Tree-Ring Isotopes Provide Clues for Sink Limitation on Treeline Formation on the Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Conditions and Sampling
2.2. Tree-Ring Width Methods
2.3. Tree-Ring δ13C and δ18O Methods
3. Results
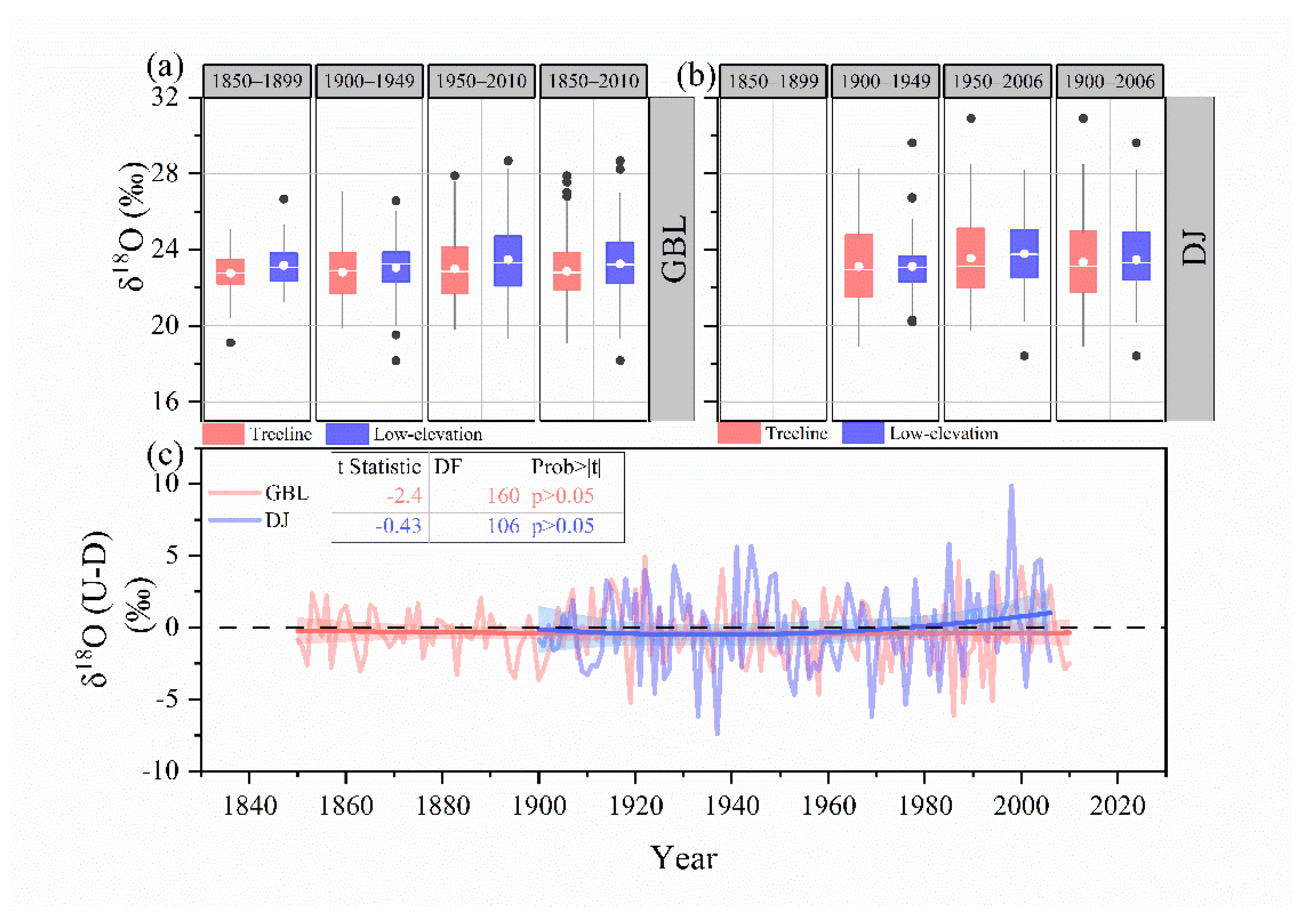
3.1. Changes in Tree Growth and Cellulose Stable Isotopes
3.2. Conceptual Model of δ13C and δ18O Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sanchez-Salguero, R.; Fajardo, A.; McIntire, E.J.B.; Gutierrez, E.; Batllori, E.; Boudreau, S.; Carrer, M.; Diez, J.; et al. Global fading of the temperature-growth coupling at alpine and polar treelines. Glob. Chang. Biol. 2021, 27, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liang, E.; Wang, Y.; Babst, F.; Camarero, J.J. Mountain treelines climb slowly despite rapid climate warming. Glob. Ecol. Biogeogr. 2021, 30, 305–315. [Google Scholar] [CrossRef]
- Susiluoto, S.; Hilasvuori, E.; Berninger, F. Testing the growth limitation hypothesis for subarctic Scots pine. J. Ecol. 2010, 98, 1186–1195. [Google Scholar] [CrossRef]
- Li, M.-H.; Xiao, W.-F.; Wang, S.-G.; Cheng, G.-W.; Cherubini, P.; Cai, X.-H.; Liu, X.-L.; Wang, X.-D.; Zhu, W.-Z. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Holtmeier, F.-K.; Broll, G. Treeline Research—From the Roots of the Past to Present Time. A Review. Forests 2020, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef]
- Hoch, G.; Popp, M.; Körner, C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 2002, 98, 361–374. [Google Scholar] [CrossRef]
- Shi, P.; Körner, C.; Hoch, G. End of season carbon supply status of woody species near the treeline in western China. Basic Appl. Ecol. 2006, 7, 370–377. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. The carbon charging of pines at the climatic treeline: A global comparison. Oecologia 2003, 135, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Viñegla, B.; Linares, J.C.; Camarero, J.J.; Cavieres, L.A.; Fajardo, A. Mediterranean and temperate treelines are controlled by different environmental drivers. J. Ecol. 2016, 104, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.P.; De Kauwe, M.G.; Bastos, A.; Belmecheri, S.; Georgiou, K.; Keeling, R.F.; McMahon, S.M.; Medlyn, B.E.; Moore, D.J.P.; Norby, R.J.; et al. Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. New Phytol. 2021, 229, 2413–2445. [Google Scholar] [CrossRef]
- Dolezal, J.; Kopecky, M.; Dvorsky, M.; Macek, M.; Rehakova, K.; Capkova, K.; Borovec, J.; Schweingruber, F.; Liancourt, P.; Altman, J. Sink limitation of plant growth determines tree line in the arid Himalayas. Funct. Ecol. 2019, 33, 553–565. [Google Scholar] [CrossRef]
