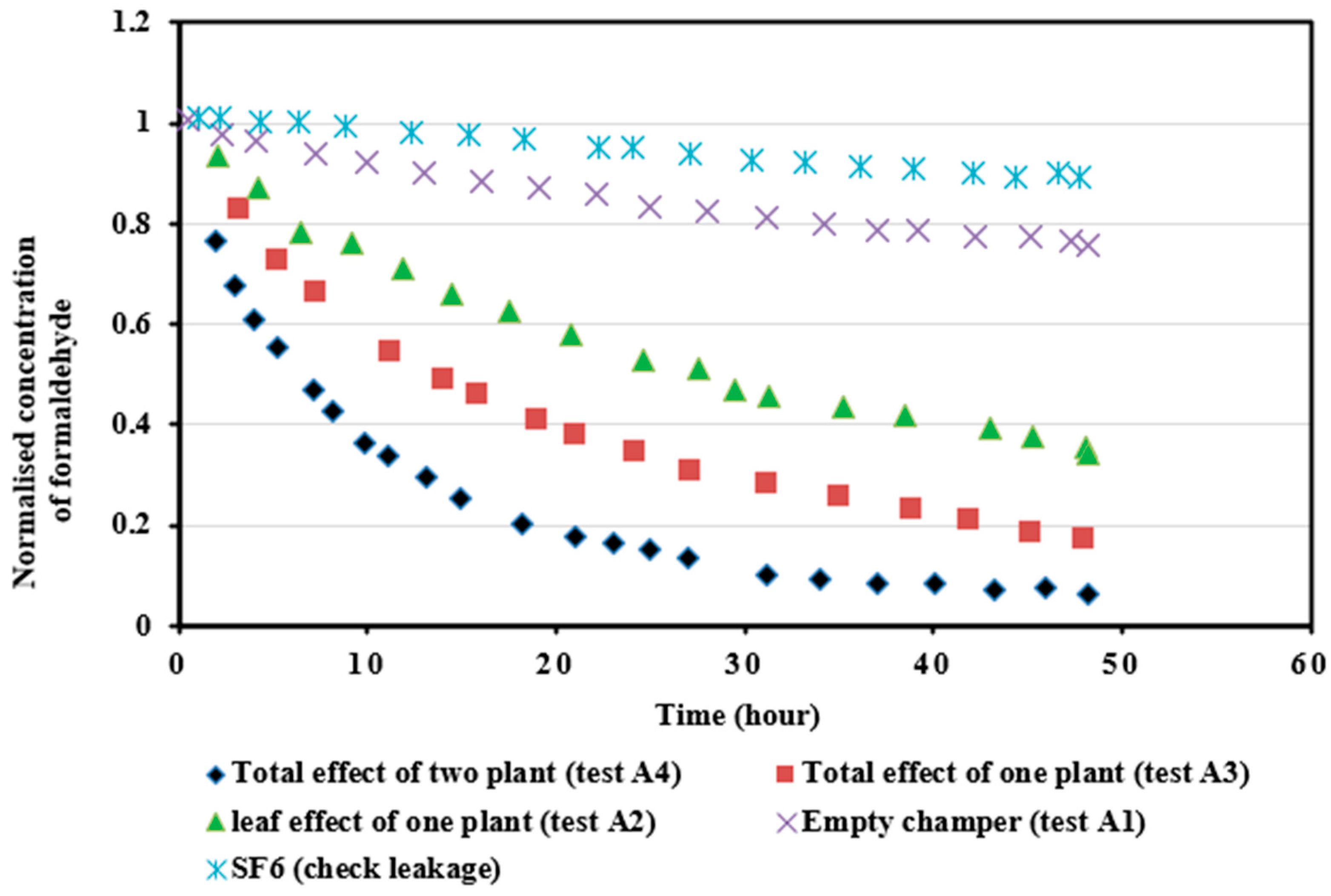
4.3.1. Formaldehyde
4.3.2. Benzene, Toluene and TVOC Compounds
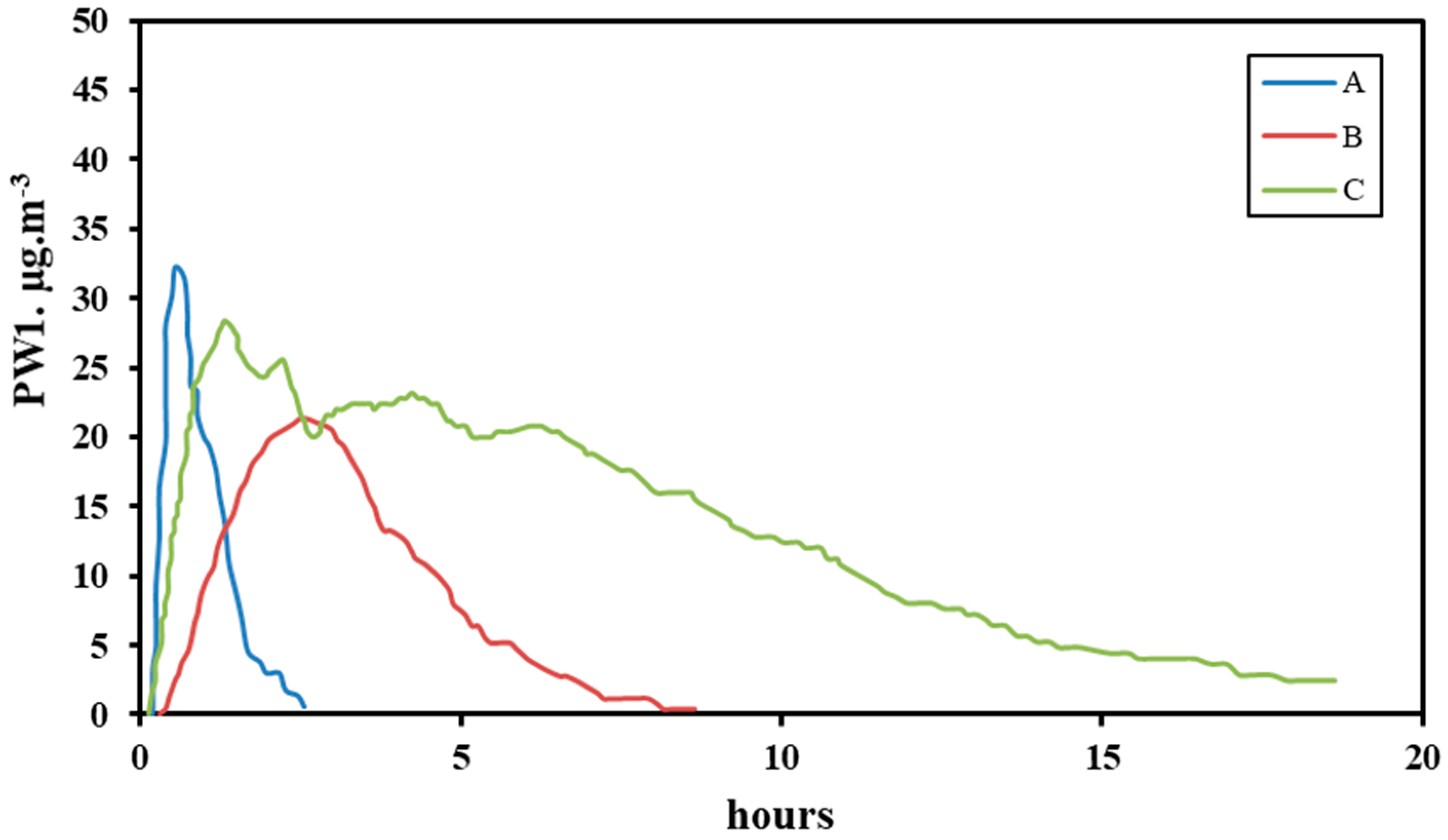
4.4. Particulate Matter Removal from Indoor Air by Plants
4.5. The Choice of Plant
5. Benefits and Economic Analysis
6. Summary, Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Hama, S.; Omidvarborna, H.; Sharma, A.; Sahani, J.; Abhijith, K.; Debele, S.E.; Zavala-Reyes, J.C.; Barwise, Y.; Tiwari, A. Temporary reduction in fine particulate matter due to ‘anthropogenic emissions switch-off’ during COVID-19 lockdown in Indian cities. Sustain. Cities Soc. 2020, 62, 102382. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Aller, M. Environmental Laws and Regulations. In Library of Congress Cataloging; CRC Press LLC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Pandey, A.K.; Pandey, M.; Tripathi, B. Air Pollution Tolerance Index of climber plant species to develop Vertical Greenery Systems in a polluted tropical city. Landsc. Urban. Plan. 2015, 144, 119–127. [Google Scholar] [CrossRef]
- Sanjuán-Herráez, D.; Rodríguez-Carrasco, Y.; Juan-Peiró, L.; Pastor, A.; De la Guardia, M. Determination of indoor air quality of a phytosanitary plant. Anal. Chim. Acta 2011, 694, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X.; Gadea, E.; Garrote, P. Impact of formaldehyde and VOCs fromwaste treatment plants upon the ambient air nearby an urban area. Sci. Total Environ. 2016, 568, 369–380. [Google Scholar] [CrossRef]
- Marć, M. Problems and challenges associated with estimating the emissions of organic compounds from indoor materials. Trac Trends Anal. Chem. 2017, 97, 297–308. [Google Scholar] [CrossRef]
- Sriprapat, W.; Thiravetyan, P. Efficacy of ornamental plants for benzene removal from contaminated air and water: Effect of plant associated bacteria. Int. Biodeterior. Biodegrad. 2016, 113, 262–268. [Google Scholar] [CrossRef]
- Schwela, P.D. Indoor Air; Kotzias, D., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2005; Volume 3, pp. 475–489. [Google Scholar]
- Schwela, D. Pollution, Indoor Air A2—Wexler, Philip. In Encyclopedia of Toxicology, 3rd ed.; Oxford University Press: Oxford, UK, 2014; pp. 1003–1017. [Google Scholar]
- Kolokotsa, D.; Santamouris, M. Review of the indoor environmental quality and energy consumption studies for low income households in Europe. Sci. Total Environ. 2015, 536, 316–330. [Google Scholar] [CrossRef] [PubMed]
- EPA. Why Indoor Air Quality Is Important to Schools. Available online: https://www.epa.gov/iaq-schools/why-indoor-air-quality-important-schools (accessed on 5 March 2021).
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2an Indoor Pollutant? Direct Effects of Low-to-Moderate CO2Concentrations on Human Decision-Making Performance. Environ. Health Perspect. 2012, 120, 1671–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.; Tribess, A.; Buonanno, G.; Stabile, L.; Scungio, M.; Baffo, I. Particle and Carbon Dioxide Concentration Levels in a Surgical Room Conditioned with a Window/Wall Air-Conditioning System. Int. J. Environ. Res. Public Health 2020, 17, 1180. [Google Scholar] [CrossRef] [Green Version]
- Torpy, F.; Irga, P.; Burchett, M. Profiling indoor plants for the amelioration of high CO2 concentrations. Urban For. Urban Green. 2014, 13, 227–233. [Google Scholar] [CrossRef]
- Australia, E. BTEX Personal Exposure Monitoring in Four Australian Cities; Technical Report No. 6; Common-Wealth of Australia: Sydney, Australia, 2003; pp. 1–62. [Google Scholar]
- Vasile, V.; Petran, H.; Dima, A.; Petcu, C. Indoor Air Quality—A Key Element of the Energy Performance of the Buildings. Energy Procedia 2016, 96, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Li, B.; Cheng, Q.; Baldwin, A.N.; Shang, Y. Investigations of indoor air quality of large department store buildings in China based on field measurements. Build. Environ. 2017, 118, 128–143. [Google Scholar] [CrossRef]
- Kazemi, A.; Ghorbanpour, M. Introduction to Environmental Challenges in All Over the World. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 25–48. [Google Scholar] [CrossRef]