- Li, M.-H.; Xiao, W.-F.; Shi, P.; Wang, S.-G.; Zhong, Y.-D.; Liu, X.-L.; Wang, X.-D.; Cai, X.-H.; Shi, Z.-M. Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 2008, 31, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, T.; Haukioja, E.; Suomela, J. Effects of Simulated Defoliation and Debudding on Needle and Shoot Growth in Scots Pine (Pinus sylvestris): Implications of Plant Source/Sink Relationships for Plant-Herbivore Studies. Funct. Ecol. 1994, 8, 631. [Google Scholar] [CrossRef]
- Myers, D.A.; Thomas, R.B.; DeLucia, E.H. Photosynthetic Responses of Loblolly Pine (Pinus Taeda) Needlesto Experimental Reduction in Sink Demand. Tree Physiol. 1999, 19, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Handa, I.T.; Körner, C.; Hättenschwiler, S. Conifer stem growth at the altitudinal treeline in response to four years of CO2 enrichment. Glob. Chang. Biol. 2006, 12, 2417–2430. [Google Scholar] [CrossRef]
- Guillemot, J.; Martin-StPaul, N.K.; Dufrene, E.; Francois, C.; Soudani, K.; Ourcival, J.-M.; Delpierre, N. The dynamic of the annual carbon allocation to wood in European tree species is consistent with a combined source–sink limitation of growth: Implications for modelling. Biogeosciences 2015, 12, 2773–2790. [Google Scholar] [CrossRef] [Green Version]
- Lyytikäinen-Saarenmaa, P. The responses of scots pine, pinus sylvestris, to natural and artificial defoliation stress. Ecol. Appl. 1999, 9, 469–474. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I.; Pfund, L.; Körner, C.; Hoch, G. Variation of mobile carbon reserves in trees at the alpine treeline ecotone is under environmental control. New Phytol. 2012, 195, 794–802. [Google Scholar] [CrossRef]
- Tranquillini, W. Physiological Ecology of the Alpine Treeline; Springer: Berlin, Germany, 1979. [Google Scholar]
- Susiluoto, S.; Perämäki, M.; Nikinmaa, E.; Berninger, F. Effects of sink removal on transpiration at the treeline: Implications for the growth limitation hypothesis. Environ. Exp. Bot. 2007, 60, 334–339. [Google Scholar] [CrossRef]
- Dang, H.S.; Zhang, K.R.; Zhang, Q.F.; Xu, Y.M. Temporal variations of mobile carbohydrates in Abies fargesii at the upper tree limits. Plant Biol. 2015, 17, 106–113. [Google Scholar] [CrossRef]
- Berninger, F.; Sonninen, E.; Aalto, T.; Lloyd, J. Modeling 13C discrimination in tree rings. Glob. Biogeochem. Cycles 2000, 14, 213–223. [Google Scholar] [CrossRef]
- Betson, N.R.; Johannisson, C.; Löfvenius, M.O.; Grip, H.; Granström, A.; Högberg, P. Variation in the δ13C of foliage of Pinus sylvestris L. in relation to climate and additions of nitrogen: Analysis of a 32-year chronology. Glob. Chang. Biol. 2007, 13, 2317–2328. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Sveinbjörnsson, B. North American and European treelines: External factors and internal processes controlling position. Ambio 2000, 29, 388–395. [Google Scholar] [CrossRef]
- Mathias, J.M.; Thomas, R.B. Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO2 and modulated by climate and plant functional types. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubic, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. PlantMol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Guerrieri, R.; Belmecheri, S.; Ollinger, S.V.; Asbjornsen, H.; Jennings, K.A.; Xiao, J.F.; Stocker, B.D.; Martin, M.E.; Hollinger, D.Y.; Brachogarrillo, R.; et al. Disentangling the role of photosynthesis and stomatal conductanceon rising forest water-use efficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 16909–16914. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhao, L.; Voelker, S.; Xu, G.; Zeng, X.; Zhang, X.; Zhang, L.; Sun, W.; Zhang, Q.; Wu, G.; et al. Warming and CO2 enrichment modified the ecophysiological responses of Dahurian larch and Mongolia pine during the past century in the permafrost of northeastern China. Tree Physiol. 2018, 39, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.C.; Ballantyne, A.P.; Cooper, L.A.; Sala, A. Limited evidence for CO2-related growth enhancement in northern Rocky Mountain lodgepole pine populations across climate gradients. Glob. Chang. Biol. 2018, 24, 3922–3937. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, X.; Chen, T.; Xu, G.; Wang, W.; Zeng, X.; Zhang, X. Elevation-dependent variations of tree growth and intrinsic water-use efficiency in Schrenk spruce (Picea schrenkiana) in the western Tianshan Mountains, China. Front. Plant Sci. 2015, 6, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, H.R.; Brooks, J.R.; Bond, B.J. Applying the dual-isotope conceptual model to interpret physiological trends under uncontrolled conditions. Tree Physiol. 2012, 32, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, Y.; Saurer, M.; Bahn, M.; Siegwolf, R. Linking stable oxygen and carbon isotopes with stomatal conductance andphotosynthetic capacity: A conceptual model. Oecologia 2000, 125, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.C.; Gao, Y.X. Meteorology of the Qinghai-Xizang (Tibet) Plateau; Science Press: Beijing, China, 1979. (In Chinese) [Google Scholar]