- Luengas, A.T.; Hort, C.; Platel, V.; Elias, A.; Barona, A.; Moynault, L. Removal of traces of toluene and p-xylene in indoor air using biofiltration and a hybrid system (biofiltration + adsorption). Environ. Sci. Pollut. Res. 2017, 24, 10674–10684. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, A.; Bogdal, C. Interactions between indoor and outdoor air pollution—Trends and scientific challenges. Environ. Pollut. 2012, 169, 150–151. [Google Scholar] [CrossRef]
- Veetil, D.S.P. Air Pollution: Sources and Effects in Urban Areas and How it Affect the Investment and Economy. Envirocities Emagazine 2012, 3, 5. [Google Scholar]
- Wetzel, T.A.; Doucette, W.J. Plant leaves as indoor air passive samplers for volatile organic compounds (VOCs). Chemosphere 2015, 122, 32–37. [Google Scholar] [CrossRef]
- Śmiełowska, M.; Marć, M.; Zabiegała, B. Indoor air quality in public utility environments—A review. Environ. Sci. Pollut. Res. 2017, 24, 11166–11176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segalin, B.; Kumar, P.; Micadei, K.; Fornaro, A.; Gonçalves, F.L. Size–Segregated particulate matter inside residences of elderly in the Metropolitan Area of São Paulo, Brazil. Atmos. Environ. 2017, 148, 139–151. [Google Scholar] [CrossRef] [Green Version]
- PM, V. Processing and Characterizations: State-of-the-Art and New Challenges. Nanostruct. Polym. Membr. 2016, 1, 1. [Google Scholar]
- Thatcher, A.; Milner, K. Is a green building really better for building occupants? A longitudinal evaluation. Build. Environ. 2016, 108, 194–206. [Google Scholar] [CrossRef]
- Ugranli, T.; Gungormus, E.; Sofuoglu, A.; Sofuoglu, S. Indoor Air Quality in Chemical Laboratories. In Elsevier Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 859–878. [Google Scholar] [CrossRef]
- Arif, M.; Katafygiotou, M.; Mazroei, A.; Kaushik, A.; Elsarrag, E. Impact of indoor environmental quality on occupant well-being and comfort: A review of the literature. Int. J. Sustain. Built Environ. 2016, 5, 1–11. [Google Scholar]
- Balmes, J.R.; Eisner, M.D. Indoor and outdoor air pollution. In Murray & Nadel’s Textbook of Respiratory Medicine E-Book; Elsevier: Amsterdam, The Netherlands, 2016; p. 2208. [Google Scholar]
- Chandrappa, R.; Kulshrestha, U.C. Sustainable Air Pollution Management Theory and Practice; Hamburg, U.F., Rulkens, W.H., Salomons, W., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Goyal, R.; Khare, M.; Kumar, P. Indoor air quality: Current status, missing links and future road map for India. J. Civ. Environ. Eng. 2012, 2, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Nazaroff, W.W. Exploring the consequences of climate change for indoor air quality. Environ. Res. Lett. 2013, 8, 015022. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.C. Outdoor-indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Kiurski, J.S.; Marić, B.B.; Aksentijević, S.M.; Oros, I.B.; Kecic, V.S.; Kovačević, I.M. Indoor air quality investigation from screen printing industry. Renew. Sustain. Energy Rev.. 2013, 28, 224–231. [Google Scholar] [CrossRef]
- Jovanović, M.; Vučićević, B.; Turanjanin, V.; Živković, M.; Spasojević, V. Investigation of indoor and outdoor air quality of the classrooms at a school in Serbia. Energy Environ. Sci. 2014, 77, 42–48. [Google Scholar] [CrossRef]
- Dėdelė, A.; Miškinytė, A. Seasonal variation of indoor and outdoor air quality of nitrogen dioxide in homes with gas and electric stoves. Environ. Sci. Pollut. Res. 2016, 23, 17784–17792. [Google Scholar] [CrossRef] [PubMed]
- Cibella, F.; Cuttitta, G.; Della Maggiore, R.; Ruggieri, S.; Panunzi, S.; De Gaetano, A.; Bucchieri, S.; Drago, G.; Melis, M.R.; La Grutta, S.; et al. Effect of indoor nitrogen dioxide on lung function in urban environment. Environ. Res. 2015, 138, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Tsai, C.M.; Yuan, C.S.; Jen, Y.H.; Ie, I.R. How incense and joss paper burning during the worship activities influences ambient mercury concentrations in indoor and outdoor environments of an Asian temple. Chemosphere 2017, 167, 530–540. [Google Scholar] [CrossRef]