- Xu, L.-X. Ecology Episode of Xi Zang 50 Year; National Publishing House: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Shi, C.; Masson-Delmotte, V.; Risi, C.; Eglin, T.; Stievenard, M.; Pierre, M.; Wang, X.; Gao, J.; Bréon, F.-M.; Zhang, Q.-B.; et al. Sampling strategy and climatic implications of tree-ring stable isotopes on the southeast Tibetan Plateau. Earth Planet. Sci. Lett. 2011, 301, 307–316. [Google Scholar] [CrossRef]
- Leavitt, S.W. Tree-ring C–H–O isotope variability and sampling. Sci. Total Environ. 2010, 408, 5244–5253. [Google Scholar] [CrossRef]
- Loader, N.J.; Robertson, I.; Barker, A.C.; Switsur, V.R.; Waterhouse, J.S. An improved technique for the batch pro-cessing of small wholewood samples to α-cellulose. Chem. Geol. 1997, 136, 313–317. [Google Scholar] [CrossRef]
- Laumer, W.; Andreu, L.; Helle, G.; Schleser, G.H.; Wieloch, T.; Wissel, H. A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses. Rapid Commun. Mass Spectrom. 2009, 23, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R. Carbon and Water Relations in Desert Plants: An Isotopic Perspective. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press: London, UK, 1993; pp. 155–172. [Google Scholar]
- Wu, G.; Liu, X.; Chen, T.; Xu, G.; Wang, W.; Zeng, X.; Wang, B.; Zhang, X. Long-term variation of tree growth and intrinsic water-use efficiency in Schrenk spruce with increasing CO2 concentration and climate warming in the western Tianshan Mountains, China. Acta Physiol. Plant. 2015, 37, 150. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z.; Van Der Sleen, P.; Zuidema, P.A. Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate. Glob. Chang. Biol. 2019, 26, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, S.R.; Wang, Y.; Camarero, J.J.; Zhu, H.; Liang, E.; Peñuelas, J. Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas. Glob. Chang. Biol. 2018, 24, 5549–5559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth inbristlecone pine at the highest elevations and possible causes. Proc. Nat. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Haverd, V.; Smith, B.; Canadell, J.G.; Cuntz, M.; Mikaloff-Fletcher, S.; Farquhar, G.; Woodgate, W.; Briggs, P.R.; Trudinger, C.M. Higher than expected CO2 fertilization inferred from leaf to global observations. Glob. Chang. Biol. 2020, 26, 2390–2402. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-G.; Bergeron, Y.; Denneler, B.; Berninger, F.; Tardif, J. Response of Forest Trees to Increased Atmospheric CO2. Crit. Rev. Plant Sci. 2007, 26, 265–283. [Google Scholar] [CrossRef]
- Streit, K.; Siegwolf, R.T.W.; Hagedorn, F.; Schaub, M.; Buchmann, N. Lack of photosynthetic or stomatal regulation after 9 years of elevated [CO2] and 4 years of soil warming in two conifer species at the alpine treeline. Plant Cell Environ. 2013, 37, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.C.R.; Horwath, W.R. Explaining Global Increases in Water Use Efficiency: Why Have We Overestimated Responses to Rising Atmospheric CO2 in Natural Forest Ecosystems? PLoS ONE 2013, 8, e53089. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sancho, E.; Dorado-Liñán, I.; Merino, E.G.; Matiu, M.; Helle, G.; Heinrich, I.; Menzel, A. Increased water-use efficiency translates into contrasting growth patterns of Scots pine and sessile oak at their southern distribution limits. Glob. Chang. Biol. 2017, 24, 1012–1028. [Google Scholar] [CrossRef]
- Lévesque, M.; Siegwolf, R.; Saurer, M.; Eilmann, B.; Rigling, A. Increased water-use efficiency does not lead to en-hanced tree growth under xeric and mesic conditions. New Phytol. 2014, 203, 94–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Guerrero, A.; Silva, L.C.R.; Barrera-Reyes, M.; Kishchuk, B.; Velázquez-Martínez, A.; Martínez-Trinidad, T.; Plascencia-Escalante, F.O.; Horwath, W.R. Growth decline and divergent tree ring isotopic composition (δ13C and δ18O) contradict predictions of CO2 stimulation in high altitudinal forests. Glob. Chang. Biol. 2013, 19, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Gazol, A.; Mayr, C.; Camarero, J.J. Recent decadal drought reverts warming-triggered growth en-hancement in contrasting climates in the southern Andes tree line. J. Biogeogr. 2019, 46, 1367–1379. [Google Scholar]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2010, 20, 597–608. [Google Scholar] [CrossRef]
- Peters, W.; Van Der Velde, I.R.; Van Schaik, E.; Miller, J.B.; Ciais, P.; Duarte, H.F.; Van Der Laan-Luijkx, I.T.; Van Der Molen, M.K.; Scholze, M.; Schaefer, K.; et al. Increased water-use efficiency and reduced CO2 uptake by plants during droughts at a continental scale. Nat. Geosci. 2018, 11, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Fajardo, A.; Hoch, G. Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiol. 2017, 37, 1001–1010. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Génard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [Green Version]
- Wieser, G. Carbon dioxide gas exchange of cembran pine (Pinus cembra) at the alpine timberline during winter. Tree Physiol. 1997, 17, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; DesLauriers, A.; Griçar, J.; Seo, J.-W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Körner, C.; Riedl, S. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springer: Basel, Switzerland, 2012; p. 357. [Google Scholar]