- Loupa, G.; Polyzou, C.; Zarogianni, A.M.; Ouzounis, K.; Rapsomanikis, S. Indoor and outdoor elemental mercury: A comparison of three different cases. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.; Morrison, G.C.; Corsi, R.L. Passive removal materials for indoor ozone control. Build. Environ. 2016, 106, 33–44. [Google Scholar] [CrossRef]
- Fadeyi, M.O. Ozone in indoor environments: Research progress in the past 15 years. Sustain. Cities Soc. 2015, 18, 78–94. [Google Scholar] [CrossRef]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606–6630. [Google Scholar] [CrossRef] [Green Version]
- Irga, P.; Paull, N.; Abdo, P.; Torpy, F. An assessment of the atmospheric particle removal efficiency of an in-room botanical biofilter system. Build. Environ. 2017, 115, 281–290. [Google Scholar] [CrossRef]
- Buczyńska, A.J.; Krata, A.; Van Grieken, R.; Brown, A.; Polezer, G.; De Wael, K.; Potgieter-Vermaak, S. Composition of PM2.5 and PM1 on high and low pollution event days and its relation to indoor air quality in a home for the elderly. Sci. Total Environ. 2014, 490, 134–143. [Google Scholar] [CrossRef]
- Defra The Air Quality Strategy for England, Scotland, Wales and Northern Ireland; Department for Environment, Food and Rural Affairs, The Stationery Office: London, UK, 2007.
- Abd El Aziz, N.G.; Mahgoub, M.H.; Azza, M.M.M.; Farahat, M.M.; Abouziena, H.F. Potentiality of Ornamental Plants and Woody Trees as Phytoremidators of Pollutants in the Air: A Review. Int. J. Chemtech Res. 2015, 8, 468–482. [Google Scholar]
- Othman, M.; Latif, M.T.; Mohamed, A.F. The PM10 compositions, sources and health risks assessment in mechanically ventilated office buildings in an urban environment. Air Qual. Atmos. Health 2015, 9, 597–612. [Google Scholar] [CrossRef]
- Bozlaker, A.; Peccia, J.; Chellam, S. Indoor/Outdoor Relationships and Anthropogenic Elemental Signatures in Airborne PM2.5 at a High School: Impacts of Petroleum Refining Emissions on Lanthanoid Enrichment. Environ. Sci. Technol. 2017, 51, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Mohammadyan, M.; Ghoochani, M.; Kloog, I.; Abdul-Wahab, S.A.; Yetilmezsoy, K.; Heibati, B.; Pollitt, K.J.G. Assessment of indoor and outdoor particulate air pollution at an urban background site in Iran. Environ. Monit. Assess. 2017, 189, 29. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Misra, A. Impact of Indoor Surface Materials and Environment on Perceived Air Quality. J. Environ. Hum. 2014, 2014, 25–35. [Google Scholar] [CrossRef]
- Mentese, S.; Mirici, N.A.; Otkun, M.T.; Bakar, C.; Palaz, E.; Tasdibi, D.; Cevizci, S.; Cotuker, O. Association between respiratory health and indoor air pollution exposure in Canakkale, Turkey. Build. Environ. 2015, 93, 72–83. [Google Scholar] [CrossRef]
- De Gennaro, G.; Dambruoso, P.R.; Loiotile, A.D.; Di Gilio, A.; Giungato, P.; Tutino, M.; Marzocca, A.; Mazzone, A.; Palmisani, J.; Porcelli, F. Indoor air quality in schools. Environ. Chem. Lett. 2014, 12, 467–482. [Google Scholar] [CrossRef]
- Sriprapat, W.; Suksabye, P.; Areephak, S.; Klantup, P.; Waraha, A.; Sawattan, A.; Thiravetyan, P. Uptake of toluene and ethylbenzene by plants: Removal of volatile indoor air contaminants. Ecotoxicol. Environ. Saf. 2014, 102, 147–151. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Recent trend in risk assessment of formaldehyde exposures from indoor air. Arch. Toxicol. 2012, 87, 73–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Re-evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Arch. Toxicol. 2016, 91, 35–61. [Google Scholar] [CrossRef] [Green Version]
- Destaillats, H.; Maddalena, R.L.; Singer, B.C.; Hodgson, A.T.; McKone, T.E. Indoor pollutants emitted by office equipment: A review of reported data and information needs. Environ. Energy Technol. Div. 2007, 42, 1371–1388. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Naphthalene, 1-Methylnapthalene, 2-Methylnapthalene; ATSDR: Atlanta, GA, USA, 2011.
- ATSDR. Trichloroethylene (TCE); ATSDR: Atlanta, GA, USA, 2011.
- Bahr, D.E.; Aldrich, T.E.; Seidu, D.; Brion, G.M.; Tollerud, D.J.; Muldoon, S.; Reinhart, N.; Youseefagha, A.; McKinney, P.; Hughes, T.; et al. Occupational exposure to trichloroethylene and cancer risk for workers at the Paducah Gaseous Diffusion Plant. Int. J. Occup. Med. Environ. Health 2011, 24, 67–77. [Google Scholar] [CrossRef]
- Environmental Health and Medicine Education. Tetrachloroethylene Toxicity, What Are the Physiological Effects of Tetrachloroethylene Exposure? In Agency for Toxic Substances and Disease Registry; ATSDR: Atlanta, GA, USA, 2008. [Google Scholar]
- Ayoko, G.A.; Wang, H. Volatile Organic Compounds. In Indoor Environments; Pluschke, P., Schleibinger, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–107. [Google Scholar] [CrossRef]
- World Health Organization. Indoor Air Quality: Organic Pollutants. Report on a WHO Meeting, Berlin, Germany, 23–27 August 1987; EURO Reports and Studies 111; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 1989. [Google Scholar]
- WHO. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- EPA. United States Environmental Protection Agency; EPA: Washington, DC, USA, 1971.
- Besis, A.; Samara, C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments—A review on occurrence and human exposure. Environ. Pollut. 2012, 169, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O.; Eriksen, G.S.; Jóhannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001, 109, 49–68. [Google Scholar]
- Hooper, K.; McDonald, T.A. The PBDEs: An emerging environmental challenge and another reason for breast-milk monitoring programs. Environ. Health Perspect. 2000, 108, 387–392. [Google Scholar] [CrossRef]
- Gaspar, F.W.; Chevrier, J.; Bornman, R.; Crause, M.; Obida, M.; Barr, D.B.; Bradman, A.; Bouwman, H.; Eskenazi, B. Corrigendum to Undisturbed dust as a metric of long-term indoor insecticide exposure: Residential DDT contamination from indoor residual spraying and its association with serum levels in the VHEMBE cohort. Environ. Int. 2016, 94, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Nuevo, M.J. Actions for remediation in cases with large concentration of radon indoor. J. Radioanal. Nucl. Chem. 2016, 311, 1219–1225. [Google Scholar] [CrossRef]
- Al-Zoughool, M.; Krewski, D. Health effects of radon: A review of the literature. Int. J. Radiat. Biol. 2009, 85, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.L.; Phillips, T.J.; Mulberg, E.J.; Hui, S.P. Activity patterns of Californians: Use of and proximity to indoor pollutant sources. Atmos. Environ. Part A Gen. Top. 1992, 26, 2141–2148. [Google Scholar] [CrossRef]
- Bruce, N.; Perez-Padilla, R.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. Bull. World Health Organ. 2000, 78, 1078–1092. [Google Scholar] [PubMed]
- Ezzati, M. Indoor Air Pollution/Developing Countries; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 547–553. [Google Scholar] [CrossRef]
- Smith, K.R.; Mehta, S.; Maeusezahl-Feuz, M. Indoor air pollution from household solid fuel use. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 1435–1493. [Google Scholar]
- Smith, K.R. Biofuels, Air Pollution, and Health: A Global Review; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Moya, T.A.; Dobbelsteen, A.V.D.; Ottelé, M.; Bluyssen, P.M. A review of green systems within the indoor environment. Indoor Built Environ. 2018, 28, 298–309. [Google Scholar] [CrossRef]
- Irga, P.J.; Pettit, T.; Irga, R.F.; Paull, N.J.; Douglas, A.N.; Torpy, F.R. Does plant species selection in functional active green walls influence VOC phytoremediation efficiency? Environ. Sci. Pollut. Res. 2019, 26, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kaunelienė, V.; Prasauskas, T.; Krugly, E.; Stasiulaitienė, I.; Čiužas, D.; Šeduikytė, L.; Martuzevičius, D. Indoor air quality in low energy residential buildings in Lithuania. Build. Environ. 2016, 108, 63–72. [Google Scholar] [CrossRef]
- Rounaghi, G.; Kakhki, R.M.; Eshghi, H. Efficient transport of lead (II) cations in natural water using a liquid membrane system with dicyclohexano-18-crown-6 as carrier. Arab. J. Chem. 2017, 10, S339–S346. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Xiao, M.; Zhang, X.; Gal, C.; Chen, X.; Liu, L.; Pan, S.; Wu, J.; Tang, L.; Clements-Croome, D. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 2017, 32, 375–396. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Influence of the inlet air in efficiency of photocatalytic devices for mineralization of VOCs in air-conditioning installations. Environ. Sci. Pollut. Res. 2014, 21, 11198–11207. [Google Scholar] [CrossRef] [PubMed]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. Reviews in Environmental Science and Bio/Technology. Rev. Environ. Sci. Biotechnol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Sarkar, M.; Chakraborty, B.; Banerjee, T. Phytoremediation of Air Pollutants: Prospects and Challenges. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–241. [Google Scholar]
- Brilli, F.; Fares, S.; Ghirardo, A.; de Visser, P.; Calatayud, V.; Muñoz, A.; Annesi-Maesano, I.; Sebastiani, F.; Alivernini, A.; Varriale, V.; et al. Plants for Sustainable Improvement of Indoor Air Quality. Trends Plant. Sci. 2018, 23, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.E.; Waring, M.S. Potted plants do not improve indoor air quality: A review and analysis of reported VOC removal efficiencies. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.-T.; Ruan, L.-W. Effects of indoor plants on air quality: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 16019–16051. [Google Scholar] [CrossRef]
- Abhijith, K.; Kumar, P.; Gallagher, J.; McNabola, A.; Baldauf, R.; Pilla, F.; Broderick, B.; Di Sabatino, S.; Pulvirenti, B. Air pollution abatement performances of green infrastructure in open road and built-up street canyon environments—A review. Atmos. Environ. 2017, 162, 71–86. [Google Scholar] [CrossRef]
- Wannomai, T.; Kemacheevakul, P.; Thiravetyan, P. Removal of Trimethylamine from Indoor Air Using Potted Plants under Light and Dark Conditions. Aerosol Air Qual. Res. 2019, 19, 1105–1113. [Google Scholar] [CrossRef]
- Aydogan, A.; Cerone, R. Review of the effects of plants on indoor environments. Indoor Built Environ. 2020. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, P.; Kalaiarasan, G.; Ottosen, T.-B. The impacts of existing and hypothetical green infrastructure scenarios on urban heat island formation. Environ. Pollut. 2021, 274, 115898. [Google Scholar] [CrossRef]
- Weyens, N.; Thijs, S.; Popek, R.; Witters, N.; Przybysz, A.; Espenshade, J.; Gawronska, H.; Vangronsveld, J.; Gawronski, S.W. The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. Mol. Sci. 2015, 16, 25576–25604. [Google Scholar] [CrossRef] [Green Version]
- Cuce, E. Thermal regulation impact of green walls: An experimental and numerical investigation. Appl. Energy 2017, 194, 247–254. [Google Scholar] [CrossRef]
- Maslauskas, T. Green Walls—The Vertical Planting Systems; VIA University College: Horsens, Denmark, 2015; p. 43. [Google Scholar]
- Pastore, L.; Corrao, R.; Heiselberg, P.K. The effects of vegetation on indoor thermal comfort: The application of a multi-scale simulation methodology on a residential neighborhood renovation case study. Energy Build. 2017, 146, 1–11. [Google Scholar] [CrossRef]
- Ravindu, S.; Rameezdeen, R.; Zuo, J.; Zhou, Z.; Chandratilake, R. Indoor environment quality of green buildings: Case study of an LEED platinum certified factory in a warm humid tropical climate. Build. Environ. 2015, 84, 105–113. [Google Scholar] [CrossRef]
- Safikhani, T.; Abdullah, A.M.; Ossen, D.R.; Baharvand, M. A review of energy characteristic of vertical greenery systems. Renew. Sustain. Energy Rev. 2014, 40, 450–462. [Google Scholar] [CrossRef]
- Gunawardena, K.; Steemers, K. Living walls in indoor environments. Build. Environ. 2019, 148, 478–487. [Google Scholar] [CrossRef]
- Green Roofs for Healthy Cities: Introduction to Green Walls, Introduction to Green Walls Technology Benefits & Design. 2008. Available online: www.greenroofs.org (accessed on 1 April 2021).