- Girardin, M.P.; Bouriaud, O.; Hogg, E.H.; Kurz, W.; Zimmermann, N.E.; Metsaranta, J.M.; Jong, R.; Frank, D.C.; Esper, J.; Buntgen, U.; et al. No growth stimulation of Canada’s boreal forets under half-century of combined warming and CO2 fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, E8406–E8414. [Google Scholar] [CrossRef] [Green Version]
- Giguère-Croteau, C.; Boucher, É.; Bergeron, Y.; Girardin, M.P.; Drobyshev, I.; Silva, L.C.R.; Hélie, J.-F.; Garneau, M. North America’s oldest boreal trees are more efficient water users due to increased [CO2], but do not grow faster. Proc. Natl. Acad. Sci. USA 2019, 116, 2749–2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.C.R.; Anand, M. Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob. Ecol. Biogeogr. 2012, 22, 83–92. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, Q.-B.; Pellatt, M.G.; Büntgen, U.; Li, M.-H.; Cherubini, P. Drought limitation on tree growth at the Northern Hemisphere’s highest tree line. Dendrochronologia 2019, 53, 40–47. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef] [Green Version]
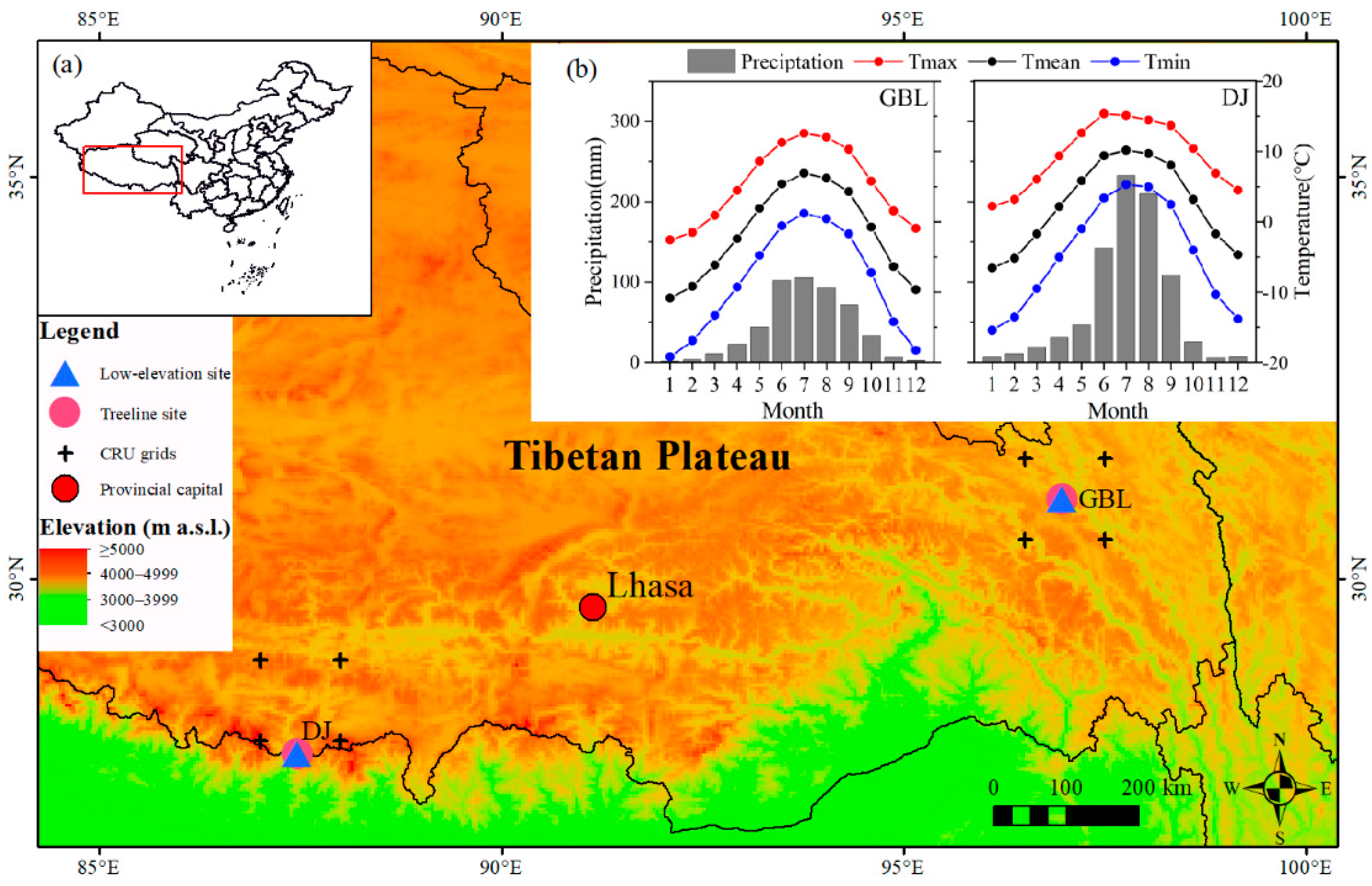




| Statistic Characteristics | GBLU | GBLD | DJU | DJD |
|---|---|---|---|---|
| Time span | 1773–2010 | 1621–2010 | 1888–2006 | 1897–2006 |
| Year since EPS > 0.85 | 1820–2010 | 1635–2010 | 1890–2006 | 1960–2006 |
| Mean sensitivity | 0.105 | 0.1 | 0.105 | 0.1 |
| Standard deviation | 0.19 | 0.173 | 0.23 | 0.137 |
| Autocorrelation order 1 | 0.765 | 0.761 | 0.833 | 0.606 |
| Signal-to-noise ratio * | 28.4 | 33 | 17.2 | 10.8 |
| Expressed population signal * | 0.94 | 0.934 | 0.931 | 0.72 |
| Variance in first eigenvector (%) * | 57 | 61.1 | 80.2 | 41.2 |
| δ13C and δ18O time span | 1850–2010 | 1850–2010 | 1900–2006 | 1900–2006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, X.; Wang, X.; Lyu, L. Tree-Ring Isotopes Provide Clues for Sink Limitation on Treeline Formation on the Tibetan Plateau. Atmosphere 2021, 12, 540. https://doi.org/10.3390/atmos12050540
Pu X, Wang X, Lyu L. Tree-Ring Isotopes Provide Clues for Sink Limitation on Treeline Formation on the Tibetan Plateau. Atmosphere. 2021; 12(5):540. https://doi.org/10.3390/atmos12050540
Chicago/Turabian StylePu, Xing, Xiaochun Wang, and Lixin Lyu. 2021. "Tree-Ring Isotopes Provide Clues for Sink Limitation on Treeline Formation on the Tibetan Plateau" Atmosphere 12, no. 5: 540. https://doi.org/10.3390/atmos12050540