- Modirrousta, S.; Mohammadi, Z. Necessity and Methods of Designing Green Buildings in Cities and its Effect on Energy Efficiency. Eur. Online J. Nat. Soc. Sci. Proc. 2015, 4, 304–314. [Google Scholar]
- Tomson, M.; Kumar, P.; Barwise, Y.; Perez, P.; Forehead, H.; French, K.; Morawska, L.; Watts, J.F. Green infrastructure for air quality improvement in street canyons. Environ. Int. 2021, 146, 106288. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, B.C.B.; NASA. Improving Indoor Air Quality with Plant-Based Systems; NASA: Washington, DC, USA, 2009.
- Darlington, A.B.; Dat, J.F.; Dixon, M.A. The Biofiltration of Indoor Air: Air Flux and Temperature Influences the Removal of Toluene, Ethylbenzene, and Xylene. Environ. Sci. Technol. 2000, 35, 240–246. [Google Scholar] [CrossRef]
- Darlington, A.B. Room Air Cleansing Using Hydroponic Plants. U.S. Patent No. 6,727,09, 27 April 2004. [Google Scholar]
- Llewellyn, D.; Darlington, A.; van Ras, N.; Kraakman, B.; Dixon, M. A hybridized membrane-botanical biofilter for improving air quality in occupied spaces. In Proceedings of the 37th COSPAR Scientific Assembly, Montreal, QC, Canada, 13–20 July 2008; Volume 37, p. 1813. [Google Scholar]
- Prodanovic, V.; Wang, A.; Deletic, A. Assessing water retention and correlation to climate conditions of five plant species in greywater treating green walls. Water Res. 2019, 167, 115092. [Google Scholar] [CrossRef]
- Sadeghian, M.M. A Review on Green Wall Classification and Function. Sci. Technol. 2016, 2, 47–51. [Google Scholar]
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco, A.; Egea, G. Influence of an active living wall on indoor temperature and humidity conditions. Ecol. Eng. 2016, 90, 120–124. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.; Torpy, F. Functional green wall development for increasing air pollutant phytoremediation: Substrate development with coconut coir and activated carbon. J. Hazard. Mater. 2018, 360, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Rev. Environ. Sci. Bio/Technol. 2018, 17, 395–415. [Google Scholar] [CrossRef]
- Sowa, J.; Hendiger, J.; Maziejuk, M.; Sikora, T.; Osuchowski, L.; Kamińska, H. Potted Plants as Active and Passive Biofilters Improving Indoor Air Quality. Iop Conf. Ser. Earth Environ. Sci. 2019, 290, 012150. [Google Scholar] [CrossRef]
- Abdo, P.; Huynh, B.P.; Irga, P.J.; Torpy, F.R. Evaluation of air flow through an active green wall biofilter. Urban For. Urban Green. 2019, 41, 75–84. [Google Scholar] [CrossRef]
- Hernandez, M.; Peden, D.B. Air Pollution: Indoor and Outdoor. In Middleton’s Allergy: Principles and Practice; Adkinson, N.F., Jr., Bochner, B.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 482–496. [Google Scholar]
- Pettit, T.; Bettes, M.; Chapman, A.R.; Hoch, L.M.; James, N.D.; Irga, P.J.; Torpy, F.R.; Plants and Environmental Quality Research Group. The botanical biofiltration of VOCs with active airflow: Is removal efficiency related to chemical properties? Atmos. Environ. 2019, 214, 116839. [Google Scholar] [CrossRef]
- Fleck, R.; Pettit, T.J.; Douglas, A.N.; Irga, P.J.; Torpy, F.R. 15—Botanical biofiltration for reducing indoor air pollution. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Pacheco-Torgal, F., Ivanov, V., Tsang, D.C.W., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 305–327. [Google Scholar]
- Curtis, L.; Stuart, M. Enhancing CHBE Indoor Air Quality: Biowall Technology; Student Report; University of British Columbia Social Ecological Economic Development Studies (SEEDS): Vancouver, BC, Canada, 2010. [Google Scholar]
- Dickinson, N.M.; Baker, A.J.; Doronila, A.; Laidlaw, S.; Reeves, R.D. Phytoremediation of inorganics: Realism and synergies. Int. J. Phytoremediat. 2009, 11, 97–114. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- Chaney, R.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Ensley, B. Rationale for use of phytoremediation. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley & Sons: New York, NY, USA, 2000; pp. 3–11. [Google Scholar]
- Prasad, M.N.V.; Freitas, H.M.D.O. Metal hyperaccumulation in plants—Biodiversity prospecting for phytoremediation technology. Electron. J. Biotechnol. 2003, 6, 285–321. [Google Scholar] [CrossRef]
- Schnoor, J.; Light, L.A.; McCutcheon, S.C.; Wolfe, N.L.; Carreia, L.H. Phytoremediation of organic and nutrient contaminants. Environ. Sci. Technol. 1995, 29, 318A–323A. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2007, 7, 47–59. [Google Scholar] [CrossRef]
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from indoor air by ornamental potted plants: A pilot study using a palm species under the controlled environment. Chemosphere 2018, 197, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gawrońska, H.; Bakera, B. Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual. Atmos. Health 2014, 8, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soreanu, G.; Dixon, M.; Darlington, A. Botanical biofiltration of indoor gaseous pollutants—A mini-review. Chem. Eng. J. 2013, 229, 585–594. [Google Scholar] [CrossRef]
- Aydogan, A.; Montoya, L.D. Formaldehyde removal by common indoor plant species and various growing media. Atmos. Environ. 2011, 45, 2675–2682. [Google Scholar] [CrossRef]
- Su, Y.; Liang, Y. Foliar uptake and translocation of formaldehyde with Bracket plants (Chlorophytum comosum). J. Hazard. Mater. 2015, 291, 120–128. [Google Scholar] [CrossRef]
- Lee, J.H. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol. Bioprocess. Eng. 2013, 18, 431–439. [Google Scholar] [CrossRef]
- Kvesitadze, E.; Sadunishvili, T.; Kvesitadze, G. Mechanisms of organic contaminants uptake and degradation in plants. World Acad. Sci. Eng. Technol. 2009, 55, 458–468. [Google Scholar]
- Pettit, T.; Irga, P.J.; Torpy, F.R. The in situ pilot-scale phytoremediation of airborne VOCs and particulate matter with an active green wall. Air Qual. Atmos. Health 2018, 12, 33–44. [Google Scholar] [CrossRef]
- Wang, Z.; Pei, J.; Zhang, J.S. Experimental investigation of the formaldehyde removal mechanismsin a dynamic botanical filtration system for indoor air purification. Hazard. Mater. 2014, 280, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Groot, F.B.; Wörtche, H. Effect of ecosystem services provided by urban green infrastructure on indoor environment: A literature review. Build. Environ. 2014, 77, 88–100. [Google Scholar] [CrossRef]
- Llewellyn, D.; Dixon, M. Can Plants Really Improve Indoor Air Quality? Reference Module in Life Sciences. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 331–337. [Google Scholar]
- Wolverton, B.C.; Johnson, A.; Bounds, K. Interior Landscape Plants for Indoor Air Pollution Abatement; NASA Stennis Space Centre: Hancock, MS, USA, 1989.
- Bondarevs, A.; Huss, P.; Gong, S.; Weister, O.; Liljedahl, R. Green Walls Utilizing Internet of Things. Sens. Transduct. 2015, 192, 16–21. [Google Scholar]
- Treesubsuntorn, C.; Thiravetyan, P. Botanical biofilter for indoor toluene removal and reduction of carbon dioxide emission under low light intensity by using mixed C3 and CAM plants. J. Clean. Prod. 2018, 194, 94–100. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.S. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build. Environ. 2011, 46, 758–768. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, N.; Wang, J.; Tong, H. Formaldehyde biofiltration as affected by spider plant. Bioresour. Technol. 2010, 101, 6930–6934. [Google Scholar] [CrossRef]
- Weidner, M.; Da Silvia, J.A.T. Potential and Limitations of Ornamental Plants for Indoor-Air Purification; Silva, J.A.T.D., Ed.; Floriculture, Ornamental and Plant Biotechnology Advances and Topical Issues; Global Science Books: London, UK, 2006; pp. 54–63. [Google Scholar]
- Liu, Y.J.; Mu, Y.J.; Zhu, Y.G.; Ding, H.; Arens, N.C. Which ornamental plant species effectively remove benzene from indoor air? Atmos. Environ. 2007, 41, 650–654. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, T.; Wang, P.; Lin, Y.; Zheng, R.; Zhao, Y.; Xu, B. Fundamentals of Ornamental Plants in Removing Benzene in Indoor Air. Atmosphere 2019, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.H.; Kwon, Y.J.; Son, K.-C.; Kays, S.J. Efficacy of Indoor Plants for the Removal of Single and Mixed Volatile Organic Pollutants and Physiological Effects of the Volatiles on the Plants. J. Am. Soc. Hortic. Sci. 2006, 131, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.S.; Pennisi, S.V.; Son, K.-C.; Kays, S.J. Screening Indoor Plants for Volatile Organic Pollutant Removal Efficiency. HortScience 2009, 44, 1377–1381. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Thiravetyan, P. Removal of benzene from indoor air by Dracaena sanderiana: Effect of wax and stomata. Atmos. Environ. 2012, 57, 317–321. [Google Scholar] [CrossRef]
- Sriprapat, W.; Thiravetyan, P. Phytoremediation of BTEX from Indoor Air by Zamioculcas zamiifolia. Water Air Soil Pollut. 2013, 224, 1–9. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, H.J.; Khalekuzzaman, M.; Yoo, E.H.; Jung, H.H.; Jang, H.S. Removal ratio of gaseous toluene and xylene transported from air to root zone via the stem by indoor plants. Environ. Sci. Pollut. Res. 2016, 23, 6149–6158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orwell, R.L.; Wood, R.L.; Tarran, J.; Torpy, F.; Burchett, M.D. Removal of Benzene by the Indoor Plant/Substrate Microcosm and Implications for Air Quality. Water Air Soil Pollut. 2004, 157, 193–207. [Google Scholar] [CrossRef]
- Tarran, J.; Torpy, F.; Burchett, M. Use of living pot-plants to cleanse indoor air—Research review. In Proceedings of the 6th International Conferece on Indoor Air Quality, Ventilation & Energy Conservation, Sustainable Built Environment, Sendai, Japan, 28–31 October 2007; pp. 249–256. [Google Scholar]
- Jen, M.S.; Hoylman, A.M.; Edwards, N.T.; Walton, B.T. Experimental method to measure gaseous uptake of 14C-toluene by foliage. Environ. Exp. Bot. 1995, 35, 389–398. [Google Scholar] [CrossRef]
- Caron, A.; Redon, N.; Thevenet, F.; Hanoune, B.; Coddeville, P. Performances and limitations of electronic gas sensors to investigate an indoor air quality event. Build. Environ. 2016, 107, 19–28. [Google Scholar] [CrossRef]
- Wood, R.A.; Burchett, M.D.; Alquezar, R.; Orwell, R.L.; Tarran, J.; Torpy, F. The Potted-Plant Microcosm Substantially Reduces Indoor Air VOC Pollution: I. Office Field-Study. Water Air Soil Pollut. 2006, 175, 163–180. [Google Scholar] [CrossRef]
- Gupta, G.P.; Kulshrestha, U. Biomonitoring and Remediation by Plants. Plant. Responses Air Pollut. 2016, 119–132. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Q.; Zhang, R. Sources, health effects and control strategies of indoor fine particulate matter (PM 2.5): A review. Sci. Total Environ. 2017, 586, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Shaneyfelt, K.M.; Anderson, A.R.; Kumar, P.; Hunt, W.F. Air quality considerations for stormwater green street design. Environ. Pollut. 2017, 231, 768–778. [Google Scholar] [CrossRef]
- Tomašević, M.; Anicic, M. Trace element content in urban tree leaves and SEM-EDAX characterization of deposited particles. Facta Univ. Ser. Phys. Chem. Technol. 2010, 8, 1–13. [Google Scholar] [CrossRef]
- Ram, S.S.; Majumder, S.; Chaudhuri, P.; Chanda, S.; Santra, S.C.; Chakraborty, A.; Sudarshan, M. A Review on Air Pollution Monitoring and Management Using Plants with Special Reference to Foliar Dust Adsorption and Physiological Stress Responses. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2489–2522. [Google Scholar] [CrossRef]
- Barwise, Y.; Kumar, P. Designing vegetation barriers for urban air pollution abatement: A practical review for appropriate plant species selection. NPJ Clim. Atmos. Sci. 2020, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Abbass, O.A.; Sailor, D.J.; Gall, E.T. Effectiveness of indoor plants for passive removal of indoor ozone. Build. Environ. 2017, 119, 62–70. [Google Scholar] [CrossRef]
- Cruz, M.D.; Müller, R.; Svensmark, B.; Pedersen, J.S.; Christensen, J.H. Assessment of volatile organic compound removal by indoor plants—A novel experimental setup. Environ. Sci. Pollut. Res. 2014, 21, 7838–7846. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.; Jung, G.J.; Seo, M.H.; Im, Y.B. Experimental study on variations of CO2 concentration in the presence of in door plants and respiration of experimental animals. Environ. Biotechnol. 2011, 52, 321–329. [Google Scholar]
- Pennisi, S.V.; van Iersel, M.W. Quantification of carbon assimilation of plants in simulated and in situ interiorscapes. HortScience 2012, 47, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Morawska, L. Energy-Pollution Nexus for Urban Buildings. Environ. Sci. Technol. 2013, 47, 7591–7592. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kacira, M.; An, L. A CFD study on improving air flow uniformity in indoor plant factory system. Biosyst. Eng. 2016, 147, 193–205. [Google Scholar] [CrossRef]
- Pichatwatana, K.; Wang, F.; Roaf, S.; Anunnathapong, M. An integrative approach for indoor environment quality assessment of large glazed air-conditioned airport terminal in the tropics. Energy Build. 2017, 148, 37–55. [Google Scholar] [CrossRef]
- Tudiwer, D.; Korjenic, A. The effect of an indoor living wall system on humidity, mould spores and CO 2 -concentration. Energy Build. 2017, 146, 73–86. [Google Scholar] [CrossRef]
- D’Alessandro, F.; Asdrubali, F.; Mencarelli, N. Experimental evaluation and modelling of the sound absorption properties of plants for indoor acoustic applications. Build. Environ. 2015, 94, 913–923. [Google Scholar] [CrossRef]
- Collins, R.; Schaafsma, M.; Hudson, M.D. The value of green walls to urban biodiversity. Land Use Policy 2017, 64, 114–123. [Google Scholar] [CrossRef]
- Coombs, K.C.; Chew, G.L.; Schaffer, C.; Ryan, P.H.; Brokamp, C.; Grinshpun, S.A.; Adamkiewicz, G.; Chillrud, S.; Hedman, C.; Colton, M.; et al. Indoor air quality in green-renovated vs. non-green low-income homes of children living in a temperate region of US (Ohio). Sci. Total Environ. 2016, 554–555, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Poddar, S.; Park, D.Y.; Chang, S. Simulation Based Analysis on the Energy Conservation Effect of Green Wall Installation for Different Building Types in a Campus. Energy Procedia 2017, 111, 226–234. [Google Scholar] [CrossRef]
- Koyama, T.; Yoshinaga, M.; Maeda, K.-I.; Yamauchi, A. Transpiration cooling effect of climber greenwall with an air gap on indoor thermal environment. Ecol. Eng. 2015, 83, 343–353. [Google Scholar] [CrossRef]
- Torpy, F.; Zavattaro, M.; Irga, P. Green wall technology for the phytoremediation of indoor air: A system for the reduction of high CO2 concentrations. Air Qual. Atmos. Health 2016, 10, 575–585. [Google Scholar] [CrossRef]





| Category Description | Acronym | Boiling Point Range, °C |
|---|---|---|
| Very volatile (gaseous) organic compounds | VVOCs | <0 to 50 |
| Volatile organic compounds | VOCs | 50 to 240 |
| Semi-volatile organic compounds | SVOCs | 240 to 380 |
| Organic compounds associated with particulate matter: Particle-bound organic compounds | POCs | >380 |
| Class | Name | Maximum Exposure Guidelines | |
|---|---|---|---|
| Organic pollutant | Carbon monoxide | 100 mg/m3 | 15 min |
| 60 mg/m3 | 30 min | ||
| 30 mg/m3 | 1 h | ||
| 10 mg/m3 | 8 h | ||
| Organic pollutant | Formaldehyde | 0.1 mg/m3 | 30 min |
| Organic pollutant | Tetrichloroethylene (TCE) | 0.25 mg/m3 | Annual |
| Organic pollutant | Tetrachloroethylene (PCE) | 100 ppm | 3 h |
| Organic pollutant | Toluene | 0.26 mg/m3 | 1 week |
| Inorganic pollutant | Asbestos | 500 F*/m3 b | -- |
| Radioactive pollutant | Radon | >1 Becquerel/m3 c | -- |
| Classical pollutant | Nitrogen dioxide | 200 μg/m3 | 1 h |
| 40 μg/m3 | Annual | ||
| 53 ppb a | Annual | ||
| Ozone | 120 μg/m3 | 8 h | |
| 0.08 ppm a | 1 h | ||
| Pollutant | Potted Plant Species (Remedy) | Results | Ref. |
|---|---|---|---|
| O3 | Peace Lily (Spathiphyllum), Ficus species (Ficus Decora Burgundy), Calathia (Calathia Species), Dieffenbachia (Dieffenbachia Species), Golden Pothos (Epipremnum aureum) | The Golden Pothos had the highest ozone deposition velocity values among plants, and the lowest value was for Peace Lily | [160] |
| Toluene and xylene | Schefflera actinophylla and Ficus benghalensis) | Removal of toluene and xylene was 13.3 and 7.0 μg·m−3·m−2 leaf area over a 24-h period in S. actinophylla and was 13.0 and 7.3 μg·m−3·m−2 leaf area in F. benghalensis. It also showed that the root zone has a vital role in toluene and xylene removal. | [148] |
| Toluene | Hedera helix | The removal rate is 66.5 μg/m2/h for toluene that is effective to remove it. | [161] |
| Benzene | Syngonium podophyllum, Sansevieria trifasciata, Euphorbia milii, Chlorophytum comosum, Epipremnum aureum, Dracaena sanderiana, Hedera helix, Clitoria ternatea | C. comosum was the highest efficient plant for removing benzene during the 96 h. | [8] |
| Trichloroethylene, tetrachloroethylene 1,2-dichloroethane benzene, toluene m, p-xylene | ficus; golden pothos; spider fern; Christmas cactus | Leaf concentrations change with air concentrations, the speed of air. It shows the potential of leaves for removing VOCs in indoor air. | [23] |
| PM | Spider plants (Chlorophytum comosum L.) | The result show accumulation of PM at a high level on surface of leaf | [126] |
| Toluene ethylbenzene | Aloe vera, Sansevieria masoniana, Sansevieria trifasciata, Sansevieria hyacinthoides, Sansevieria ehrenbergii, Kalanchoe blossfeldiana, Dracaenaderemensis, Codiaeum variegatum, Chlorophytum comosum, Dracaena sanderiana, Cordyline fruticosa, Aglaonema commutatum | S. trifasciata had the highest value for removing toluene, C. comosum. for removal of ethylbenzene, S. trifasciata and S. hyacinthoides had a high value in the absorption of toluene and ethylbenzene. | [54] |
| Benzene Trichloroethylne Formaldehyde | Chamaedorea seifritzii, Aglaonema modestum, Hedera helix, Ficus benjamina, Gerbera jamesonii, Dracaena deremensis, Dracaena marginata, Dracaena massangeana, Sansevieria laurentii, Spathiphyllum, Chrysanthemum morifolium, Dracaena deremensis | These plants require low light and low metabolic rates. These plants are a suitable selection to decrease sick building syndrome containing many new, energy-efficient buildings. The plant root-soil zone showed high efficiency for removal of VOCs | [136] |
| Formaldehyde | Golden Pothos | Dynamic airflow through the root bed and microbes were essential for removing high efficiency; moisture of bed root has a vital role in removing VOCs. | [133] |
| Benzenen-hexane | Janet Craig S. Sweet Chico | The highest value for removing TVOCs (75%) by potted-plants is when indoor average TVOC concentrations are higher than 100 ppb. | [153] |
| Benzene | Indoor ornamental plants representing 73 species and cultivars (35 families and 60 genera) | Thirteen species removed between 0.1–9.99% of benzene in contaminated air, 17 species removed 10–20%, and 17 species removed 20–40%. Three species removed 60–80% of benzene in the experimental air. | [142] |
| Benzene | Spathiphyllum, Howea forsteriana, Dracaena marginata, Epipremnum aureum, Spathiphyllum, | These potted-plants decrease VOCs, even if the level of VOCs has very low. | [149] |
| Schefflera, Dracaena | |||
| Toluene toxicity, 14C-toluene uptake | Soybean (Glycine max) | 14C concentrations in Leaf tissue increased during the light phases and decreased during 12-hr dark phases. | [151] |
| Benzene CO2, CO | Zamioculcas, Aglaonema Dracaena | CO2 concentration increases 10% in offices in the air-conditioned building. The CO level reduces with or without air-conditioning. Higher value removing of benzene appearance by these plants. | [150] |
| CO2, acetone methyl acetate Formaldehyde PM | Green wall | The active green wall has high efficiency to increase indoor air quality by absorbing VOC and PM but has not been highly effective for carbon dioxide adsorption. The green wall increases the relative humidity, which is a suitable selection to use in a dry environment. | [137] |
| CO2 | Aglaonema commutatum Schott, Aspidistra elatior Blume, Castanospermum australe A.CunnexHoo., Chamaedorea elegans Wild., Dracaena deremensis Engl., Dypsis lutescens, Beentje and J.Dransf., Ficus benjamina L. Howea forsteriana Becc. | These plants can be used in high-intensity light, but if the light intensity is higher than the optimal value, but will become photoinhibited, and possibly be etched off chlorophyll | [15] |
| CO2 | Peace lily, weeping fig, areca palm | The rate of photosynthesis change with the variation of CO2 concentration in light indoor. The leaf area is effective to decrease CO2. for 3 plants, with the area of the leaf up to 15,000 cm2, the CO2 concentration decreases (just leaf area is effective in the reduction of CO2. | [162] |
| CO2 | Sweet Chico, Hahnii, Chamaedorea elegans, Dracaena marginata, Florida Beauty, Lemon Lime, Janet Craig, Ctenanthe, oppenheimiana, Ficus repens, Hedera helix, Epipremnum, aureus, Philodendron, scandens, Dizygotheca, elegantissima | Woody plants species accumulate dry mass (and carbon) better than smaller, herbaceous species. | [163] |
| Other Effects | Green Wall | Results | Ref. |
|---|---|---|---|
| Alcohols, CO, O3, CO2, NO2, VOC | – | The performances and limitations of electronic gas sensors to investigate an indoor air quality event were evaluated. | [152] |
| PM2.5, black carbon, ultrafine particles, sulfur, total volatile organic compounds (VOCs), formaldehyde | – | Households (with green wall and without the green wall) were compared. | [170] |
| Simulation-Based Analysis of the Energy Conservation Effect of Green Wall. | H. helix | Green wall induced loss and gains energy in winter and summer | [171] |
| Effects of vegetation on indoor thermal comfort; simulation. | Hedera helix | Improved consumption energy and decreased temperature up to 4.8 °C. | [96] |
| The effect of an indoor living wall system on humidity, mold spores, and CO2 concentration. | – | Indoor living wall caused a higher comfort level in the winter months. In summer, the humidity was also higher, ehile the temperature was similar. The concentration of CO2 decrease by 3.49% | [167] |
| Thermal regulation impact | Hedera helix | Decreasing temperature by 6.1 °C, 4.0 °C for sunny and cloudy days, respectively | [94] |
| The incident acoustic absorption coefficient | Nephrolepis Exaltata (Boston Fern) and Helxine soleirolii (Baby Tears) | The combination of Fern and substrate absorption coefficient reached a value of 0.75 at 300 Hz, then decreased at 700 Hz, after it has steadily increased up 0.9 at 1600 Hz. The absorption coefficient of the combination of Baby Tears and soil increased less steeply than the other one, reaching 0.95 at 1600 Hz. | [168] |
| Cooling effect | Kudzu (Pueraria lobata) | The average transpiration cooling effect calculated from the indoor wall surface temperatures was 0.23 °C, although the average shading cooling effect was 8.55 °C under global solar radiation on a vertical south surface between 400 and 600 Wm−2. Also, those calculated from room temperatures under the same environmental conditions were 0.15 and 4.00 °C, respectively. | [172] |
| CO2, acetone methyl acetate Formaldehyde and PM | Green wall | The active green wall had high efficiency to increase indoor air quality, such as absorbing VOC and PM. But it has not been highly effective for carbon dioxide adsorption. The green wall increased the relative humidity, which is a suitable selection to use in a dry environment. | [137] |
| CO2 removal | Chlorophytum comosum and Epipremnum aureum | Both of the plants were effective in CO2 removal at densities higher than 50 μmol m−2 s−1. when the intensity of the light increased, the green wall was capable of significant quantifiable reductions in high CO2 concentrations within a sealed room environment. | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandehali, S.; Miri, T.; Onyeaka, H.; Kumar, P. Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls. Atmosphere 2021, 12, 473. https://doi.org/10.3390/atmos12040473
Bandehali S, Miri T, Onyeaka H, Kumar P. Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls. Atmosphere. 2021; 12(4):473. https://doi.org/10.3390/atmos12040473
Chicago/Turabian StyleBandehali, Samaneh, Taghi Miri, Helen Onyeaka, and Prashant Kumar. 2021. "Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls" Atmosphere 12, no. 4: 473. https://doi.org/10.3390/atmos12040473
APA StyleBandehali, S., Miri, T., Onyeaka, H., & Kumar, P. (2021). Current State of Indoor Air Phytoremediation Using Potted Plants and Green Walls. Atmosphere, 12(4), 473. https://doi.org/10.3390/atmos12040473









